Abstract
There is increasing evidence for the involvement of blood–brain barrier (BBB) in vascular dementia (VaD) and Alzheimer´s disease (AD) pathogenesis. However, the role of endothelial function-related genes in these disorders remains unclear. We evaluated the association of four single-nucleotide polymorphisms (VEGF, VEGFR2 and NOS3) with diagnosis and rate of cognitive decline in AD and VaD in a Spanish case–control cohort (150 VaD, 147 AD and 150 controls). Participants carrying -604AA genotype in VEGFR2 (rs2071559) were less susceptible to VaD after multiple testing. Further analysis for VaD subtype revealed a significant difference between small-vessel VaD patients and controls, but not for large-vessel VaD patients. In addition, -2578A and -460C alleles in VEGF (rs699947 and rs833061) showed to decrease the risk of AD, whereas NOS3 (rs1799983) influenced disease progression. Our study supports previous findings of a deleterious effect of VEGFR2 reduced expression on small-vessel disease, but not on large-vessel disease; as well as a detrimental effect of down-regulating VEGF and eNOS in AD, affecting vascular permeability and neuronal survival. These data highlight the relevance of endothelial function and, therefore, BBB in both VaD and AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia, followed by vascular dementia (VaD). AD is characterized by extracellular deposits of amyloid-β peptide (Aβ), intracellular neurofibrillary tangles containing hyperphosphorylated tau protein and neuronal loss, whereas VaD is due to clinical stroke or subclinical vascular brain injury1,2. Beyond the monogenic forms, the majority of AD and VaD cases are sporadic disorders resulting from the interaction of multiple genetic and environmental factors. To date, apolipoprotein E (APOE)-ε4 is the only genetic factor consistently associated with both disorders3,4. In VaD, heterogeneity of the cerebrovascular mechanisms underlying this condition (e.g., cardioembolic, atherosclerotic, ischemic, haemorrhagic, etc.) creates challenges for research. In AD, genome-wide association studies (GWAS) have proposed several new susceptibility genes, but these variants only suppose a modest level of risk and its mechanisms of action in AD pathogenesis are partially unknown5.
Other genetic risk factors of AD and VaD still need to be found, and it seems likely that genetic variants in relation to critical biological processes constitute potential candidates6. Interestingly, recent evidence supports an important role of blood–brain barrier (BBB) dysfunction in both entities7,8,9. In that regard, three BBB endothelial proteins, vascular endothelial growth factor (VEGF), type 2 VEGF receptor (VEGFR2), and endothelial nitric oxide synthase (eNOS), have been proposed to underlie the onset and progression of the pathological hallmarks in AD as well as affect the response of the brain to vascular disease10,11,12.
VEGF is a cytokine induced by hypoxia that favours vascular permeability and angiogenesis, neuroprotection, neuronal survival, regeneration, differentiation and axonal outgrowth13,14. Increased concentrations of VEGF have been reported in cerebral vessels, neurons and reactive astrocytes in the neocortex of AD patients, and especially within Aβ plaques. Hence, continuous sequestration of VEGF into amyloid plaques during the progression of AD has been suggested to provoke deficiency of available VEGF, and therefore vascular dysfunction and neurodegeneration18. Additionally, high levels of VEGF have been found in cerebrospinal fluid (CSF) of patients with VaD19. A few studies have investigated the association between the VEGF gene (6p21.3) and AD with inconsistent results; whereas the haplotype GTC at G-1154A, G-7A, and C13553T of the VEGF gene has been associated with VaD in Koreans20,21.
VEGFR2 (also called kinase insert domain-containing receptor [KDR]) is a key receptor for VEGF. VEGF-VEGFR2 signalling has been involved in the development of vascular diseases, as VEGF-VEGFR2 binding promotes angiogenesis, i.e., proliferation, migration and survival of the endothelial cell13,22. Besides, an anti-angiogenic effect of Aβ peptides in AD have been partially attributed to the fact that Aβ1-42 is able to compete with VEGF by interacting directly with VEGFR223. Despite the biological and pathological significance of VEGFR2, research concerning the role of VEGFR2 gene (4p12) on the risk of dementias is lacking.
eNOS catalyzes the conversion of amino acid L-arginine to nitric oxide (NO) in endothelial cells where it helps maintain homeostasis by inducing vasodilatation, anti-inflammatory, antithrombotic and antiproliferative properties24. In AD, loss of eNOS contributes to cerebral blood vessels stiffening thereby diminishing clearance of Aβ and, in cultured human brain microvascular endothelium, increasing expression of β-amyloid protein precursor (AβPP) and β-site APP-cleaving enzyme 1 (BACE) thus favouring production of cytotoxic Aβ peptides25,26,27. eNOS also modulates synaptic function in the hippocampus, which is the first and most severely affected brain region in the pathogenesis of AD28. Therefore, the NOS3 gene (7q35) has been proposed as candidate for association with VaD and AD. So far, studies in several AD populations yielded conflicting results29. An increased risk of incident dementia in stroke survivors older than 75 years from the UK has been reported, but this study evaluated poststroke dementia (not only VaD)30.
On this basis we investigated whether the VEGF rs699947, VEGF rs833061, VEGFR2 rs2071559 and NOS3 rs1799983 polymorphisms influence or not the susceptibility to VaD and late-onset AD, as well as disease progression, in a Spanish population.
Results
This study included 150 VaD patients (74 large-vessel VaD and 76 small-vessel VaD), 147 AD patients, and 150 controls. Baseline characteristics of patients and controls are summarized in Table 1. Mean age at onset was 75,5 (SD 6,8) years for AD and 74,2 (SD 7,4) years for VaD. Compared with controls separately, VaD cases were well-matched in terms of gender, but were significantly younger and less educated. On the contrary, AD patients showed a higher number of females than controls (63.3 vs. 49.3, P = 0.016), while no differences were found in terms of age or education. As expected, VaD patients had a higher prevalence of hypertension, diabetes mellitus, hypercholesterolemia, and heavier alcohol consumption. Analyzing demographic and vascular risk factors by VaD subtype, both large-vessel and small-vessel VaD cases were younger and had higher alcohol intake history as compared with controls, albeit only small-vessel VaD patients had a significantly lower education, more hypertension, diabetes mellitus and hypercholesterolemia than controls (all P < 0.05). In contrast, AD participants had less hypertension and diabetes mellitus percentage compared to the control group. For this reason, SNPs analyses were calculated with an adjustment for these variables in the different groups.
A comparison of genotype frequencies of the VEGF rs699947 and rs833061, VEGFR2 rs2071559 and NOS3 rs1799983 polymorphisms between VaD and AD patients and control group is displayed in Table 2. Genotype distributions of each polymorphism in the control samples did not deviate from those expected based on the Hardy–Weinberg equilibrium. There was strong linkage disequilibrium of the VEGF polymorphisms at loci -2578 (rs699947) and -460 (rs833061) in the three groups (D´ = 1.0).
When the genetic data were analyzed adjusting for age and gender with multivariate logistic regression analysis, carrying the A allele of the VEGFR2 rs2071559 polymorphism was found to diminish in a half the risk of developing overall VaD in the recessive model, P = 0.014 OR = 0.51 (0.30–0.87). None of the SNPs rs699947 and rs833061 for VEGF and rs1799983 for NOS3 was related to VaD risk (Table 2).
The global study of susceptibility in AD patients showed that carriers of AA genotype and CA + AA (A-allele bearing) genotypes in the SNP VEGF rs699947 polymorphism as well as carriers of CC genotype and TC + CC (C-allele bearing) genotypes in the SNP VEGF rs833061 polymorphism had a decreased risk to develop AD, both in the codominant model, P = 0.027 OR = 0.45 (0.22–0.91), and the dominant model, P = 0.040 OR = 0.56 (0.33–0.97) after adjustment for age and gender with multivariate logistic regression analysis. No other associations were found in the rest of SNPs between AD cases and controls (Table 2).
Analysis according to the VaD subtype (small-vessel VaD or large-vessel VaD) showed an association between the GG genotype of VEGFR2 rs2071559 and higher risk to suffer from small-vessel VaD, P = 0.011 in codominant model, OR = 2.91 (1.28–6.63); likewise, those with the allele A had a lower risk to develop this subtype of VaD, P = 0.002 in recessive model, OR = 0.37 (0.20–0.70), which persisted statistically significant after controlling for education and vascular risk factors as covariates (Table 3). Conversely, no associations were found for VEGFR2 rs2071559 polymorphism when genotypes were compared between large-vessel VaD and control participants.
Due to the great significant differences in carriers of the APOE ε4 allele between AD patients (40.8%) and controls (11.3%), P = 0.0001 OR = 5.90 (3.16–11.01), a second analysis in these groups was proposed by APOE rs429358 ε4 status. Among the subgroup of APOE ε4 non-carriers, VEGF rs699947 AA and rs833061 CC genotypes were significantly associated with a decreased AD risk in the codominant model, P = 0.021 OR = 0.38 (0.17–0.86); P = 0.021 and, similarly, participants with the C allele in the SNP VEGF rs699947 or the T allele in the SNP VEGF rs833061 had higher risk to suffer from AD in our sample, P = 0.033 in recessive model, OR = 2.17 (1.06–4.42), which remained statistically significant after controlling for vascular risk factors. Among the subgroup of APOE ε4 carriers, although not statistically significant a tendency in VEGF rs699947 and rs833061 was found, that reached significant differences under the heterozygous additive and codominant models (P = 0.026) after correction for education and vascular risk factors as covariates (Table 4).
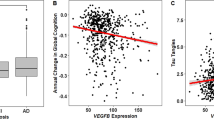
We next investigated the role of SNPs in APOE, VEGF, VEGFR2 and NOS3 genes on disease progression. The NOS3 rs1799983 GG genotype was found to be an independent protective factor of rapid progression, P = 0.048 OR = 0.22 (0.05–0.99) after adjusting for classic factors usually affecting AD progression (Table 5). The MMSE rate (MMSE decay/follow-up time expressed in years) values were also significantly lower in NOS3 rs1799983 GG when compared with GT + TT genotypes (P = 0.035) (Fig. 1). Further a general lineal model confirmed that NOS3 rs1799983 T allele was an independent marker for faster decline, P = 0.028, B 0.556 (0.061–1.051), after covariate adjustment. However, for APOE, VEGF and VEGFR2 genotypes, the lack of association was the rule among analyzed end points (Table 5).
NOS3 (also so-called eNOS) rs1799983NOS G/G genotype determines a less rapid cognitive decline in Alzheimer´s disease. The study included 147 (GG:48; GT:69; TT:30) patients with AD. Scores of the MMSE rate (MMSE decay/follow-up time expressed in years) derived from GG and GT + TT genotypes were measured. Box plots show median values (horizontal line inside the box), quartiles (box boundaries), and the largest and smallest observed values (error bars). Statistical significance for difference using Mann–Whitney U test: **P < 0.05 compared with GG genotype.
Discussion
We conducted a case–control study to investigate the relationship between several endothelial function-related gene polymorphisms, VaD and AD in a Spanish population. At the epidemiological level, there were more women in the group of participants with AD than in the control group, in agreement with several studies in Europe. The higher prevalence of AD in women could be in some extent explained due to differences in the following factors: a) longevity and survival bias -there are more women at older ages, when the development of AD is more likely-, b) comorbidities -e.g., women have twice the risk of depression at midlife, which is believed to increase the risk of AD-, c) biological hormonal factors -indeed, oophorectomy, menopause and androgen-deprivation therapy have been associated with deleterious cognitive changes in the literature-; and d) sociocultural factors -in the past century, women had fewer opportunities for higher education and occupational attainment and also exercise less than men at midlife, and both education and exercise are associated with a decreased risk of AD31.
The major findings in our study are that VEGFR2 rs2071559 A allele protects against VaD, whereas VEGF rs699947 A and rs833061 C alleles show to decrease the risk of AD and NOS3 rs1799983 influences disease progression. Our data also show strong linkage disequilibrium between the SNPs in VEGF gene analyzed (rs699947 A and rs833061 C), which is consistent with prior studies32.
Noteworthy, when we grouped the patients by VaD subtype, the VEGFR2 -604A allele conferred a significant decreased risk for small-vessel VaD, but not for large-vessel VaD, suggesting a difference in the pathophysiological mechanisms underlying both VaD subtypes. Although there have been no prior reports of an association between VEGFR2 polymorphisms and VaD in any population, these results are in agreement with existing literature in stroke patients, which is limited to Asian population. Oh et al. observed that individuals carrying the VEGFR2 + 1719 T allele had an increased risk of ischemic stroke in small-vessel disease (SVD) patients. Despite the fact that there was no association between SNP − 604 and SNP + 1192 and ischemic stroke risk, GGT, GAT and GGT haplotypes of -604A > G, + 1192G > A, and + 1719A > T VEGFR2 polymorphisms increased risk of ischemic stroke33. In another Chinese study, the + 1192A allele was associated not only with increased susceptibility to intracerebral haemorrhage (ICH), but also it was a prognostic factor for stroke recurrence, whereas the -604G allele predicted a reduced susceptibility to atherothrombotic stroke and stroke recurrence, and was reversely correlated with carotid artery intima media thickness34. Thereafter, Han et al. described a higher risk of silent brain infarcts in men and younger than 65 years carrying the -604G allele in a Korean population35.
VEGFR2 is the main receptor for VEGF in endothelial cells and endothelial progenitor cells. Binding of VEGF to VEGFR2 is followed by activation of downstream signalling pathways such as PI3-K/Akt, PLC/PKC, Src, MEK/ERK and eNOS, which are essential for migration, proliferation and survival of endothelial cells, thereby stimulating angiogenesis14,36. VEGF can also prevent oxidized-LDL–induced endothelial cell damage via an intracellular glutathione-dependent mechanism through VEGFR237. Several SNPs of VEGR2 are known to inhibit the activity of VEGF-VEGFR2 signalling pathway. The minor G allele of SNP –604A > G leads to structural alteration of the binding site for transcriptional factor E2F (involved in cell cycle regulation, interacting with Rb p107 protein) in VEGFR2 gene promoter region, which suppresses VEGFR2 expression by 68%. The minor A allele of SNP + 1192G > A in exon 7 and the minor T allele of SNP + 1719A > T in exon 11 have been found to reduce binding affinity of VEGF to VEGFR232,38.
Interestingly, the different consequences for different strokes subtypes of genetic variants in VEGFR2 gene could be explained by a dual role of the VEGF-VEGFR2 system, enhancing both physiological and pathological angiogenesis35. It has been proposed that the allele -604G in VEGFR2 gene, by down-regulating VEGF-VEGFR2 signalling, causes disrupted endothelial cell development and defective blood vessel formation, decreases the integrity of vascular endothelium as well as inhibits endothelial repair, eventually leading to small-arterial occlusion and SVD34,35,39. Similarly, vascular degeneration and formation of weak, thin-walled vasculature can reduce vessel compliance and increase the risk of spontaneous vessel wall rupture and ICH under some stresses such as hypertension and increased shear stress34,40. In contrast, the allele -604G could exert a protective effect on large-artery atherosclerosis by reducing neovascularisation and inflammation, retarding atherosclerotic lesions growth and the plaque destabilization leading to rupture in major cranial arteries34,35. Albeit the decrease in VEGFR2 function could inhibit atherosclerosis, a damaging effect diminishing the maintenance of endothelial integrity may be more profound35. Noteworthy, though the degree of VEGFR2 expression in small artery myocytes has been associated with the aging brain, the relationship between VEGFR2 and small-vessel VaD in our research is preserved irrespective of age41.
Besides its involvement in the vascular system, neurotrophic effects of VEGF have also been ascribed to VEGFR2 signal36. In ischemic stroke, VEGF is induced in the ischemic border zone and acts on local neurons to promote neuroprotection42. VEGF also stimulates neurogenesis in the subventricular zone of the lateral ventricles and in the subgranular zone of the hippocampal dentate gyrus, from which new neurons migrate to the site of ischemia43. Hence, functional recovery following stroke depends partly on neuronal plasticity in non-ischemic regions44. In this regard, one study evidenced that the beneficial effects of bone marrow mononuclear cells inoculation in an animal model of VaD (which increase level of VEGF as well as levels of p-Raf1 and p-ERK –downstream proteins in the VEGFR2 signalling pathway–, increase vascular density, reduce white matter lesions, and finally, lead to a better cognitive outcome) were abolished by using VEGFR2 inhibitor SU541645.
On the other hand, carriers of the VEGF -2578A and -460C alleles, both SNPs in linkage disequilibrium in the promoter region of the gene, decreased the risk to suffer from AD in our study. In vitro experiments have demonstrated that VEGF binding to Aβ-40 and Aβ-42 within the amyloid plaques in the brain of AD patients might result in sequestration and local deficiency of available VEGF and, subsequently, contribute to insufficient vascularisation and reduced cerebral perfusion16,17,18. Cerebral hypoperfusion is common in AD, initially in the posterior cingulate and precuneus areas, and later in medial temporal regions46. VEGF elevations have been postulated to counteract these deleterious effects of the AD pathological cascade by enhancing vascular survival47. In fact, patients with AD exhibited lower levels of cerebral capillary VEGF expression in the hippocampus, superior temporal cortex, and brainstem than controls, whereas treating AD mice models with cells secreting VEGF yielded reductions in memory impairment, tau and amyloid burden48,49,50. In addition, Hohman et al. reported that increased levels of VEGF in CSF were associated with improved hippocampal volume, episodic memory, and executive function51.
Nevertheless, CSF levels of VEGF in patients with AD are discordant. Tarkowski et al. evidenced higher CSF levels of VEGF in AD and VaD than controls; Blasko et al. demonstrated no difference in CSF VEGF levels between AD and controls; and data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) found lower CSF levels of VEGF capable to distinguish AD from controls with 76% sensitivity and a 84% specificity19,52,53. This inconsistence agrees with the results from a meta-analysis containing 7 studies (2731 AD patients and 2442 controls), in which 3 studies observed an association between VEGF-2578C > A polymorphisms and risk of AD and 4 studies did not20. Similarly, contradictory results have also been reported about serum VEGF levels in AD patients, independently of VEGF genotypes18,54. We consider some concurrent diseases that might up-regulate VEGF (e.g., tumours, ischemia, trauma and inflammation), differences in technologies used, sample sizes and populations included would explain variations in CSF and serum levels of VEGF55. We excluded participants with these disorders mentioned above in our work in order to avoid confusion factors.
Finally, we demonstrated that a rapid decline occurs in patients with AD if the NOS3 rs1799983 T allele is present. This category of patients consistently seems to have a more aggressive disease and thus needs particular attention at follow-up. It has been suggested that the 894G > T polymorphism in the NOS3 gene influences AD development by increasing the production of endothelial NO56,57. In AD brains, the deposits of Aβ can generate superoxide radicals that react with NO to form peroxynitrate, which can cause oxidative stress to further accelerate neurodegenerative changes leading to AD26,27,28,58,59,60. In this sense, Chrysohoou et al. observed that compared with the NOS3 894GG genotype, carriers of the 894TT genotype had higher levels of inflammatory and oxidative stress markers including fibrinogen, leukocytes, oxidized low-density lipoprotein cholesterol, homocysteine, C-reactive protein and Aβ levels, all of them often observed in neurodegenerative processes61.
Although APOE is well characterized as a disease risk modulator, its importance as a predictor of progression is not confirmed in the present study, which supports the results of a meta-analysis suggesting that the presence of the APOE ε4 allele does not contribute to the rate of cognitive decline in persons with AD62. The strength of our analysis is that we kept in mind many factors that have been related to disease progression (i.e., education, psychotic symptoms, treatment with any cholinesterase inhibitor, memantine, or both) and could distort observed associations63.
The main limitation of this study is that it is only of moderate size. Therefore, future studies with a larger subject size would be necessary to examine the potential importance of VEGFR2 and VEGF genes as novel genetic risk markers for VaD (particularly small-vessel VaD) and AD, respectively, in addition to the influence of NOS3 gene on AD progression. Our findings would also need to be validated in other ethnic groups.
In conclusion, our results provide evidence of the putative role of some polymorphisms in endothelial function-related genes as genetic susceptibility factors in both VaD and AD and boost to further investigate angiogenesis as a new target for dementia prevention and treatment.
Materials and methods
Study design and population
Patients enrolled in this case–control study (160 VaD patients and 160 AD patients) were recruited consecutively from September 2005 to January 2007 within the Neurology Department, Complejo Asistencial Universitario de Salamanca [CAUSA] (Salamanca, Spain) and from March 2011 to January 2012 within the Neurology Division, Complejo Asistencial de Ávila (Avila, Spain) and Outpatients Departments from which we receive referrals. The inclusion of AD patients started in September 2011. Neuropsychological protocols, a detailed structured interview, and clinical examinations were performed. All patients had morphologic and/or functional neuroradiological testing together with the usual battery of screening blood tests to exclude treatable causes of dementia. The National Institute on Aging and Alzheimer's Association (NIA-AA) criteria were fulfilled by patients with AD64, and the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria by patients with VaD65. In addition, VaD patients were classified according to the radiological NINDS-AIREN criteria as having large-vessel or cortical VaD [cVaD] (strategic large-vessel infarct of the dominant hemisphere or bilateral hemispheric strokes) or small-vessel or subcortical VaD [sVaD] (white-matter hyperintensities involving at least a quarter of the white matter, multiple lacunes or bilateral thalamic lesions)65. We exclude patients with ischemic-hypoperfusive or hemorrhagic VaD, as well as those presenting both vascular and Alzheimer features (mixed dementia). All dementia cases were defined as sporadic VaD or late-onset AD because there was neither an autosomal dominant dementia trait nor a first degree relative diagnosed with familial dementia. Cognitive impairment was assessed using the Mini-Mental State Examination (MMSE)66. Cognitively healthy controls (n = 160) were recruited consecutively from individuals older than 75 years who attended a health screening in the outpatient clinics of the participating institutions, from June 2011 to November 2011. Assessment of controls included a full medical history and a physical examination. All the healthy controls that had MMSE scores of ≤ 28 and a history of neurological or psychiatric disease were excluded. Furthermore, none of the patients or controls was affected by cancer or chronic inflammatory diseases.
All the individuals included in the study were of Caucasian origin and live in Castilla y Leon, a central-western region of Spain. The study protocol was in accordance with the Declaration of Helsinki, approved by the clinical research ethics committees of the healthcare areas of Salamanca and Avila and complied with Spanish data protection law (LO 15/1999) and specifications (RD 1720/2007). Written informed consent was obtained from the patients or their legal guardians when patients had serious cognitive impairment and control participants. For each participant, a questionnaire was administered to gather information on demography, vascular risk factors and life style. The following variables were included in the analyses: a history of hypertension (blood pressure ≥ 140/90 mmHg measured on the right arm in supine position at two different occasions in an interval of at least two weeks in between or diagnosis of hypertension previous to the use of anti-hypertensive medication), type 2 diabetes mellitus (symptoms with random glucose > 200 mg/dL [11,1 mmol/L], fasting plasma glucose > 126mg/dL [7 mmol/L], plasma glucose two hours after a 75 g oral glucose tolerance test > 200 mg/dL [11,1 mmol/L] or diagnosis of diabetes previous to the use of diabetic medication), hypercholesterolemia (total cholesterol > 190 mg/dL or LDL-cholesterol > 115 mg/dL, or previous diagnosis and cholesterol lowering diet or drugs), tobacco use (any current smoking status or ex-smokers for less than 5 years), and alcohol consumption (alcohol intake ≥ 40 g per day). We collected a blood sample from each participant in a tube containing sodium EDTA.
Moreover, 152 AD patients from the AD group underwent neurological evaluation by a neurologist at least in three different evaluations (basal plus two follow-up examinations). For the disease progression calculation, we used MMSE variation during follow-up. More precisely, for each patient, we computed MMSE values and their timing of administration, for example, at the time of AD diagnosis (basal) and at the last available follow-up data point for MMSE scale. AD patients were classified according to the cut-off established by Cortes et al.67 as having rapid progression (individuals with MMSE rate, MMSE decay/follow-up time expressed in years, higher than 4.5) or normal progression (AD patients with MMSE score point decrease per year lower or equal to 4.5).
After further exclusion of participants without blood samples and patients without clinical follow-up data available, a total of 150 VaD patients (sVaD-74, cVaD-76) 147 AD patients and 150 controls were included for data analysis (response rate: 93.8% for VaD and controls and 91.9% for AD).
DNA isolation and genotyping
DNA was extracted from peripheral blood leukocytes following the phenol–chloroform method. Determination of the single nucleotide polymorphisms (SNPs) of + 334 T > C (E4; rs429358) in apolipoprotein E (APOE), -2578C > A (rs699947) and -460C > T (rs833061) in the promoter region of VEGF, -604A > G (rs2071559) in the promoter region of VEGFR2 and + 894G > T (rs1799983) in exon seven of eNOS were carried out with TaqMan_ SNP Genotyping Assays (assay ID: c_3084793_20, c_1647381_10, c_8311602_10, c_158969271_10 and C_3219460_20, respectively) on a ABI Prism 7300 HT Real-Time polymerase chain reaction (RT-PCR) System (Applied Biosystems Inc., Foster City, CA). The reactions were set up in a 96-well plate and were performed in a final volume of 10 μl with 0.5 μl of sample DNA, 0.25 μl TaqMan-specific assay, 4.25 μl distilled H20 and 5 μl Taq-Man Genotyping Master Mix. The amplification conditions were as follows: 60 ºC initial denaturation for 30 s followed by 40 cycles of 95 ºC denaturation for 10 min, 95 ºC annealing for 15 s and elongation at 60 ºC for 1 min. In all PCR reactions, a final elongation step was applied at 60 ºC for 30 s. To ensure the reproducibility, a 5% of random samples were re-genotyping.
Statistical analysis
In baseline characteristics, categorical variables − presented as numbers and percentages − were analyzed by using the χ2 test, whereas the Student´s t test was required for continuous variables − expressed as mean values ± standard deviation (SD) − when the normality of the distribution was determined by the Kolmogorov–Smirnov test. The χ2 test was also used to assess the Hardy–Weinberg equilibrium was accomplished in control group participants for each polymorphism and to compare genotype frequencies between cases and controls. The relative risk of dementia for each polymorphism was estimated in odds ratios (ORs) and 95% confidence intervals (CIs). Three genetic models were taken into account: the dominant model (Mm + mm vs. MM), the codominant model (mm vs. Mm vs. MM), and the recessive model (mm vs. MM + Mm); in which “M” indicates the major allele and “m” the minor allele. Associations of SNPs with VaD and AD were analyzed by multivariate logistic regression adjusted for possible confounders, including age, gender, education, APOE ε4 allele and vascular risk factors. We applied the Bonferroni´s correction for multiple comparisons since some stratification of the samples was performed.
Three different measurements were employed to assess the influence of SNPs on disease progression, in order to determine consistent effects regardless of statistical method or covariates selected. The Mann–Whitney U test and a general lineal model were used to evaluate the role of each SNP in MMSE rate (MMSE decay/follow-up time [in years]), whereas logistic regression analysis, after adjusting for factors generally affecting AD progression68 (age at diagnosis, gender, education, depression, psychosis, treatment with cholinesterase inhibitors, memantine, or both, and follow-up time), was calculated to investigate genotype effects on disease progression phenotypes. . Depression and psychosis (delusions/hallucinations) were assessed at an interview with a responsible caregiver by using the corresponding sub-scales in the Neuropsychiatric Inventory69. Each sub-scale has an entry question inquiring whether the disturbance had been present in the last month. If the answer is affirmative, the caregiver is asked to rate the specific symptom on a four-point frequency and on a three-point severity scale; subsequently, frequency and severity scores are multiplied (composite score). A composite score of 4 or more on an individual subscale (delusions or hallucinations) or 6 or more on a combined subscale (delusions plus hallucinations) was used to identify the presence of psychosis clinically relevant, whereas a composite score of 4 or more on the depression domain was required for a diagnosis of depression.
Statistics were carried out with the statistical software package SPSS (version 21.0. SPSS Inc. [Chicago, IL]). For all statistical analyses, P-values < 0.05 were considered to reach statistical significance.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gatz, M. et al. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63, 168–174 (2006).
Gorelick, P.B., et al; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2, 2672–2713 (2011).
Corder, E. H. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923 (1993).
Manso-Calderón, R. & González-Sarmiento, R. Genetic susceptibility to vascular cognitive impairment: A pathophysiological view. Fut. Neurol. 11, 119–134 (2016).
Ikram, M. A. et al. Genetics of vascular dementia–review from the ICVD working group. BMC Med. 15, 48. https://doi.org/10.1186/s12916-017-0813-9 (2017).
Hachinski, V. et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37, 2220–2241 (2006).
Zlokovic, B. V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201 (2008).
Stanimirovic, D. B. & Friedman, A. Pathophysiology of the neurovascular unit: Disease cause or consequence?. J. Cereb. Blood Flow Metab. 32, 1207–1221 (2012).
Enciu, A. M., Constantinescu, S. N., Popescu, L. M., Mureşanu, D. F. & Popescu, B. O. Neurobiology of vascular dementia. J Aging Res 2011, 401604. https://doi.org/10.4061/2011/401604 (2011).
Provias, J. & Jeynes, B. Neurofibrillary tangles and senile plaques in Alzheimer´s brains are associated with reduced capillary expression of vascular endothelial growth factor and endothelial nitric oxide synthase. Curr. Neurovasc. Res. 5, 199–205 (2008).
Hermann, D. M. & Zechariah, A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J. Cereb Blood Flow Metab. 29, 1620–1643 (2009).
Moro, M. A., Cárdenas, A., Hurtado, O., Leza, J. C. & Lizasoain, I. Role of nitric oxide after brain ischaemia. Cell Calcium 36, 265–275 (2004).
Ferrara, N., Gerber, H. P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Ma, Y., Qu, Y. & Fei, Z. Vascular endothelial growth factor in cerebral ischemia. J. Neurosci. Res. 89, 969–978 (2011).
Kalaria, R. N. et al. Vascular endothelial growth factor in Alzheimer’s disease and experimental cerebral ischemia. Brain Res. Mol. Brain Res. 62, 101–105 (1998).
Storkebaum, E. & Carmeliet, P. VEGF: A critical player in neurodegeneration. J. Clin. Invest. 113, 14–18 (2004).
Yang, S. P. et al. Co-accumulation of vascular endothelial growth factor with beta-amyloid in the brain of patients with Alzheimer’s disease. Neurobiol. Aging 25, 283–290 (2004).
Mateo, I. et al. Low serum VEGF levels are associated with Alzheimer’s disease. Acta Neurol. Scand 116, 56–58 (2007).
Tarkowski, E. et al. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol. Aging 23, 237–243 (2002).
He, D. et al. Vascular endothelial growth factor polymorphisms and risk of Alzheimer’s disease: A meta-analysis. Gene 518, 296–302 (2013).
Kim, Y., Nam, Y. J. & Lee, C. Haplotype analysis of single nucleotide polymorphisms in VEGF gene for vascular dementia. Am. J. Med. Genet. B Neuropsychiatr. Gene 141B, 332–335 (2006).
Takahashi, H. & Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. (Lond) 109, 227–241 (2005).
Patel, N. S. et al. Alzheimer’s beta-amyloid peptide blocks vascular endothelial growth factor mediated signaling via direct interaction with VEGFR-2. J. Neurochem. 112, 66–76 (2010).
Endres, M., Laufs, U., Liao, J. K. & Moskowitz, M. A. Targeting eNOS for stroke protection. Trends Neurosci. 27, 283–289 (2004).
Li, S., Wang, W., Wang, C. & Tang, Y. Y. Possible involvement of NO/NOS signaling in hippocampal amyloid-β production induced by transient focal cerebral ischemia in aged rats. Neurosci. Lett. 470, 106–110 (2010).
Aliev, G. et al. Nitric oxide as an initiator of brain lesions during the development of Alzheimer disease. Neurotox Res. 16, 293–305 (2009).
de la Monte, S. M. et al. Nitric oxide synthase-3 overexpression causes apoptosis and impairs neuronal mitochondrial function: Relevance to Alzheimer’s-type neurodegeneration. Lab Invest. 83, 287–298 (2003).
Malinski, T. Nitric oxide and nitroxidative stress in Alzheimer’s disease. J. Alzheimers Dis. 11, 207–218 (2007).
Alzheimer Research Forum, Drugs in Clinical Trials: AAB-001, http://www.alzforum.org/drg/drc/detail.asp?id=101 (2012).
Morris, C. M. et al. NOS3 gene rs1799983 polymorphism and incident dementia in elderly stroke survivors. Neurobiol. Aging 32(554), e1-6 (2011).
Nebel, R. A. et al. Understanding the impact of sex and geneder in Alzheimer´s disease: A call to action. Alzheimers Dement 14, 1171–1183 (2018).
Yang, X. et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol. Vis. 17, 3088–3096 (2011).
Oh, S. H. et al. Association between kinase insert domain-containing receptor gene polymorphism and haplotypes and ischemic stroke. J. Neurol. Sci. 308, 62–66 (2011).
Zhang, W. et al. VEGF receptor-2 variants are associated with susceptibility to stroke and recurrence. Stroke 40, 2720–2726 (2009).
Han, I. B. et al. Association between kinase insert domain containing receptor gene polymorphisms and silent brain infarction: A Korean study. J. Neurol. Sci. 318, 85–89 (2012).
Wittko-Schneider, I. M., Schneider, F. T. & Plate, K. H. Brain homeostasis: VEGF receptor 1 and 2-two unequal brothers in mind. Cell Mol. Life Sci. 70, 1705–1725 (2013).
Kuzuya, M. et al. VEGF protects against oxidized LDL toxicity to endothelial cells by an intracellular glutathione-dependent mechanism through the KDR receptor. Arterioscler. Thromb. Vasc. Biol. 21, 765–770 (2001).
Wang, Y. et al. Polymorphisms of KDR gene are associated with coronary heart disease. J. Am. Coll. Cardiol. 50, 760–767 (2007).
Shibuya, M. Tyrosine kinase receptor Flt/VEGFR family: Its characterization related to angiogenesis and cancer. Genes Cancer 1, 1119–1123 (2010).
Qureshi, A. I. et al. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 344, 1450–1460 (2001).
Ahmed-Jushuf, F. et al. Age-dependent expression of VEGFR2 in deep brain arteries in small vessel disease, CADASIL, and healthy brains. Neurobiol. Aging 42, 110–115 (2016).
Wang, Y. et al. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain 128, 52–63 (2005).
Stowe, A. M. et al. VEGF protein associates to neurons in remote regions following cortical infarct. J. Cereb Blood Flow Metab 27, 76–85 (2007).
Reitmeir, R. et al. Vascular endothelial growth factor induces contralesional corticobulbar plasticity and functional neurological recovery in the ischemic brain. Acta Neuropathol. 123, 273–284 (2012).
Wang, J. et al. Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signalling pathway in a rat model of vascular dementia. Behav. Brain Res. 265, 171–180 (2014).
Matsuda, H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann. Nucl. Med. 15, 85–92 (2001).
Religa, P. et al. VEGF significantly restores impaired memory behavior in Alzheimer’s mice by improvement of vascular survival. Sci. Rep. 3, 2053. https://doi.org/10.1038/srep02053 (2013).
Provias, J. & Jeynes, B. Reduction in vascular endothelial growth factor expression in the superior temporal, hippocampal, and brainstem regions in Alzheimer’s disease. Curr. Neurovasc. Res. 11, 202–209 (2014).
Garcia, K. O. et al. Therapeutic effects of the transplantation of VEGF overexpressing bone marrow mesenchymal stem cells in the hippocampus of murine model of Alzheimer’s disease. Front. Aging Neurosci. 6, 30. https://doi.org/10.3389/fnagi.2014.00030 (2014).
Wang, P. et al. VEGF-induced angiogenesis ameliorates the memory impairment in APP transgenic mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 411, 620–626 (2011).
Hohman, T. J., Bell, S. P. & Jefferson, A. L. Alzheimer’s Disease Neuroimaging Initiative. The role of vascular endothelial growth factor in neurodegeneration and cognitive decline: Exploring interactions with biomarkers of Alzheimer disease. JAMA Neurol. 72, 520–529 (2014).
Blasko, I. et al. Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement Geriatr. Cogn. Disord. 21, 9–15 (2006).
Guo, L.-H., Alexopoulos, P. & Perneczky, R. Heart-type fatty acid binding protein and vascular endothelial growth factor: Cerebrospinal fluid biomarker candidates for Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 263, 553–560 (2013).
Zhang, J. B. et al. Association of serum vascular endothelial growth factor levels and cerebral microbleeds in patients with Alzheimer’s disease. Eur. J. Neurol. 23, 1337–1342 (2016).
Huang, L., Jia, J. & Liu, R. Decreased serum levels of the angiogenic factors VEGF and TGF-1 inAlzheimer’s disease and amnestic mild cognitive impairment. Neurosci. Lett. 550, 60–63 (2013).
Veldman, B. A. et al. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J. Hypertens 20, 2023–2027 (2002).
Liu, S. et al. The nitric oxide synthase 3 G894T polymorphism associated with Alzheimer’s disease risk: A meta-analysis. Sci. Rep. 5, 13598. https://doi.org/10.1038/srep13598 (2015).
Patel, V. P. & Chu, C. T. Nuclear transport, oxidative stress, and neurodegeneration. Int. J. Clin. Exp. Pathol. 4, 215–229 (2011).
Austin, S. A., Santhanam, A. V., Hinton, D. J., Choi, D. S. & Katusic, Z. S. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 127, 691–700 (2013).
Austin, S. A., Santhanam, A. V. & Katusic, Z. S. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ. Res. 107, 1498–1502 (2010).
Chrysohoou, C. et al. Evidence for association between endothelial nitric oxide synthase gene polymorphism (G894T) and inflammatory markers: The ATTICA study. Am. Heart J. 148, 733–738 (2004).
Allan, C. L. & Ebmeier, K. P. The influence of ApoE4 on clinical progression of dementia: A meta-analysis. Int. J. Geriatr. Psychiatry 26, 520–526 (2011).
Ruiz, A. et al. Exploratory analysis of seven Alzheimer’s disease genes: Disease progression. Neurobiol. Aging 34(4), 1310–1317 (2013).
McKhann, G. M. et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269 (2011).
Román, G. C. et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260 (1993).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. ‘“Mini-mental state”’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Cortes, F. et al. REAL-FR Group. Prognosis of Alzheimer’s disease today: A two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement 4, 22–29 (2008).
de Oliveira, F. F. et al. Lifetime risk factors for functional and cognitive outcomes in patients with Alzheimer’s disease. J. Alzheimers Dis. 65, 1283–1299 (2018).
Cummings, J. L. et al. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314 (1994).
Acknowledgements
This research was funded by a grant (FIS PI 10/00219) belonging to the Institute of Health Carlos III, Government of Spain. Moreover, the authors gratefully acknowledge all the staff at Hospital Universitario de Salamanca and Hospital Nuestra Señora de Sonsoles who contributed to recruitment of participants, as well as Centro de Referencia Estatal de Atención a Personas con Enfermedad de Alzheimer y otras Demencias (Salamanca, Spain), Asociación de familiares de enfermos de Alzheimer (Avila, Spain) and Centro Residencial Decanos (Avila, Spain) for providing a subset of the AD patients in this study. The authors also thank the individuals who participated in this study and Mercedes Sánchez-Barba for assistance in statistical analysis.
Author information
Authors and Affiliations
Contributions
The study conception and design were elaborated by R.M.C., P.C.P. & R.G.S. R.M.C., P.C.P., M.D.S.G. & M.E.H.P. recruited the patients and collected the data. The genetic analysis of the samples was performed by R.M.C. R.M.C., P.C.P. & R.G.S. analysed the results. R.M.C. did the statistical analysis and the study supervision or coordination was done by P.C.P. & R.G.S. R.M.C. did the drafting of the article. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manso-Calderón, R., Cacabelos-Pérez, P., Sevillano-García, M.D. et al. Analysis of endothelial gene polymorphisms in Spanish patients with vascular dementia and Alzheimer´s disease. Sci Rep 13, 13441 (2023). https://doi.org/10.1038/s41598-023-39576-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39576-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.