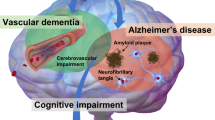
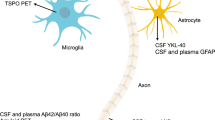
Abstract
As disease-specific interventions for dementia are being developed, the ability to identify the underlying pathology and dementia subtypes is increasingly important. Vascular cognitive impairment and dementia (VCID) is the second most common cause of dementia after Alzheimer disease, but progress in identifying molecular biomarkers for accurate diagnosis of VCID has been relatively limited. In this Review, we examine the roles of large and small vessel disease in VCID, considering the underlying pathophysiological processes that lead to vascular brain injury, including atherosclerosis, arteriolosclerosis, ischaemic injury, haemorrhage, hypoperfusion, endothelial dysfunction, blood–brain barrier breakdown, inflammation, oxidative stress, hypoxia, and neuronal and glial degeneration. We consider the key molecules in these processes, including proteins and peptides, metabolites, lipids and circulating RNA, and consider their potential as molecular biomarkers alone and in combination. We also discuss the challenges in translating the promise of these biomarkers into clinical application.
Key points
-
VCID has multiple underlying pathologies that can contribute to cognitive impairment.
-
Identification of molecular biomarkers that can differentiate VCID from healthy ageing and Alzheimer disease remains challenging.
-
Multiple molecular biomarkers have been associated with VCID, but none has yet been translated into clinical application.
-
The heterogeneity and complexity of VCID means that use of multiple biomarkers in combination tends to be necessary.
-
Promising biomarkers related to various pathophysiological pathways could be combined into panels to optimize sensitivity and specificity; machine learning could be useful for constructing these panels.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gorelick, P. B. et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713 (2011). An overall guide for practitioners to gain a better understanding of VCID.
Kalaria, R. N. & Ballard, C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis. Assoc. Disord. 13, S115–S123 (1999).
Iadecola, C. et al. Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 73, 3326–3344 (2019). A critical appraisal of the epidemiology, pathobiology, neuropathology and neuroimaging of VCID.
Simrén, J. et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement. 17, 1145–1156 (2021).
Jack, C. R. Jr. et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Iadecola, C. The pathobiology of vascular dementia. Neuron 80, 844–866 (2013). A review of the pathophysiology of VCID.
van der Flier, W. M. et al. Vascular cognitive impairment. Nat. Rev. Dis. Prim. 4, 18003 (2018). A comprehensive review of VCID.
Zhou, X. J., Vaziri, N. D., Wang, X. Q., Silva, F. G. & Laszik, Z. Nitric oxide synthase expression in hypertension induced by inhibition of glutathione synthase. J. Pharmacol. Exp. Ther. 300, 762–767 (2002).
Dowsett, L. et al. ADMA: a key player in the relationship between vascular dysfunction and inflammation in atherosclerosis. J. Clin. Med. 9, 3026 (2020).
Iadecola, C. & Gottesman, R. F. Neurovascular and cognitive dysfunction in hypertension. Circ. Res. 124, 1025–1044 (2019).
Iadecola, C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42 (2017).
Kernagis, D. N. & Laskowitz, D. T. Evolving role of biomarkers in acute cerebrovascular disease. Ann. Neurol. 71, 289–303 (2012).
Faraco, G. et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 21, 240–249 (2018).
Faraco, G. et al. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 574, 686–690 (2019).
Shimokawa, H. & Godo, S. Nitric oxide and endothelium-dependent hyperpolarization mediated by hydrogen peroxide in health and disease. Basic Clin. Pharmacol. Toxicol. 127, 92–101 (2020).
Zlokovic, B. V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 12, 723–738 (2011). A review of pathophysiology of the neurovascular unit.
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018). A review of the blood–brain barrier in Alzheimer disease and other neurodegenerative disorders.
Nation, D. A. et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276 (2019).
Sweeney, M. D. et al. A novel sensitive assay for detection of a biomarker of pericyte injury in cerebrospinal fluid. Alzheimers Dement. 16, 821–830 (2020).
Rosenberg, G. A. Willis lecture: biomarkers for inflammatory white matter injury in Binswanger disease provide pathways to precision medicine. Stroke 53, 3514–3523 (2022).
Nikolakopoulou, A. M. et al. Pericyte loss leads to circulatory failure and pleiotrophin depletion causing neuron loss. Nat. Neurosci. 22, 1089–1098 (2019).
Procter, T. V., Williams, A. & Montagne, A. Interplay between brain pericytes and endothelial cells in dementia. Am. J. Pathol. 191, 1917–1931 (2021).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Touyz, R. M. & Briones, A. M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens. Res. 34, 5–14 (2011).
Mayhan, W. G., Arrick, D. M., Sharpe, G. M. & Sun, H. Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation 15, 225–236 (2008).
Dong, Y. F. et al. Attenuation of brain damage and cognitive impairment by direct renin inhibition in mice with chronic cerebral hypoperfusion. Hypertension 58, 635–642 (2011).
Santhanam, A. V., d’Uscio, L. V. & Katusic, Z. S. Erythropoietin increases bioavailability of tetrahydrobiopterin and protects cerebral microvasculature against oxidative stress induced by eNOS uncoupling. J. Neurochem. 131, 521–529 (2014).
Prasad, K. AGE-RAGE stress: a changing landscape in pathology and treatment of Alzheimer’s disease. Mol. Cell Biochem. 459, 95–112 (2019).
Tang, Y. & Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194 (2016).
Guo, S., Wang, H. & Yin, Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 14, 815347 (2022).
Kim, E., Otgontenger, U., Jamsranjav, A. & Kim, S. S. Deleterious alteration of glia in the brain of Alzheimer’s disease. Int. J. Mol. Sci. 21, 6676 (2020).
Chen, B., Cheng, Q., Yang, K. & Lyden, P. D. Thrombin mediates severe neurovascular injury during ischemia. Stroke 41, 2348–2352 (2010).
Zoia, A., Drigo, M., Caldin, M., Simioni, P. & Piek, C. J. Fibrinolysis in dogs with intracavitary effusion: a review. Animals 12, 2487 (2022).
Chen, Z. L. & Strickland, S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell 91, 917–925 (1997).
Ihara, M. et al. Chronic cerebral hypoperfusion induces MMP-2 but not MMP-9 expression in the microglia and vascular endothelium of white matter. J. Cereb. Blood Flow Metab. 21, 828–834 (2001).
Arai, K. & Lo, E. H. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 29, 4351–4355 (2009).
Ihara, M. & Tomimoto, H. Lessons from a mouse model characterizing features of vascular cognitive impairment with white matter changes. J. Aging Res. 2011, 978761 (2011).
Rosenberg, G. A. Binswanger’s disease: biomarkers in the inflammatory form of vascular cognitive impairment and dementia. J. Neurochem. 144, 634–643 (2018).
Wardlaw, J. M. et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology 82, 1331–1338 (2014).
Saadoun, D., Vautier, M. & Cacoub, P. Medium- and large-vessel vasculitis. Circulation 143, 267–282 (2021).
Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 65, 252–256 (2015).
Gaengel, K., Genové, G., Armulik, A. & Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 630–638 (2009).
Silva, I. T., Mello, A. P. & Damasceno, N. R. Antioxidant and inflammatory aspects of lipoprotein-associated phospholipase A2 (Lp-PLA2): a review. Lipids Health Dis. 10, 170 (2011).
Huang, Y. T., Hong, F. F. & Yang, S. L. Atherosclerosis: the culprit and co-victim of vascular dementia. Front. Neurosci. 15, 673440 (2021).
Velican, C. Studies on the age-related changes occurring in human cerebral arteries. Atherosclerosis 11, 509–529 (1970).
Ritz, K., Denswil, N. P., Stam, O. C., van Lieshout, J. J. & Daemen, M. J. Cause and mechanisms of intracranial atherosclerosis. Circulation 130, 1407–1414 (2014). A review of the pathophysiology of intracranial atherosclerosis.
Resch, J. A. & Baker, A. B. Etiologic mechanisms in cerebral atherosclerosis. Preliminary study of 3,839 cases. Arch. Neurol. 10, 617–628 (1964).
D’Armiento, F. P. et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke 32, 2472–2479 (2001).
Fatkin, D., Kelly, R. P. & Feneley, M. P. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J. Am. Coll. Cardiol. 23, 961–969 (1994).
Blann, A. D., Nadar, S. K. & Lip, G. Y. The adhesion molecule P-selectin and cardiovascular disease. Eur. Heart J. 24, 2166–2179 (2003).
Kamel, H., Okin, P. M., Elkind, M. S. & Iadecola, C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke 47, 895–900 (2016).
Magid-Bernstein, J. et al. Cerebral hemorrhage: pathophysiology, treatment, and future directions. Circ. Res. 130, 1204–1229 (2022). A review of the pathophysiology of cerebral haemorrhage.
Jolink, W. M. T. et al. Location-specific risk factors for intracerebral hemorrhage: systematic review and meta-analysis. Neurology 95, e1807–e1818 (2020).
Kremer, P. H., Jolink, W. M., Kappelle, L. J., Algra, A. & Klijn, C. J. Risk factors for lobar and non-lobar intracerebral hemorrhage in patients with vascular disease. PLoS ONE 10, e0142338 (2015).
Magaki, S. et al. Charcot-Bouchard aneurysms revisited: clinicopathologic correlations. Mod. Pathol. 34, 2109–2121 (2021).
Jin, J. et al. Inflammation and immune cell abnormalities in intracranial aneurysm subarachnoid hemorrhage (SAH): relevant signaling pathways and therapeutic strategies. Front. Immunol. 13, 1027756 (2022).
Aoki, T. & Nishimura, M. Targeting chronic inflammation in cerebral aneurysms: focusing on NF-kappaB as a putative target of medical therapy. Expert Opin. Ther. Targets 14, 265–273 (2010).
Aoki, T. & Narumiya, S. Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 33, 304–311 (2012).
Zhang, Z. et al. New mechanisms and targets of subarachnoid hemorrhage: a focus on mitochondria. Curr. Neuropharmacol. 20, 1278–1296 (2022).
Budohoski, K. P. et al. The pathophysiology and treatment of delayed cerebral ischaemia following subarachnoid haemorrhage. J. Neurol. Neurosurg. Psychiatry 85, 1343–1353 (2014). A review of the pathophysiology of subarachnoid haemorrhage.
National Center for Advancing Translational Sciences (NCATS). Toolkit for patient-focused therapy development. https://toolkit.ncats.nih.gov/module/discovery/developing-translational-research-tools/biomarkers/ (accessed 19 October 2023).
Shoamanesh, A. et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology 84, 825–832 (2015).
Zuliani, G. et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer’s disease or vascular dementia. J. Neurol. Sci. 272, 164–170 (2008).
El Husseini, N. et al. Vascular cellular adhesion molecule-1 (VCAM-1) and memory impairment in African-Americans after small vessel-type stroke. J. Stroke Cerebrovasc. Dis. 29, 104646 (2020).
Engelhart, M. J. et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch. Neurol. 61, 668–672 (2004).
Lavallee, P. C. et al. Circulating markers of endothelial dysfunction and platelet activation in patients with severe symptomatic cerebral small vessel disease. Cerebrovasc. Dis. 36, 131–138 (2013).
Janes, F. et al. ADMA as a possible marker of endothelial damage. A study in young asymptomatic patients with cerebral small vessel disease. Sci. Rep. 9, 14207 (2019).
Pikula, A. et al. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham Offspring Study. Stroke 40, 2959–2964 (2009).
Gao, Q. et al. S100B and ADMA in cerebral small vessel disease and cognitive dysfunction. J. Neurol. Sci. 354, 27–32 (2015).
Notsu, Y. et al. Evaluation of asymmetric dimethylarginine and homocysteine in microangiopathy-related cerebral damage. Am. J. Hypertens. 22, 257–262 (2009).
Ihara, M., Washida, K., Yoshimoto, T. & Saito, S. Adrenomedullin: a vasoactive agent for sporadic and hereditary vascular cognitive impairment. Cereb. Circ. Cogn. Behav. 2, 100007 (2021).
Kis, B. et al. Cerebral endothelial cells are a major source of adrenomedullin. J. Neuroendocrinol. 14, 283–293 (2002).
Kuriyama, N. et al. Association between mid-regional proadrenomedullin levels and progression of deep white matter lesions in the brain accompanying cognitive decline. J. Alzheimers Dis. 56, 1253–1262 (2017).
Carmeliet, P. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7, 575–583 (2001).
Hinman, J. D. et al. Placental growth factor as a sensitive biomarker for vascular cognitive impairment. Alzheimers Dement. 19, 3519–3527 (2023).
Luna, R. L. et al. Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Mol. Hum. Reprod. 22, 130–142 (2016).
Erhardt, E. B. et al. Inflammatory biomarkers aid in diagnosis of dementia. Front. Aging Neurosci. 13, 717344 (2021).
Wang, J. et al. Dynamic changes of CSF sPDGFRβ during ageing and AD progression and associations with CSF ATN biomarkers. Mol. Neurodegener. 17, 9 (2022).
Sagare, A. P., Sweeney, M. D., Makshanoff, J. & Zlokovic, B. V. Shedding of soluble platelet-derived growth factor receptor-β from human brain pericytes. Neurosci. Lett. 607, 97–101 (2015).
Montagne, A. et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Miners, J. S., Kehoe, P. G., Love, S., Zetterberg, H. & Blennow, K. CSF evidence of pericyte damage in Alzheimer’s disease is associated with markers of blood-brain barrier dysfunction and disease pathology. Alzheimers Res. Ther. 11, 81 (2019).
Lv, X. et al. Changes in CSF sPDGFRβ level and their association with blood-brain barrier breakdown in Alzheimer’s disease with or without small cerebrovascular lesions. Alzheimers Res. Ther. 15, 51 (2023).
Bjerke, M. et al. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J. Alzheimers Dis. 27, 665–676 (2011).
Adair, J. C. et al. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke 35, e159–e162 (2004).
Candelario-Jalil, E. et al. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke 42, 1345–1350 (2011).
Erhardt, E. B. et al. Biomarkers identify the Binswanger type of vascular cognitive impairment. J. Cereb. Blood Flow Metab. 39, 1602–1612 (2019).
Gong, M. & Jia, J. Contribution of blood-brain barrier-related blood-borne factors for Alzheimer’s disease vs. vascular dementia diagnosis: a pilot study. Front. Neurosci. 16, 949129 (2022).
Wallin, A. et al. Biochemical markers in vascular cognitive impairment associated with subcortical small vessel disease — a consensus report. BMC Neurol. 17, 102 (2017). A review of biomarkers associated with VCID.
Skillback, T. et al. CSF/serum albumin ratio in dementias: a cross-sectional study on 1861 patients. Neurobiol. Aging 59, 1–9 (2017).
Kettunen, P. et al. Blood-brain barrier dysfunction and reduced cerebrospinal fluid levels of soluble amyloid precursor protein-β in patients with subcortical small-vessel disease. Alzheimers Dement. 14, e12296 (2022).
Li, L. et al. Oxidative LDL modification is increased in vascular dementia and is inversely associated with cognitive performance. Free Radic. Res. 44, 241–248 (2010).
Murr, J., Carmichael, P. H., Julien, P. & Laurin, D. Plasma oxidized low-density lipoprotein levels and risk of Alzheimer’s disease. Neurobiol. Aging 35, 1833–1838 (2014).
Fitzpatrick, A. L. et al. Lipoprotein-associated phospholipase A2 and risk of dementia in the Cardiovascular Health Study. Atherosclerosis 235, 384–391 (2014).
Wright, C. B. et al. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke 40, 3466–3471 (2009).
Esterbauer, H., Schaur, R. J. & Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 (1991).
Gustaw-Rothenberg, K., Kowalczuk, K. & Stryjecka-Zimmer, M. Lipids’ peroxidation markers in Alzheimer’s disease and vascular dementia. Geriatr. Gerontol. Int. 10, 161–166 (2010).
Polidori, M. C. et al. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dement. Geriatr. Cogn. Disord. 18, 265–270 (2004).
Helmy, A. A., Naseer, M. M., Shafie, S. E. & Nada, M. A. Role of interleukin 6 and alpha-globulins in differentiating Alzheimer and vascular dementias. Neurodegener. Dis. 9, 81–86 (2012).
Wada-Isoe, K., Wakutani, Y., Urakami, K. & Nakashima, K. Elevated interleukin-6 levels in cerebrospinal fluid of vascular dementia patients. Acta Neurol. Scand. 110, 124–127 (2004).
Nagai, K., Kozaki, K., Sonohara, K., Akishita, M. & Toba, K. Relationship between interleukin-6 and cerebral deep white matter and periventricular hyperintensity in elderly women. Geriatr. Gerontol. Int. 11, 328–332 (2011).
Hoshi, T. et al. Serum inflammatory proteins and frontal lobe dysfunction in patients with cardiovascular risk factors. Eur. J. Neurol. 17, 1134–1140 (2010).
Aribisala, B. S. et al. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 45, 605–607 (2014).
De Luigi, A. et al. Peripheral inflammatory response in Alzheimer’s disease and multiinfarct dementia. Neurobiol. Dis. 11, 308–314 (2002).
Tarkowski, E., Blennow, K., Wallin, A. & Tarkowski, A. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J. Clin. Immunol. 19, 223–230 (1999).
Sproston, N. R. & Ashworth, J. J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754 (2018).
Shang, J. et al. Different associations of plasma biomarkers in Alzheimer’s disease, mild cognitive impairment, vascular dementia, and ischemic stroke. J. Clin. Neurol. 14, 29–34 (2018).
Vishnu, V. Y. et al. Role of inflammatory and hemostatic biomarkers in Alzheimer’s and vascular dementia — a pilot study from a tertiary center in Northern India. Asian J. Psychiatry 29, 59–62 (2017).
Ke, X. J. & Zhang, J. J. Changes in HIF-1alpha, VEGF, NGF and BDNF levels in cerebrospinal fluid and their relationship with cognitive impairment in patients with cerebral infarction. J. Huazhong Univ. Sci. Technol. Med. Sci. 33, 433–437 (2013).
Satizabal, C. L., Zhu, Y. C., Mazoyer, B., Dufouil, C. & Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 78, 720–727 (2012).
van Dijk, E. J. et al. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 112, 900–905 (2005).
Mitaki, S., Nagai, A., Oguro, H. & Yamaguchi, S. C-reactive protein levels are associated with cerebral small vessel-related lesions. Acta Neurol. Scand. 133, 68–74 (2016).
Wada, M. et al. Cerebral small vessel disease and C-reactive protein: results of a cross-sectional study in community-based Japanese elderly. J. Neurol. Sci. 264, 43–49 (2008).
Miralbell, J. et al. Cognitive patterns in relation to biomarkers of cerebrovascular disease and vascular risk factors. Cerebrovasc. Dis. 36, 98–105 (2013).
Masumura, M., Hata, R., Nagai, Y. & Sawada, T. Oligodendroglial cell death with DNA fragmentation in the white matter under chronic cerebral hypoperfusion: comparison between normotensive and spontaneously hypertensive rats. Neurosci. Res. 39, 401–412 (2001).
De Luigi, A. et al. Inflammatory markers in Alzheimer’s disease and multi-infarct dementia. Mech. Ageing Dev. 122, 1985–1995 (2001).
Ali, M. & Bracko, O. VEGF paradoxically reduces cerebral blood flow in Alzheimer’s disease mice. Neurosci. Insights 17, 26331055221109254 (2022).
Tarkowski, E. et al. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer’s disease and vascular dementia. Neurobiol. Aging 23, 237–243 (2002).
Chakraborty, A. et al. Vascular endothelial growth factor remains unchanged in cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Alzheimers Res. Ther. 10, 58 (2018).
Rapisarda, A. & Melillo, G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv. Cancer Res. 114, 237–267 (2012).
Trares, K. et al. Association of the inflammation-related proteome with dementia development at older age: results from a large, prospective, population-based cohort study. Alzheimers Res. Ther. 14, 128 (2022).
Lazarovici, P., Marcinkiewicz, C. & Lelkes, P. I. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF) — implications in drug development. Curr. Pharm. Des. 12, 2609–2622 (2006).
Kandasamy, M. et al. TGF-β signaling: a therapeutic target to reinstate regenerative plasticity in vascular dementia? Aging Dis. 11, 828–850 (2020).
Malaguarnera, L., Motta, M., Di Rosa, M., Anzaldi, M. & Malaguarnera, M. Interleukin-18 and transforming growth factor-beta 1 plasma levels in Alzheimer’s disease and vascular dementia. Neuropathology 26, 307–312 (2006).
Dong, H., Zhang, Y., Huang, Y. & Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 13, 931473 (2022).
Tang, S. C. et al. Elevated plasma level of soluble form of RAGE in ischemic stroke patients with dementia. Neuromol. Med. 19, 579–583 (2017).
Qian, L. et al. Early biomarkers for post-stroke cognitive impairment. J. Neurol. 259, 2111–2118 (2012).
Kim, O. Y. & Song, J. The importance of BDNF and RAGE in diabetes-induced dementia. Pharmacol. Res. 160, 105083 (2020).
Huang, L. K. et al. Plasma phosphorylated-tau181 is a predictor of post-stroke cognitive impairment: a longitudinal study. Front. Aging Neurosci. 14, 889101 (2022).
Pinto-Benito, D., Paradela-Leal, C., Ganchala, D., de Castro-Molina, P. & Arevalo, M. A. IGF-1 regulates astrocytic phagocytosis and inflammation through the p110α isoform of PI3K in a sex-specific manner. Glia 70, 1153–1169 (2022).
Toth, P. et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J. Cereb. Blood Flow Metab. 34, 1887–1897 (2014).
Quinlan, P., Horvath, A., Nordlund, A., Wallin, A. & Svensson, J. Low serum insulin-like growth factor-I (IGF-I) level is associated with increased risk of vascular dementia. Psychoneuroendocrinology 86, 169–175 (2017).
Ban, Y. et al. Impact of increased plasma serotonin levels and carotid atherosclerosis on vascular dementia. Atherosclerosis 195, 153–159 (2007).
Kang, J. et al. Positive association between serum insulin-like growth factor-1 and cognition in patients with cerebral small vessel disease. J. Stroke Cerebrovasc. Dis. 30, 105790 (2021).
Suk, K. Lipocalin-2 as a therapeutic target for brain injury: an astrocentric perspective. Prog. Neurobiol. 144, 158–172 (2016).
Llorens, F. et al. Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia. Nat. Commun. 11, 619 (2020).
Ojala, J. O., Sutinen, E. M., Salminen, A. & Pirttilä, T. Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells. J. Neuroimmunol. 205, 86–93 (2008).
Altendahl, M. et al. An IL-18-centered inflammatory network as a biomarker for cerebral white matter injury. PLoS ONE 15, e0227835 (2020).
Mossanen Parsi, M., Duval, C. & Ariëns, R. A. S. Vascular dementia and crosstalk between the complement and coagulation systems. Front. Cardiovasc. Med. 8, 803169 (2021).
Pyun, J. M., Ryoo, N., Park, Y. H. & Kim, S. Fibrinogen levels and cognitive profile differences in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 49, 489–496 (2020).
Gallacher, J. et al. Is sticky blood bad for the brain?: hemostatic and inflammatory systems and dementia in the Caerphilly Prospective Study. Arterioscler. Thromb. Vasc. Biol. 30, 599–604 (2010).
Carcaillon, L. et al. Elevated plasma fibrin D-dimer as a risk factor for vascular dementia: the Three-City cohort study. J. Thromb. Haemost. 7, 1972–1978 (2009).
Staszewski, J., Piusinska-Macoch, R., Brodacki, B., Skrobowska, E. & Stepien, A. Association between hemostatic markers, serum lipid fractions and progression of cerebral small vessel disease: a 2-year follow-up study. Neurol. Neurochir. Pol. 52, 54–63 (2018).
Wang, X. et al. Endothelial function, inflammation, thrombosis, and basal ganglia perivascular spaces in patients with stroke. J. Stroke Cerebrovasc. Dis. 25, 2925–2931 (2016).
van Oijen, M., Witteman, J. C., Hofman, A., Koudstaal, P. J. & Breteler, M. M. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke 36, 2637–2641 (2005).
Mari, D. et al. Hemostasis abnormalities in patients with vascular dementia and Alzheimer’s disease. Thromb. Haemost. 75, 216–218 (1996).
Tomimoto, H. et al. Coagulation activation in patients with Binswanger disease. Arch. Neurol. 56, 1104–1108 (1999).
Tomimoto, H. et al. The coagulation-fibrinolysis system in patients with leukoaraiosis and Binswanger disease. Arch. Neurol. 58, 1620–1625 (2001).
Nagai, M., Hoshide, S. & Kario, K. Association of prothrombotic status with markers of cerebral small vessel disease in elderly hypertensive patients. Am. J. Hypertens. 25, 1088–1094 (2012).
Tran, N. D., Wong, V. L., Schreiber, S. S., Bready, J. V. & Fisher, M. Regulation of brain capillary endothelial thrombomodulin mRNA expression. Stroke 27, 2304–2310 (1996).
Giwa, M. O. et al. Neuropathologic evidence of endothelial changes in cerebral small vessel disease. Neurology 78, 167–174 (2012).
Cesari, M., Pahor, M. & Incalzi, R. A. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc. Ther. 28, e72–e91 (2010).
Gaffney, P. J. Fibrin(-ogen) interactions with plasmin. Haemostasis 6, 2–25 (1977).
Ban, Y. et al. Increased plasma urotensin-II and carotid atherosclerosis are associated with vascular dementia. J. Atheroscler. Thromb. 16, 179–187 (2009).
Norgren, N., Rosengren, L. & Stigbrand, T. Elevated neurofilament levels in neurological diseases. Brain Res. 987, 25–31 (2003).
Ma, W. et al. Elevated levels of serum neurofilament light chain associated with cognitive impairment in vascular dementia. Dis. Markers 2020, 6612871 (2020).
Peters, N. et al. Serum neurofilament light chain is associated with incident lacunes in progressive cerebral small vessel disease. J. Stroke 22, 369–376 (2020).
Marchegiani, F. et al. Diagnostic performance of new and classic CSF biomarkers in age-related dementias. Aging 11, 2420–2429 (2019).
Sjögren, M. et al. Neurofilament protein in cerebrospinal fluid: a marker of white matter changes. J. Neurosci. Res. 66, 510–516 (2001).
Jonsson, M. et al. Cerebrospinal fluid biomarkers of white matter lesions — cross-sectional results from the LADIS study. Eur. J. Neurol. 17, 377–382 (2010).
Huss, A. et al. Association of serum GFAP with functional and neurocognitive outcome in sporadic small vessel disease. Biomedicines 10, 1869 (2022).
Duering, M. et al. Serum neurofilament light chain levels are related to small vessel disease burden. J. Stroke 20, 228–238 (2018).
Egle, M. et al. Neurofilament light chain predicts future dementia risk in cerebral small vessel disease. J. Neurol. Neurosurg. Psychiatry 92, 582–589 (2021).
Escartin, C. et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 24, 312–325 (2021).
Mecocci, P. et al. Serum autoantibodies against glial fibrillary acidic protein in brain aging and senile dementias. Brain Behav. Immun. 6, 286–292 (1992).
Gattringer, T. et al. Serum glial fibrillary acidic protein is sensitive to acute but not chronic tissue damage in cerebral small vessel disease. J. Neurol. 270, 320–327 (2023).
Fortin, L. J. & Genest, J. Jr. Measurement of homocyst(e)ine in the prediction of arteriosclerosis. Clin. Biochem. 28, 155–162 (1995).
Moretti, R., Giuffré, M., Caruso, P., Gazzin, S. & Tiribelli, C. Homocysteine in neurology: a possible contributing factor to small vessel disease. Int. J. Mol. Sci. 22, 2051 (2021).
Zhang, F., Slungaard, A., Vercellotti, G. M. & Iadecola, C. Superoxide-dependent cerebrovascular effects of homocysteine. Am. J. Physiol. 274, R1704–R1711 (1998).
Weekman, E. M., Woolums, A. E., Sudduth, T. L. & Wilcock, D. M. Hyperhomocysteinemia-induced gene expression changes in the cell types of the brain. ASN Neuro 9, 1759091417742296 (2017).
Malaguarnera, M. et al. Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clin. Chem. Lab. Med. 42, 1032–1035 (2004).
Lehmann, M., Gottfries, C. G. & Regland, B. Identification of cognitive impairment in the elderly: homocysteine is an early marker. Dement. Geriatr. Cogn. Disord. 10, 12–20 (1999).
Nagga, K. et al. Cobalamin, folate, methylmalonic acid, homocysteine, and gastritis markers in dementia. Dement. Geriatr. Cogn. Disord. 16, 269–275 (2003).
Davis, G. K. et al. Potential biomarkers for dementia in Trinidad and Tobago. Neurosci. Lett. 424, 27–30 (2007).
Davis, G., Baboolal, N., Nayak, S. & McRae, A. Sialic acid, homocysteine and CRP: potential markers for dementia. Neurosci. Lett. 465, 282–284 (2009).
Zhang, L., Liu, N., Zhang, J., Zhang, H. & Zhang, Y. Clinical research of homocysteine, high-sensitive C-reactive protein and D-Dimer in patients with vascular dementia. Pak. J. Pharm. Sci. 30, 1445–1447 (2017).
Kloppenborg, R. P. et al. Homocysteine and progression of generalized small-vessel disease: the SMART-MR study. Neurology 82, 777–783 (2014).
Seshadri, S. et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch. Neurol. 65, 642–649 (2008).
Yoo, J. S. et al. Homocysteinemia is associated with the presence of microbleeds in cognitively impaired patients. J. Stroke Cerebrovasc. Dis. 29, 105302 (2020).
Pavlovic, A. M. et al. Increased total homocysteine level is associated with clinical status and severity of white matter changes in symptomatic patients with subcortical small vessel disease. Clin. Neurol. Neurosurg. 113, 711–715 (2011).
Björkhem, I. et al. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J. Lipid Res. 39, 1594–1600 (1998).
Lutjohann, D. et al. Plasma 24S-hydroxycholesterol (cerebrosterol) is increased in Alzheimer and vascular demented patients. J. Lipid Res. 41, 195–198 (2000).
Zuliani, G. et al. Plasma 24S-hydroxycholesterol levels in elderly subjects with late onset Alzheimer’s disease or vascular dementia: a case-control study. BMC Neurol. 11, 121 (2011).
Ference, B. A. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38, 2459–2472 (2017).
Schilling, S. et al. Plasma lipids and cerebral small vessel disease. Neurology 83, 1844–1852 (2014).
Axelsson, E., Wallin, A. & Svensson, J. Patients with the subcortical small vessel type of dementia have disturbed cardiometabolic risk profile. J. Alzheimers Dis. 73, 1373–1383 (2020).
McGrath, E. R. et al. Circulating ceramide ratios and risk of vascular brain aging and dementia. Ann. Clin. Transl. Neurol. 7, 160–168 (2020).
Liu, Y. et al. Plasma lipidomic biomarker analysis reveals distinct lipid changes in vascular dementia. Comput. Struct. Biotechnol. J. 18, 1613–1624 (2020).
Guo, Q. et al. miR-130b-3p regulates M1 macrophage polarization via targeting IRF1. J. Cell Physiol. 236, 2008–2022 (2021).
Varendi, K., Kumar, A., Härma, M. A. & Andressoo, J. O. miR-1, miR-10b, miR-155, and miR-191 are novel regulators of BDNF. Cell Mol. Life Sci. 71, 4443–4456 (2014).
Ragusa, M. et al. miRNAs plasma profiles in vascular dementia: biomolecular data and biomedical implications. Front. Cell Neurosci. 10, 51 (2016).
Liang, C. et al. MicroRNA-146a switches microglial phenotypes to resist the pathological processes and cognitive degradation of Alzheimer’s disease. Theranostics 11, 4103–4121 (2021).
Dong, H. et al. Serum microRNA profiles serve as novel biomarkers for the diagnosis of Alzheimer’s disease. Dis. Markers 2015, 625659 (2015).
Bai, Y. Y. & Niu, J. Z. miR-222 regulates brain injury and inflammation following intracerebral hemorrhage by targeting ITGB8. Mol. Med. Rep. 21, 1145–1153 (2020).
Ouyang, Y. B. et al. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 61, 1784–1794 (2013).
Barbagallo, C. et al. Specific signatures of serum miRNAs as potential biomarkers to discriminate clinically similar neurodegenerative and vascular-related diseases. Cell Mol. Neurobiol. 40, 531–546 (2020).
Shigemizu, D. et al. Risk prediction models for dementia constructed by supervised principal component analysis using miRNA expression data. Commun. Biol. 2, 77 (2019).
Joo, H. S., Jeon, H. Y., Hong, E. B., Kim, H. Y. & Lee, J. M. Exosomes for the diagnosis and treatment of dementia. Curr. Opin. Psychiatry 36, 119–125 (2023).
Han, X. et al. Circulating exo-miR-154-5p regulates vascular dementia through endothelial progenitor cell-mediated angiogenesis. Front. Cell Neurosci. 16, 881175 (2022).
Zhao, W. et al. Exosomal miRNA-223-3p as potential biomarkers in patients with cerebral small vessel disease cognitive impairment. Ann. Transl. Med. 9, 1781 (2021).
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E. & Bousser, M. G. Cadasil. Lancet Neurol. 8, 643–653 (2009).
Cho, B. P. H. et al. Association of vascular risk factors and genetic factors with penetrance of variants causing monogenic stroke. JAMA Neurol. 79, 1303–1311 (2022).
Chen, C. H., Cheng, Y. W., Chen, Y. F., Tang, S. C. & Jeng, J. S. Plasma neurofilament light chain and glial fibrillary acidic protein predict stroke in CADASIL. J. Neuroinflammation 17, 124 (2020).
Gravesteijn, G. et al. Serum neurofilament light correlates with CADASIL disease severity and survival. Ann. Clin. Transl. Neurol. 6, 46–56 (2019).
Pescini, F. et al. Circulating biomarkers in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy patients. J. Stroke Cerebrovasc. Dis. 26, 823–833 (2017).
Rufa, A. et al. Plasma levels of asymmetric dimethylarginine in cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy. Cerebrovasc. Dis. 26, 636–640 (2008).
Primo, V. et al. Blood biomarkers in a mouse model of CADASIL. Brain Res. 1644, 118–126 (2016).
Yamamoto, Y., Liao, Y. C., Lee, Y. C., Ihara, M. & Choi, J. C. Update on the epidemiology, pathogenesis, and biomarkers of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J. Clin. Neurol. 19, 12–27 (2023).
Yamamoto, Y., Craggs, L., Baumann, M., Kalimo, H. & Kalaria, R. N. Review: molecular genetics and pathology of hereditary small vessel diseases of the brain. Neuropathol. Appl. Neurobiol. 37, 94–113 (2011).
Branyan, K. et al. Elevated TGFβ signaling contributes to cerebral small vessel disease in mouse models of Gould syndrome. Matrix Biol. 115, 48–70 (2023).
Dagonnier, M., Donnan, G. A., Davis, S. M., Dewey, H. M. & Howells, D. W. Acute stroke biomarkers: are we there yet? Front. Neurol. 12, 619721 (2021). A review of biomarkers associated with stroke.
Bjerke, M. et al. Subcortical vascular dementia biomarker pattern in mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 28, 348–356 (2009).
Staszewski, J., Piusinska-Macoch, R., Brodacki, B., Skrobowska, E. & Stepien, A. IL-6, PF-4, sCD40 L, and homocysteine are associated with the radiological progression of cerebral small-vessel disease: a 2-year follow-up study. Clin. Interv. Aging 13, 1135–1141 (2018).
Rosenberg, G. A. et al. Validation of biomarkers in subcortical ischaemic vascular disease of the Binswanger type: approach to targeted treatment trials. J. Neurol. Neurosurg. Psychiatry 86, 1324–1330 (2015).
Winder, Z. et al. Hierarchical clustering analyses of plasma proteins in subjects with cardiovascular risk factors identify informative subsets based on differential levels of angiogenic and inflammatory biomarkers. Front. Neurosci. 14, 84 (2020).
Kong, D. H., Kim, Y. K., Kim, M. R., Jang, J. H. & Lee, S. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int. J. Mol. Sci. 19, 1057 (2018).
Singh, J., Lee, Y. & Kellum, J. A. A new perspective on NO pathway in sepsis and ADMA lowering as a potential therapeutic approach. Crit. Care 26, 246 (2022).
Steinert, M., Ramming, I. & Bergmann, S. Impact of Von Willebrand factor on bacterial pathogenesis. Front. Med. 7, 543 (2020).
Saki, N., Javan, M., Moghimian-Boroujeni, B. & Kast, R. E. Interesting effects of interleukins and immune cells on acute respiratory distress syndrome. Clin. Exp. Med. https://doi.org/10.1007/s10238-023-01118-w (2023).
Hachinski, V. et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37, 2220–2241 (2006).
Sachdev, P. S. et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology 62, 912–919 (2004).
Au, R. et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch. Neurol. 63, 246–250 (2006).
Sachdev, P. S. et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat. Rev. Neurol. 10, 634–642 (2014).
Markus, H. S. et al. Framework for Clinical Trials in Cerebral Small Vessel Disease (FINESSE): a review. JAMA Neurol. 79, 1187–1198 (2022).
Banks, W. A., Reed, M. J., Logsdon, A. F., Rhea, E. M. & Erickson, M. A. Healthy aging and the blood-brain barrier. Nat. Aging 1, 243–254 (2021).
Davis, K. D. et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat. Rev. Neurol. 16, 381–400 (2020).
Custodero, C. et al. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: a systematic review and meta-analysis. Geroscience 44, 1373–1392 (2022).
Hosoki, S., Tanaka, T. & Ihara, M. Diagnostic and prognostic blood biomarkers in vascular dementia: from the viewpoint of ischemic stroke. Neurochem. Int. 146, 105015 (2021). A review of biomarkers associated with VCID and stroke.
Ihara, M. & Yamamoto, Y. Transcriptomic mapping of the human cerebrovasculature. Nat. Rev. Neurol. 18, 319–320 (2022).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sperling, R. A. et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 7, 367–385 (2011).
Dichgans, M., Beaufort, N., Debette, S. & Anderson, C. D. Stroke genetics: turning discoveries into clinical applications. Stroke 52, 2974–2982 (2021).
Román, G. C. et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260 (1993).
Sachdev, P. et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis. Assoc. Disord. 28, 206–218 (2014).
Skrobot, O. A. et al. Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 14, 280–292 (2018).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41 (1993).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 4th edn (American Psychiatric Association, 1994).
World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders (World Health Organization, 1992).
Chui, H. C. et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology 42, 473–480 (1992).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Association, 2013).
Rost, N. S. et al. Post-stroke cognitive impairment and dementia. Circ. Res. 130, 1252–1271 (2022).
Acknowledgements
The search strategies were peer reviewed by the UNSW librarian.
Author information
Authors and Affiliations
Contributions
S.H. and P.S.S. conceived the idea of the Review. S.H. performed the literature search, prepared the original figures and tables, and wrote the first draft. G.K.H. and T.J. assisted with the literature search by assessing manuscripts against the review criteria. A.P., K.A.M., V.S.C., R.R., A.S., J.C.K., A.B., A.W., B.V.Z., M.I. and P.S.S. critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Neurology thanks G. J. Biessels and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
We searched for papers using PubMed, Embase and Scopus with the terms (‘vascular dementia’ [Text Word]) OR (‘Vascular cognitive impairment’ [Text Word]) OR (‘vascular contributions to cognitive impairment and dementia’ [Text Word]) OR (‘Vascular cognitive impairment and Dementia’ [Text Word]) OR (Dementia, Vascular [Mesh]) OR (Cerebral Small Vessel Diseases [Mesh]) AND (Biomarker [Text Word] OR Biomarkers [Mesh] OR RNA [Text Word] OR RNA [Mesh]) AND (Plasma [Text Word] OR Serum [Mesh] OR Blood [Text Word] OR Cerebrospinal Fluid [Mesh] OR Circulating [Text Word]) on 16th December 2022. The reference lists of identified papers were used to identify additional papers on VCID biomarkers.
Supplementary information
Glossary
- Cerebral microbleeds
-
Small areas of signal void with associated blooming on T2*-weighted MRI or susceptibility-weighted imaging.
- Lacunes
-
Round or ovoid subcortical, fluid-filled cavities of 3–15 mm in diameter; the MRI signal is similar to that of cerebrospinal fluid.
- Perivascular spaces
-
Fluid-filled spaces that surround blood vessels in the grey or white matter.
- White matter hyperintensities
-
Abnormalities of variable size in the white matter that appear as hyperintensity without cavitation on T2-weighted MRI.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosoki, S., Hansra, G.K., Jayasena, T. et al. Molecular biomarkers for vascular cognitive impairment and dementia. Nat Rev Neurol 19, 737–753 (2023). https://doi.org/10.1038/s41582-023-00884-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-023-00884-1