Abstract
Diabetes medications may modify the risk of certain cancers. We systematically searched MEDLINE, Embase, Web of Science, and Cochrane CENTRAL from 2011 to March 2021 for studies evaluating associations between diabetes medications and the risk of breast, lung, colorectal, prostate, liver, and pancreatic cancers. A total of 92 studies (3 randomized controlled trials, 64 cohort studies, and 25 case–control studies) were identified in the systematic review, involving 171 million participants. Inverse relationships with colorectal (n = 18; RR = 0.85; 95% CI = 0.78–0.92) and liver cancers (n = 10; RR = 0.55; 95% CI = 0.46–0.66) were observed in biguanide users. Thiazolidinediones were associated with lower risks of breast (n = 6; RR = 0.87; 95% CI = 0.80–0.95), lung (n = 6; RR = 0.77; 95% CI = 0.61–0.96) and liver (n = 8; RR = 0.83; 95% CI = 0.72–0.95) cancers. Insulins were negatively associated with breast (n = 15; RR = 0.90; 95% CI = 0.82–0.98) and prostate cancer risks (n = 7; RR = 0.74; 95% CI = 0.56–0.98). Positive associations were found between insulin secretagogues and pancreatic cancer (n = 5; RR = 1.26; 95% CI = 1.01–1.57), and between insulins and liver (n = 7; RR = 1.74; 95% CI = 1.08–2.80) and pancreatic cancers (n = 8; RR = 2.41; 95% CI = 1.08–5.36). Overall, biguanide and thiazolidinedione use carried no risk, or potentially lower risk of some cancers, while insulin secretagogue and insulin use were associated with increased pancreatic cancer risk.
Similar content being viewed by others
Introduction
The prevalence of type II diabetes, herein referred to as diabetes, continues to grow and, globally, will affect 7.7% (439 million) of adults aged 20 to 79 years old by 20301. Epidemiological evidence suggests that diabetes is associated with a higher risk for many cancers, from 20 to 30% elevated breast or colorectal cancer to 97% elevated risk of intrahepatic cholangiocarcinoma or endometrial cancer2,3. These associations likely reflect shared etiology. For instance, tobacco use, physical inactivity, overweight, and poor diet are closely linked to cancer risk and progression4 and risk of diabetes5,6,7. Several biological mechanisms have been proposed to explain why diabetes may be associated with cancer, including inflammation, hyperglycemia, and hyperinsulinemia8,9. Furthermore, medications for diabetes may modify the association. Multiple epidemiologic studies have evaluated the association of diabetes medications with the risk of site-specific cancers in patients with diabetes10,11.
Previous systematic reviews either synthesized the effect of a single medication class on multiple cancer sites12,13,14 or the effects of multiple medication classes on overall cancer15. No systematic reviews have synthesized multiple associations among different diabetes medication classes and cancer sites in patients with diabetes, which limits our capacity to analyze evidence in the context of various mechanisms involved with diabetes treatment and underlying causes of cancers. To this end, we conducted a systematic review and meta-analyses of the latest randomized controlled trials (RCTs) and observational studies (cohort and case–control studies) published in the last 10 years that evaluated the effect of diabetes medications, namely biguanides, incretin-based medicines, alpha-glucosidase inhibitors (AGIs), insulin secretagogues, thiazolidinediones, and insulins, on the risk of developing any of study cancers, specifically breast, lung, colorectal, prostate, liver, or pancreatic cancers.
Results
Literature search
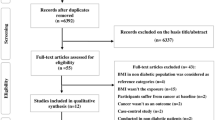
The literature search and study selection process are summarized in Fig. 1. Of the 10,540 citations uniquely identified from databases, 323 were eligible for full-text review. After review, 92 studies were selected for inclusion. A detailed description of these studies can be found in Supplementary file 1. A total of 226 separate associations between various diabetes medication classes and cancer sites were evaluated in the 92 studies (Table 1). Sodium-glucose cotransporter 2 inhibitors were included in the meta-analysis but will not be discussed in this review as no studies on cancer sites of interest were identified.
Study characteristics
All three RCTs were categorized as having a high risk of bias due to either lack of allocation concealment or potential selective reporting (Table 1 and supplementary references in Supplementary file 1). Among the 64 cohort studies, nine and 55 were categorized as moderate and low risk of bias, respectively. For the 55 retrospective cohort studies, observation time ranged from two to 25 years and 43 studies had data spanning more than five years. The follow-up period in nine prospective cohort studies varied from two to 14 years, and six studies followed participants for more than five years. Of the 25 case–control studies, nine and 16 were categorized as moderate and low risk of bias, respectively. Five of the case–control studies were conducted in hospital settings.
Overall, colorectal (n = 57) and pancreatic cancers (n = 42) were the most commonly evaluated outcomes across included studies (Table 1). A cohort design was used for the majority of the breast (89%) and lung cancer studies (81%). Studies of lung and prostate cancer were generally of the highest quality, with only 7% of lung and 13% of prostate cancer studies characterized as susceptible to moderate or high risk of bias. Regarding lung, colorectal, and liver cancers, approximately equivalent numbers of the studies were conducted in populations from Asian and Western regions. The majority of studies on breast, prostate, and pancreatic cancer were conducted in populations from Western regions.
Of the studies that reported specific diabetes medication classes, the majority studied biguanides and insulins, while only six studies evaluated the effect of AGIs on cancer risk. Most studies (67%) were of a cohort design. The proportions of studies with a low risk of bias were similar across diabetes medication classes. The majority of studies of biguanides and insulin secretagogues involved populations from Western regions, while the majority of studies of AGIs were conducted in populations from Asian regions.
Meta-analysis
Individual associations among medication classes and cancer sites and subgroup analysis results are summarized in Tables 2, 3, 4, 5, 6 and 7. Forest plots for pooled analyses are shown in Supplementary file 2. Overall, a number of inverse associations were found. Biguanide use was significantly associated with lower risks of colorectal (n = 18; RR = 0.85; 95% CI = 0.78–0.92; Table 4) and liver cancers (n = 10; RR = 0.55; 95% CI = 0.46–0.66; Table 6). Use of thiazolidinediones was associated with lower risks of breast (n = 6; RR = 0.87, 95% CI = 0.80–0.95; Table 2), lung (n = 6; RR = 0.77; 95% CI = 0.61–0.96; Table 3) and liver cancers (n = 8; RR = 0.83; 95% CI = 0.72–0.95; Table 6). Lower risks of breast (n = 15; RR = 0.90; 95% CI = 0.82–0.98; Table 2) and prostate cancers (n = 7; RR = 0.74; 95% CI = 0.56–0.98; Table 5) were found among insulin users compared with nonusers. On the other hand, elevated risk of pancreatic cancer was observed with insulin secretagogue use (n = 5; RR = 1.26; 95% CI = 1.01–1.57; Table 7), and elevated risks of liver (n = 7; RR = 1.74; 95% CI = 1.08–2.80; Table 6) and pancreatic cancers (n = 8; RR = 2.41; 95% CI = 1.08–5.36; Table 7) were observed with insulin use.
Effects were largely consistent across studies with different designs, quality levels, and population regions, though there are some notable differences. Subgroup analyses revealed that the positive association between insulin use and liver cancer was restricted to studies conducted in populations from Western regions (n = 4; RR = 2.48, 95% CI = 1.31–4.70; Table 6). The use of insulin secretagogues was significantly associated with higher colorectal cancer risk in cohort studies (n = 4; RR = 1.21; 95% CI = 1.04–1.40; Table 4) but not in case–control studies. Biguanide use was associated with a higher risk of pancreatic cancer in case–control studies but not in cohort studies (n = 3; RR = 1.25; 95% CI = 1.03–1.50; Table 7). Thiazolidinedione use was associated with a lower risk of pancreatic cancer in cohort studies (n = 4; RR = 0.59; 95% CI = 0.39–0.89; Table 7), whereas, in case–control studies, a non-significant increased risk of pancreatic cancer was observed (n = 2; RR = 1.26, 95% CI = 0.79–2.02).
Discussion
This comprehensive meta-analysis examined associations among the most commonly prescribed diabetes medication classes and the risk of cancer in patients with diabetes. The meta-analysis included 92 primary studies which involved 171 million participants, of which 1.3 million were diagnosed with cancer, from across 18 countries or regions. Evidence of increased and decreased risks of cancer was found. For example, biguanide use was associated with a moderately decreased risk of colorectal cancer and a considerably lower risk of liver cancer. Thiazolidinedione use was found to moderately reduce the risks of breast, lung, and liver cancers. Conversely, the use of insulin secretagogues was associated with a moderately elevated risk of pancreatic cancer. Opposing associations with different cancer sites within a medication class were also evident; insulin use was associated with a higher risk of liver and pancreatic cancers but a lower risk of breast and prostate cancers. In addition to varied cancer-specific biologic mechanisms, these differences may be attributed to variations in research instruments and the composition of study populations (e.g., age differences) across the included studies.
Several biological mechanisms have been speculated to contribute to cancer modifying effects of diabetes medications. The protective effects of biguanides on colorectal cancer may be attributed to their ability to regulate upstream and downstream molecular mechanisms involved in cellular metabolism and energy homeostasis, cell cycle arrest, oxidative stress, inflammation, and apoptosis16. The potential protective association of biguanides with liver cancer might be further attributed to decreased lipogenic enzyme gene expression and suppressed lipogenesis17. By stimulating endogenous insulin production, insulin secretagogues increase insulin-like growth factor-1 levels which may promote pancreatic cancer development by interfering with cell metabolism and stimulating cell proliferation18. Thiazolidinediones have been shown to suppress breast cancer cell proliferation, stimulate apoptosis, and impede tumor angiogenesis with PPAR-γ ligands19. Thiazolidinediones-induced PPAR-γ activation has also been shown to hinder lung tumor progression through G0/G1 cell cycle arrest20. The inverse association observed between thiazolidinediones and liver cancer may be attributed to p27Kip1 protein accumulation which is associated with reduced liver cell growth21.
A protective effect of insulin was observed on breast cancer in the review, but the etiology is unclear. The lack of adjustment for mammography screening suggested detection bias might be a concern. Our finding of lower prostate cancer risk in men with diabetes using insulins is similar to a previous study. Men who were on insulins were more likely to have severe diabetes which might be linked to lower prostate-specific antigen levels and reduced risk of prostate cancer22. The cancer-promoting effect of insulins on the liver might be partly explained by portal circulation, which exposes the liver to high levels of insulin in people who have insulin-resistant diabetes23. In addition, insulin use has growth-promoting and mitogenic effects on pancreatic cancer cells, which may lead to pancreatic cancer development or acceleration24. However, the higher risk of pancreatic cancer might also be attributable to severe diabetes conditions rather than insulin use.
Although Noto et al. 25 found that individuals with diabetes had an overall increased cancer risk, our study highlights the impact of specific diabetes medications on site-specific cancer risk. In alignment with the systematic review and meta-analysis by Noto et al. 26, our findings underscore the potential protective effect of biguanides, such as metformin, in reducing the risk of colorectal and liver cancers. Similarly, the protective effect of metformin in our study echoes DeCensi et al. 27, implying a robust consensus across studies regarding this association. Our findings that insulin and insulin secretagogue use can be linked to an elevated risk of certain cancers align with the results from Karlstad et al. 28 and Singh et al. 29. These consistent findings across studies highlight the potentially harmful effects of these medication classes on cancer risk, meriting careful clinical consideration. In addition to confirming results from previous systematic reviews, our review broadens the understanding by incorporating more recent studies, diverse geographical regions, and differing study designs, thus enhancing the comprehensiveness and applicability of the findings.
Our meta-analysis reveals associations between diabetes medication use and cancer risk that were generally consistent across diverse study designs, quality levels, and population regions. However, regional differences were discovered, such as the stronger association between insulin use and liver cancer risk in populations from the Western regions. The inconsistent association between insulin secretagogues and colorectal cancer and biguanides and pancreatic cancer, which varied by study design, underscores the need to interpret results cautiously. Our findings indicate that geographical factors and study design might influence observed associations, necessitating additional research to enhance our understanding.
This review represents the latest examination of associations among all currently prescribed classes of diabetes medications and the risk of cancer across multiple sites. A myriad of new diabetes medications has become available over the past two decades30. Our approach, considering different classes of medications separately across individual cancer sites, is an important advance over previous literature reviews12,13,14,15 that have grouped all diabetes medications and/or cancer sites together. The large sample size of included studies augmented the precision of summary effect estimates, and the inclusion of large numbers of individuals from Asian and Western regions enhances the generalizability of our findings.
Our review has some limitations. Evidence of heterogeneity in summary risk estimates was observed for biguanides and insulins across all cancer sites, but subgroup analyses did not reveal clear sources of this heterogeneity. It is possible that heterogeneity reflects differences in study population demographics such as varying proportions of males/females and differences in age or unmeasured confounding within studies, for example, few studies adjusted for lifestyle factors like nutritional status that may confound associations. It also appeared that study design impacted results. For instance, the use of insulin secretagogues showed a more prominent effect on liver and pancreatic cancers in case–control studies than in cohort studies. When the majority of studies were case–control studies, differences observed in results between cohort and case–control studies might be attributed to recall bias. Effects of sodium-glucose cotransporter 2 inhibitors on cancer risk and AGIs on breast and prostate cancer could not be explored due to the absence of data. In general, few studies of cancer risk in association with AGIs and incretin-based medicines were identified. Differences in the standard of care for diabetes across jurisdictions and the use of combination drug therapies may have impacted results. Because the severity of diabetes is typically a driver of specific medication use, the possibility of confounding by indication cannot be ruled out in the individual studies that were included in this review. We did not evaluate dose–response relationships by incorporating the duration of diabetes medication use because it is beyond the scope of this review.
In conclusion, various diabetes medications might be linked to the risk of study cancers; however, the strengths of associations showed variation to some extent across studies with different designs, quality levels, and population regions. On the other hand, the preventive effect of biguanides against colorectal and liver cancers was consistent across different studies. A potential cancer-promoting effect of insulins on the liver was only found in populations from Western regions, whereas the increased risk associated with insulin on the pancreas was only found in populations from Asian regions. Although further studies are required, these findings suggest that it may be important to weigh the potential harms of insulin among patients with diabetes who are at high risk of liver or pancreatic cancers due to family history or other risk factors. The choice of medication for patients with diabetes may incorporate individuals’ susceptibility to individual cancers in conjunction with established clinical considerations. Future studies may further consider the duration of medication use and dose–response relationships to consolidate current knowledge in observed associations with study cancers.
Methods
The search adhered to the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)31 statement. The completed PRISMA checklist can be found in Supplementary file 3. This systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) in March 2021 (registration number: CRD42021239348).
Study design
We included original human experimental and observational studies that evaluated the associations between diabetes medications and six site-specific cancers: breast, lung, colorectal, prostate, liver, and pancreatic cancers. The first four cancers are the most prevalent cancers worldwide 32, and thus it is critical to understand possible risk factors. As a result of the high prevalence, we also expected to identify a larger body of evidence for breast, lung, colorectal and prostate cancer from which to synthesize findings. Although cancers of the liver and pancreas are less common, they have high fatality rates. Moreover, liver and pancreatic cancers were included in this review due to biological plausibility of diabetes medication and liver and pancreatic cancer, reflecting the roles of the liver and pancreas in blood glucose regulation and diabetes. Studies using a cross-sectional design were excluded as they could not provide clear evidence of temporality. In this study, we reviewed the results of RCTs, cohort, and case–control studies. Reviews, commentaries, opinion pieces, letters, and case reports were also excluded.
Participants
The study population for breast cancer was restricted to female biological sex due to the rareness of breast cancer in males. People with prevalent cancer or a previous history of study cancers were excluded. Studies focusing on pediatric or adolescent populations were also excluded. No other restrictions were placed on the study population.
Exposures
In RCTs, diabetes medications were randomly assigned. For observational studies, the determination of the exposure to diabetes medications was made on the basis of medical records, pharmacy prescription records, or insurance claims databases. Self-reported exposure was also included, recognizing its inherent limitations. Studies were excluded if they did not provide explicit methodologies for establishing exposure to diabetes medication.
Comparators
Studies without an appropriate comparator group were excluded. In addition to people receiving no treatment or placebo, for each diabetes medication of interest, people using one or more other diabetes medications were considered to be eligible as controls. Control groups were from population-based and hospital settings in observational studies and in RCTs, respectively.
Outcomes
Cancer incidence or mortality was determined through a national record system, cancer registry or death certificates. Eligible measures of the effect included odds ratio (OR), relative risk (RR), and hazard ratio (HR). The absence of these specific effect measures led to exclusion.
Publication characteristics
This systematic review focuses on research published in the last ten years. Searches were limited to publications dating from January 2011 onwards. The timeframe was chosen to reflect advances in pharmaceutical treatment of diabetes; the introduction of new medications e.g., GLP-1 receptor agonists and SGT-2 inhibitors with distinct mechanisms of action from existing diabetes medication classes. The focus on recent literature is thus more likely to capture evidence on diabetes medications currently prescribed and have greater clinical relevance. No language restrictions were imposed. After the titles/abstracts screening, the full text of selected studies in languages other than English was translated using Google Translate.
Search strategies
With the assistance of an institutional librarian, we systematically searched MEDLINE (Ovid), Embase (Ovid), Web of Science Core Collection, and Cochrane CENTRAL (Ovid). Search terms included controlled vocabulary and keywords related to various diabetes medications and cancer at multiple sites. Details of the search strategy for each database are provided in Supplementary file 4.
Study selection
All titles and abstracts were imported to Covidence (https://www.covidence.org). The study selection comprised two stages. Firstly, all titles and abstracts were screened by reviewers (Y.C., F.M., and S.S.) for potentially eligible papers according to the above-mentioned inclusion and exclusion criteria. Studies that were excluded by more than one reviewer were excluded from this review. Secondly, full texts of all relevant studies were retrieved, and two reviewers (Y.C., F.M., or S.S.) independently screened each study for eligibility. Reasons for exclusion were documented at the full-text screening stage.
Data extraction
Two reviewers, Y.C. and F.M. or Y.C. and S.S., extracted data from each included study using Covidence independently. Discrepancies in extracted data were resolved through discussion or consensus with a third reviewer.
Quality assessment
The methodological quality of RCTs and observational studies was scrutinized independently by two reviewers and collated by the lead reviewer using Cochrane Collaboration’s tool for assessing the risk of bias33 and the Newcastle–Ottawa scale (NOS)34. If randomization, allocation concealment, and blinding were uncertain in an RCT, it was categorized as a high-risk study15. Three domains assessed in observational studies were the selection of study groups, the comparability of groups, and the ascertainment of exposure or outcome of interest. A score of less than 4 or more than 6 represented a high or low risk of bias, respectively15.
Data synthesis
We performed meta-analyses to obtain the pooled relative risk (RR) using Stata software version 17.0 (Stata Corp., College Station, TX, USA). First, the findings were summarized in a narrative form, with textual description and tabulation to compare potential differences among studies. We employed a DerSimonian-Laird random-effects model35 to pool the data from two or more studies evaluating the same class of diabetes medication and the same site of cancer. Cochran’s Q was used to test the statistical heterogeneity of the included studies, with a significance level of 0.136. The magnitude of heterogeneity was measured using the I2 statistic37, with < 25% and > 75% corresponding to low and high heterogeneity, respectively. Analyses were performed to quantify individual associations among different medication classes and cancer sites. We performed subgroup analyses (study design, quality levels, and population regions) to determine possible sources of between-study heterogeneity.
Data availability
Data are available upon request from Y.C. (ychen153@student.ubc.ca).
Abbreviations
- AGIs:
-
Alpha-glucosidase inhibitors
- Cochrane CENTRAL:
-
Cochrane Central Register of Controlled Trials
- EMBASE:
-
Excerpta Medica DataBase
- HR:
-
Hazard ratio
- MEDLINE:
-
Medical Literature Analysis and Retrieval System Online
- NOS:
-
Newcastle–Ottawa scale
- OR:
-
Odds ratio
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
Prospective Register of Systematic Reviews
- RCT:
-
Randomized controlled trial
- RR:
-
Relative risk
- 95% CI:
-
95% Confidence interval
References
Shaw, J. E., Sicree, R. A. & Zimmet, P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 (2010).
Collins, K. K. The diabetes-cancer link. Diabetes Spectr 27, 276–280 (2014).
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S., Ntzani, E. E. & Ioannidis, J. P. A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 350, 7607 (2015).
Stein, C. J. & Colditz, G. A. Modifiable risk factors for cancer. Br. J. Cancer 90, 299–303 (2004).
Adeghate, E., Schattner, P. & Dunn, E. An update on the etiology and epidemiology of diabetes mellitus. Ann. N. Y. Acad. Sci. 1084, 1–29 (2006).
Kao, W. H. L., Puddey, I. B., Boland, L. L., Watson, R. L. & Brancati, F. L. Alcohol consumption and the risk of type 2 diabetes mellitus: Atherosclerosis risk in communities study. Am. J. Epidemiol. 154, 748–757 (2001).
Jee, S. H., Foong, A. W., Hur, N. W. & Samet, J. M. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care 33, 2567–2572 (2010).
Cignarelli, A. et al. Diabetes and cancer: Pathophysiological fundamentals of a ‘dangerous affair’. Diabetes Res. Clin. Pract. 143, 378–388 (2018).
Klil-Drori, A. J., Azoulay, L. & Pollak, M. N. Cancer, obesity, diabetes, and antidiabetic drugs: Is the fog clearing?. Nat. Rev. Clin. Oncol. 14, 85–99 (2017).
Butler, P. C., Elashoff, M., Elashoff, R. & Gale, E. A. M. A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe?. Diabetes Care 36, 2118–2125 (2013).
Yang, X. et al. Use of sulphonylurea and cancer in type 2 diabetes–The Hong Kong Diabetes Registry. Diabetes Res. Clin. Pract. 90, 343–351 (2010).
Zhang, P., Li, H., Tan, X., Chen, L. & Wang, S. Association of metformin use with cancer incidence and mortality: A meta-analysis. Cancer Epidemiol. 37, 207–218 (2013).
Zhao, Y., Wang, Y., Lou, H. & Shan, L. Alpha-glucosidase inhibitors and risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Oncotarget 8, 81027–81039 (2017).
Bosetti, C. et al. Cancer risk for patients using thiazolidinediones for type 2 diabetes: A meta-analysis. Oncologist 18, 148–156 (2013).
Wu, L., Zhu, J., Prokop, L. J. & Murad, M. H. Pharmacologic therapy of diabetes and overall cancer risk and mortality: A meta-analysis of 265 studies. Sci. Rep. 5, 10147 (2015).
Kamarudin, M. N. A., Sarker, Md. M. R., Zhou, J.-R. & Parhar, I. Metformin in colorectal cancer: Molecular mechanism, preclinical and clinical aspects. J. Exp. Clin. Cancer Res. 38, 491 (2019).
Bhalla, K. et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev. Res. 5, 544–552 (2012).
Gallagher, E. J. & LeRoith, D. Diabetes, antihyperglycemic medications and cancer risk: Smoke or fire?. Endocr. Pract. 1, 1–28 (2013).
Blanquicett, C., Roman, J. & Hart, C. M. Thiazolidinediones as anti-cancer agents. Cancer Ther. 6, 25–34 (2008).
Keshamouni, V. G. et al. Peroxisome proliferator-activated receptor-γ activation inhibits tumor progression in non-small-cell lung cancer. Oncogene 23, 100–108 (2004).
Motomura, W. et al. Growth arrest by troglitazone is mediated by p27Kip1 accumulation, which results from dual inhibition of proteasome activity and Skp2 expression in human hepatocellular carcinoma cells. Int. J. Cancer 108, 41–46 (2004).
Müller, H., Raum, E., Rothenbacher, D., Stegmaier, C. & Brenner, H. Association of diabetes and body mass index with levels of prostate-specific antigen: Implications for correction of prostate-specific antigen cutoff values?. Cancer Epidemiol. Biomarkers Prev. 18, 1350–1356 (2009).
Heuson, J.-C., Legros, N. & Heimann, R. Influence of insulin administration on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in intact, oophorectomized, and hypophysectomized rats. Cancer Res. 32, 7 (1972).
Kazakoff, K. et al. Effects of voluntary physical exercise on high-fat diet-promoted pancreatic carcinogenesis in the hamster model. Nutr. Cancer 26, 265–279 (1996).
Noto, H., Tsujimoto, T., Sasazuki, T. & Noda, M. Significantly increased risk of cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Endocr. Pract. 17, 616–628 (2011).
Noto, H., Goto, A., Tsujimoto, T. & Noda, M. Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis. PLoS ONE 7, e33411 (2012).
DeCensi, A. et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 3, 1451–1461 (2010).
Karlstad, Ø. et al. Use of insulin and insulin analogs and risk of cancer–Systematic review and meta-analysis of observational studies. Curr. Drug Saf. 8, 333–348 (2014).
Singh, S. et al. Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Am. J. Gastroenterol. 108, 510–519 (2013).
White, J. R. A brief history of the development of diabetes medications. Diabetes Spectr. 27, 82–86 (2014).
Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71 (2021).
World Health Organization/International Agency for Research on Cancer. Worldwide Cancer Data. https://www.wcrf.org/cancer-trends/worldwide-cancer-data/.
Higgins, J. P., Savović, J., Page, M. J., Elbers, R. G. & Sterne, J. A. in Cochrane Handbook for Systematic Reviews of Interventions. 205–228. https://doi.org/10.1002/9781119536604.ch8 (Wiley, 2019).
Wells, G. A. et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2013).
DerSimonian, R. & Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 28, 105–114 (2007).
Dickersin, K. & Berlin, J. A meta-analysis: State-of-the-science. Epidemiol Rev 14, 154–176 (1992).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003).
Acknowledgements
We would like to thank Ursula Ellis (Librarian, Woodward Library of the University of British Columbia) who assisted with the development of the search strategy and provided valuable feedback and advice.
Funding
This study was funded by Michael Smith Foundation for Health Research (Grant no. 17644).
Author information
Authors and Affiliations
Contributions
Y.C., R.A.M., P.B., and T.D. contributed to the initial research question and created the study design. Y.C., F.M., and S.S. searched the electronic databases to identify eligible primary studies in accordance with inclusion and exclusion criteria. Y.C. and a second reviewer (F.M. or S.S.) worked independently on data extraction and quality evaluation for each primary study. F.M. and S.S. contributed equality to this review. Y.C. performed statistical analyses, interpreted results, and drafted the manuscript. R.A.M. provided critical comments on the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Mushashi, F., Son, S. et al. Diabetes medications and cancer risk associations: a systematic review and meta-analysis of evidence over the past 10 years. Sci Rep 13, 11844 (2023). https://doi.org/10.1038/s41598-023-38431-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-38431-z
This article is cited by
-
Poly-Agonist Pharmacotherapies for Metabolic Diseases: Hopes and New Challenges
Drugs (2024)
-
Diabetes medications may increase or decrease risk of cancer
Reactions Weekly (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.