Abstract
Nanotechnology is used to overcome fundamental flaws in today's marketed pharmaceuticals that obstruct therapy, like restricted solubility and quick release of drugs into the bloodstream. In both human and animal researches, melatonin was demonstrated to regulate glucose levels. Despite the fact that melatonin is quickly transported through the mucosa, its sensitivity to be oxidized creates a difficulty in achieving the required dose. Additionally, due to its variable absorption and poor oral bioavailability necessitates the development of alternative delivery methods. The study aimed to synthesize melatonin loaded chitosan/lecithin (Mel-C/L) nanoparticles to be assessed in the treatment of streptozotocin (STZ)-induced diabetes in rats. The antioxidant, anti-inflammatory, and cytotoxicity properties of nanoparticles were estimated to determine the safety of manufactured nanoparticles for in vivo studies. In addition, Mel-C/L nanoparticles were administered to rats for eight weeks after inducing hyperglycemia. The therapeutic effect of Mel-C/L nanoparticles was assessed in all experimental groups by detecting insulin and blood glucose levels; observing improvements in liver and kidney functions as well as histological and immunohistochemical evaluation of rats’ pancreatic sections. The results proved that Mel-C/L nanoparticles showed remarkable anti-inflammatory, anti-coagulant, and anti-oxidant effects, in addition to its efficiency in reducing blood glucose levels of STZ-induced diabetic rats and great ability to promote the regeneration of pancreatic beta (β)-cells. Furthermore, Mel-C/L nanoparticles elevated the insulin level; and decreased the elevated levels of urea, creatinine and cholesterol. In conclusion, nanoparticles application decreased the administrated melatonin dose that in turn can diminish the side effects of free melatonin administration.
Similar content being viewed by others
Introduction
Diabetes mellitus (DM) is a chronic endocrine condition defined by a persistent hyperglycemia that is frequently caused by absolute or relative insulin secretion insufficiency and/or insulin resistance1. Chronic hyperglycemia reduces the antioxidant defense mechanisms in the pancreatic beta (β) cells leading to severe oxidative stress as a result of the excessive reactive oxygen species (ROS) production; and leads to DNA oxidation, cell malfunction and finally cell death2,3,4,5,6,7,8,9,10,11. Aside from the macromolecular damage, ROS can activate a number of cellular stress-sensitive pathways that have been associated to insulin resistance and reduced insulin production1,8.
DM has been linked to lower blood melatonin levels in GotoKakizaki rats (rats with type II diabetes) according to Frese et al.12 Rodrigues et al.13 and Sun et al.14 who reported that melatonin has been used to control blood glucose levels in both animal and human studies; where, expression of the glucose transporter type 4 gene was diminished in pinealectomized animals leading to hyperglycemia. They added that melatonin administration has relieved these concerns. Milosavljevi et al.15 has reported the protective role of melatonin in dental pulp of diabetic patients due to its stimulation of superoxide dismutase (SOD) activity. de Oliveira et al.16 showed the synergistic effect of insulin and melatonin in streptozotocin (STZ)-induced diabetic rats; the authors found improvements in glucose metabolism and sensitivity of white adipose tissue to insulin. Moreover, melatonin administration reduced insulin resistance and blood glucose level in high fat diet diabetic mice according to Sartori et al.17. At night, individuals with type II diabetes release less melatonin than healthy individuals18. The studies of Heo et al.19 and Xu et al.20 showed that one dose of melatonin modified the glucose tolerance test in healthy pre-menopausal and post-menopausal females.
Melatonin also has immunomodulatory properties, which are based on its ability to increase cytokine production and anti-apoptotic and antioxidant properties21,22,23,24,25. Melatonin enhances the immune system through affecting the release of cytokines from healthy lymphocytes26. According to previous studies, melatonin can also lower blood glucose by activating the insulin signaling pathway (IRS-1/PI3kinase pathway) via G-protein-coupled membrane receptors (MT1 and MT2) which are expressed in islets of Langerhans, also it has the ability to regulate glucose homeostasis which leads to glucose uptake into skeletal muscle27,28. Melatonin also has an indirect antioxidant effect by upregulating antioxidant enzymes including (glutathione peroxidase, glutathione reductase, and glucose 6-phosphate dehydrogenase) and downregulating oxidant enzymes (e.g. NO synthase and lipoxygenase)28,29, resulting in suppression of nitric oxide formation which facilitates the decrease of the inflammatory response30,31.
According to the review of Foley and Steel32, migraine, disorientation, vomiting, and sleepiness are the most prevalent melatonin adverse effects. Short-term depression, slight trembling, anxiety, stomach discomfort, annoyance, lower alertness, confusion, and reduced blood pressure are some of the popular melatonin adverse effects33. Melatonin supplements can also interact with a variety of pharmaceuticals, including oral contraceptives, anticoagulants, anticonvulsants, and immunosuppressive drugs. Also, driving and machinery operation after receiving melatonin should be avoided34. In addition, although melatonin is rapidly absorbed across the mucosa, its oxidation sensitivity poses a substantial challenge in reaching a therapeutic dose. Furthermore, its limited oral bioavailability, owing to fluctuating absorption and significant 1st pass processing, necessitates the development of new administration routes and delivery systems35.
Polymeric nanoparticles have been studied extensively as a drug delivery system because of their multiple benefits, including the capacity to enhance bio-availability, transport of water insoluble chemicals, and shielding of the mucosa from drug toxicity. Schaffazick et al.36,37 showed that polysorbate eighty coated Eudragit nanoparticles loaded with melatonin have enhanced the in vitro action of melatonin towards lipid oxidation. Therefore, polymeric nanoparticles could be a useful melatonin delivery platform35. Many researches have chosen lecithin and chitosan as constitutive ingredients to attain these goals35,38. Chitosan is a polycationic polymer, positively charged, that is biodegradable and biocompatible; with a pH of 5.5–6.5 and a pKa of 6.539. Chitosan interacts electrostatically with negatively charged mucosa due to its positive charges40. Lecithin is a phospholipid-rich natural lipid that has been employed to make a variety of delivery nanostructures41, liposomes42, micelles43, and lipid solid nanoparticles44.
The study aimed to synthesize melatonin loaded chitosan/lecithin (Mel-C/L) nanoparticles to be assessed in the treatment of STZ-induced diabetes in rats. Mel-C/L nanoparticles were administrated for eight weeks after induction of hyperglycemia in rats; followed by the determination of blood glucose and insulin level.
Materials and methods
Materials
Chitosan (C) (≥ 75% deacetylated and low molecular weight), melatonin (Mel) and streptozotocin (STZ) were purchased from Sigmaaldrich, UK. Lecithin (L) (lipoid S45) was purchased from Lipoid GmbH, Germany).
Preparation of chitosan-lecithin (C/L) nanoparticles loaded with melatonin (Mel)
The used method was in accordance to Hafner et al.35; where the C/L ratio was 1:10. Ten mg/ml of chitosan (≥ 75% deacetylated, low molecular weight) was diluted in HCl (0.275 N). Forty ml of distilled water was mixed with 500 μl of the prepared chitosan solution; followed by adjusting the pH (3) and completing the volume to 46 ml. Lecithin was dissolved in absolute ethanol to reach 1.25 mg/ml concentration; followed by the addition of 5 mg/ml melatonin (Sigma). Four ml of lecithin–melatonin ethanolic solution was injected into chitosan solution (46 ml) by the aid of a syringe (0.75 mm inner diameter). Melatonin was replaced by ultrapure water during the preparation of unloaded C/L nanoparticles. The produced Mel-C/L nanoparticles were freeze-dried to obtain nanoparticles powder.
Physical characterization
The physical characterization was performed at 25 °C; where zeta potential was measured to determine the surface charge of prepared Mel-C/L nanoparticles35. The size of nanoparticles was determined by dynamic light scattering (DLS) using zetasizer (Malvern Panalytical). X-ray diffraction analyses (XRD) for Mel, C, L, unloaded and loaded nanoparticles were performed to determine the crystallographic structure. High resolution transmission electron microscope (Hitachi H-600, Tokyo, Japan) was used to measure the particle size and morphology.
Measurement of Mel entrapment efficiency (EE)
Briefly, the nanoparticle suspension was filtered through Amicon Ultra-15 filters (30 kDa, Millipore®). The filtrate was collected and utilised to quantify the non-entrapped Mel. The EE was measured indirectly by calculating the amount of melatonin that was not entrapped in nanoparticles. Absorbance at 279 nm was used for the determination of unloaded Mel; where, prepared nanoparticles solution was filtered and centrifuged for 45 min at 3000 rpm and 4 °C. The EE % was calculated from: EE % = [(Total Mel-Free Mel)/Total Mel] × 100. The drug loading (DL) % was calculated from: DL % = [(Total Mel-Free Mel)/quantity of prepared nanoparticles] × 10035.
Determination of Mel release % from C/L nanoparticles
The Mel release (%) from nanoparticles was carried out according to the sink conditions for Mel that considers its solubility in water of 100 μg/ml35. The nanoparticles were resuspended, at a concentration of 90 μg/ml, in acetate buffer. Twelve ml of the nanoparticles suspension were agitated at 37 °C on a rotary shaker (70 rpm). The Mel release was recorded at intervals of 1, 2, 4, 6, and 24 h. Each time, the whole volume was transferred to the 30 kDa Amicon Ultra-15 filters (Millipore®) and spun for half an hour. Absorbance at 279 nm was used for the determination of released Mel.
Determination of the antioxidant activity
The antioxidant activity of prepared nanoparticles was determined from the free radical scavenging effect using 1,1-diphenyl-2-picryl hydrazyl (DPPH)45. Briefly, one ml of DPPH/ethanol solution (0.1 mM) was added to different concentration of nanoparticles (3.9, 7.8, 15.62, 31.25, 62.5, 125, 250, 500, 1000 μg/ml); followed by shaking and standing for half an hour at 25 °C. Ascorbic acid was used as a standard compound and the absorbance was measured at 517 nm. IC50 was determined from Log dose-inhibition curve; and DPPH scavenging activity % = [(A0 − A1)/A0] × 100. A0: absorbance of control reaction and A1: absorbance of sample.
In vitro coagulation assay
Measurement of clotting time in seconds was used to determine the anticoagulant activity of Mel-C/L nanoparticles; where heparin was used as control46. Partial thromboplastin time (PTT) and prothrombin time (PT) reagents were used according to the manufacturers’ instructions. Briefly, 900 μl rat plasma was mixed with 100 μl of different concentrations of nanoparticles (25, 50 and 75 μg/ml) or heparin dissolved in saline. All reagents were pre-incubated at 37 °C for three min and the whole test was performed at 37 °C. The clotting time were recorded; and the test was repeated three times.
In vitro anti-inflammatory (membrane stabilization) assay
Three ml of fresh rat heparinized blood were centrifuged for ten min at 3000 rpm; followed by dissolving the formed pellet (40% v/v) with isotonic buffer (equal in volume to the supernatant)47. Mel-C/L nanoparticles was mixed with 5 ml distilled water, as a hypotonic solution, with different doses (100, 200, 400, 600, 800 and 1000 μg/ml). The same nanoparticles doses were mixed with 5 ml isotonic solution; and 5 ml of 200 μg/ml indomethacin was used as a control. 0.1 ml of the prepared erythrocytes suspension was added to nanoparticles solutions and control; followed by one hour of incubation at 37 °C then centrifugation for three min at 1500 rpm. The released haemoglobin in the supernatant was measured by spectrophotometer at 540 nm; where percent of hemolysis inhibition was calculated from: hemolysis inhibition (%) = 1 − [(ODb − ODa)/(ODc − ODa)] × 100. ODa: absorbance of samples in isotonic solution, ODb: absorbance of samples in hypotonic solution and ODc: absorbance of control in hypotonic solution.
In vitro cytotoxicity (MTT) assay
In tissue culture plates, one hundred µl/well of 105 CaCO2 cells were incubated at 37 °C for one day to allow cells’ monolayer formation. The monolayers were washed, after media decantation, with a washing media. Mel-C/L nanoparticles were mixed with RPMI media to form graded concentrations (31.25, 62.5, 125, 250, 500 and 1000 µg/ml). 0.1 ml of the prepared nanoparticles dilution was added to the wells followed by incubation for 24 h. Twenty µl of 5 mg/ml of MTT (3-(4,5-dimethylthiazol-2-yl)-2–5-diphenyltetrazolium bromide) was added to the wells. To ensure MTT mixing, plates were placed for five min on the plates’ shaker followed by incubation (5% CO2 and 37 °C) for four hours. Two hundreds µl of DMSO were added to the plates to solubilize the formed formazan. The absorbance, directly correlated to formazan, was read at 560 nm35.
Animals and experimental design
Male Sprague dawley rats, weighting 180–240 g and ten weeks age, were obtained from National Organization for Drug Control and Research (NODCAR, Cairo, Egypt). All experimental procedures were carried out in accordance with the international guidelines for the care and use of laboratory animals, and were given permission by Cairo University's Institutional Animal Care and Use Committee (CUIF9921). The study was carried out in compliance with the ARRIVE guidelines. Animals were divided into two main groups [healthy (HG) and diabetic (DG) group]. Each group was, furtherly, divided into seven subgroups (6 rats each): control untreated rats (I), treated rats that received free Mel [5 mg/kg (II) or 10 mg/kg (III)], unloaded C/L nanoparticles [5 mg/kg (IV) or 10 mg/kg (V)], Mel-C/L nanoparticles [5 mg/kg (VI) or 10 mg/kg (VII)]. Treatment was orally administrated, after induction of hyperglycemia, for 8 weeks between 5 and 7 pm daily. At the end of study, rats were terminally anesthetized with pentobarbital (80 mg/kg). Blood and organs (liver, kidney, and pancreas) from several experimental groups were collected. After allowing the blood to coagulate, serum was separated into aliquots and kept at − 20 °C.
Induction of diabetes
Animals were fasted for 6 h before receiving STZ on the first experimental day. STZ was dissolved, to a final concentration of 32.5 mg/ml, in 50 mM sodium citrate buffer (pH 4.5)48,49. Rats were administered with 65 mg/kg (2.0 ml/kg) of STZ solution through intravenous injection. Sucrose water (10%) were given to rats only on the first day after STZ administration. On the tenth day, animals were fasted for 6 h and blood samples were taken from the tail vein to determine the level of fasting blood glucose (FBG). Diabetic rats were defined as those having an FBG level more than 150 mg/dl as compared to the control group. Using a Rat FTA ELISA Kit (MBS2601586, MyBioSource, USA), serum fructosamine (FTA) was measured at the end of the study to determine the average blood sugar level over the previous three weeks.
Preparation of pancreatic homogenate
Half gram of pancreatic tissue was homogenized in 4.5 ml of cold Tris–HCl buffer (10 mmol, pH = 7.4); followed by ten-minute centrifugation at 4 °C at 2500 rpm. Protein content, in supernatant, was measured according to Lowry’s protocol50.
Biochemical and immunological measurements
Levels of serum insulin was determined by Insulin Rat ELISA Kit (Invitrogen, ERINS). Levels of cholesterol and triglycerides, in serum and liver, were estimated by Cholesterol Assay Kit (Abcam ab65390) and Triglyceride Assay Kit (Zen-Bio STG-1-NC). Liver function enzymes, Alanine transaminase (ALT) and aspartate aminotransferase (AST), were measured by Rat ELISA Kit (Abcam ab234579 and MyBioSource MBS264975; respectively). Levels of urea and creatinine were determined by rat ELISA kit (MyBioSource MBS2600001 and MBS007289; respectively). The antioxidant capacity of prepared Mel-C/L nanoparticles, in vivo, was evaluated from measuring levels of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPx) activity in pancreatic tissue by rat ELISA Kit (MyBioSource MBS268427, MBS266897 and MBS727547; respectively). Levels of interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-12 were determined by Rat ELISA Kit (MyBioSource MBS843359, Biolegend 438206, and MyBioSource MBS2507061, respectively).
Histopathological and immunohistochemical examination
Organs (pancreas, kidney and liver) from all experimental groups were fixed in buffered formalin (10%) for 24 h; followed by dehydration in decreasing concentration of alcohol, infiltration, embedding and staining with hematoxylin and eosin stain51. Immunohistochemical studies were performed according to Farid et al.29. Where, four μm long pancreatic sections, formalin fixed, were deparaffinized, rehydrated, and washed by saline. The activity of endogenous peroxidases was blocked by adding 3 percent H2O2/methanol to pancreatic tissues, followed by washing with saline and blocking with bovine serum albumin (5%). Anti-insulin antibody (Sigma I2018) and anti-NF-kB p65 antibody (Abcame ab86299) were applied as primary antibodies for NF-kB and insulin, respectively. After 60 min of incubation, pancreatic sections were washed and treated for 120 min with HRP-conjugated rabbit anti-rat IgG (Abcam ab6734) as a secondary antibody. To visualize the response, 3,3-diaminobenzidine (DAB) was employed till a brown colour developed. Pancreatic sections were counterstained using Mayer's hematoxyline.
Statistical analysis
Results were analyzed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Data was represented as mean ± SD and investigated by one way analysis of variance (ANOVA). Differences between means was evaluated by Tukey post hoc test; where, values with p less than 0.05 was considered statistically significant.
Ethics approval
All experimental procedure and animal maintenance were approved by the Institutional Animal Care and Use Committee, Cairo University, Egypt (CUIF9921).
Results
Physical characterization
According to our results, EE% of Mel in C/L nanoparticles was 28.4% and the DL% was 5.1%; the Mel release was represented in Fig. 1A. The zeta potential of the prepared nanoparticles was − 23 mV (Fig. 1C). DLS showed that Mel loaded nanoparticles have a size of 20 nm (Fig. 1B); on the other hand, TEM showed that the nanoparticles size ranged from 75 to 98 nm (Fig. 1D). Figure 1G showed the XRD pattern of Mel (a), where sharp peaks appeared at 17.4, 24.4, 25.6 and 26.1°. XRD patterns of C (b) and L (c) (Fig. 1G) showed broad bands at around 20 and 20.1°, respectively. Mel-C/L nanoparticles (d) showed XRD diffraction superimposition of L, C and Mel; where the diffraction peaks of Mel appeared with the characteristic broad peaks of both C and L. Unloaded C/L nanoparticles (e) showed broad band in the range of 15°–24°.
(A) Mel release (%) from C/L nanoparticles, results were expressed as mean ± SD (n = 3), (B) Size of Mel-C/L nanoparticles, (C) Zeta potential, (D) TEM image, (E) DPPH scavenging % of Mel-C/L nanoparticles in comparison to ascorbic acid (data was represented as mean ± SD and investigated by one way analysis of variance), (F) % of viability of Caco 2 cells in different concentrations of nanoparticles (data was represented as mean ± SD and investigated by one way analysis of variance) and G] XRD of Mel (a), C (b), L (c), Mel-C/L nanoparticles (d) and unloaded C/L nanoparticles (e).
Antioxidant activity
DPPH free radical scavenging assay was used for screening the antioxidant activity of nanoparticles. DPPH scavenging % was represented in Fig. 1E; where, the prepared nanoparticles have a potential antioxidant activity when compared to ascorbic acid (control).
MTT assay
Caco 2 cells displayed high viability, after 24 h, with Mel-C/L nanoparticles up to the concentration of 31.25 μg/ml, respectively (Fig. 1F). The IC50 was 160.35 µg/ml.
Anti-coagulant activity
Mel loaded nanoparticles have significantly increased PT and PTT, in a dose dependent way, at concentration of 25 µg/ml (Table 1).
Anti-inflammatory activity
Table 2 demonstrated that Mel-C/L nanoparticles have significantly (p < 0.05) inhibited the lysis of red blood cells. Where, high hemolysis inhibition % (18.04, 31.14, 45.35, 60.98 and 73.99%) were observed at concentration of 100, 200, 400, 600 and 800 µg/ml, respectively. The results showed that hemolysis inhibition occurred in a dose dependent way; where, 1000 µg/ml of nanoparticles achieved similar results to 200 µg/ml indomethacin (94.63 and 99.3%, respectively).
In vivo anti-diabetic effect of Mel-C/L nanoparticles
Body weight, blood glucose and insulin level
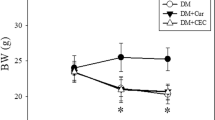
Diabetes induction significantly reduced body weight in untreated subgroup DGI in comparison to healthy control subgroup HGI. Also, an increase in levels of FBG and FTA with a significant reduction in insulin level were observed. Mel-C/L nanoparticles administration, in DGVI and DGVII, improved the rats’ body weight to be comparable to those of corresponding healthy control subgroups (HGVI and HGVII). Moreover, a reduction in blood glucose level and an elevation in insulin level were observed in DGVI and DGVII (Table 3).
Liver function enzymes (ALT and AST)
Diabetes induction, in DGI, led to a significant increase in levels of liver function enzymes (ALT and AST) when compared to healthy control subgroups. Treatment with free Mel (DGII and DGIII) or C/L nanoparticles (DGIV and DGV) has, slightly, reduced both of ALT and AST. Subgroup DGVI and DGVII (treated with 5 and 10 mg/kg Mel-C/L nanoparticles, respectively) showed a significant decrease in liver function enzymes when compared to diabetic untreated subgroup DGI (Fig. 2).
Liver function enzymes (ALT and AST) levels in different experimental groups. Data were presented as mean ± SEM, *represented a significance difference in comparison to diabetic untreated subgroup DGI and #represented a significance difference in comparison to healthy control subgroup HGI at p < 0.05 by One Way ANOVA and Tukey post hoc test (n = 6). HGI Control untreated rats, HGII Healthy rats that received free Mel (5 mg/kg), HGIII Healthy rats that received free Mel (10 mg/kg), HGIV Healthy rats that received unloaded C/L nanoparticles (5 mg/kg), HGV Healthy rats that received unloaded C/L nanoparticles (10 mg/kg), HGVI Healthy rats that received Mel-C/L nanoparticles (5 mg/kg), HGVII Healthy rats that received Mel-C/L nanoparticles (10 mg/kg), DGI Control untreated rats, DGII Diabetic rats that received free Mel (5 mg/kg), DGIII Diabetic rats that received free Mel (10 mg/kg), DGIV Diabetic rats that received unloaded C/L nanoparticles (5 mg/kg), DGV Diabetic rats that received unloaded C/L nanoparticles (10 mg/kg), DGVI Diabetic rats that received Mel-C/L nanoparticles (5 mg/kg), DGVII Diabetic rats that received Mel-C/L nanoparticles (10 mg/kg).
Kidney function parameters (urea and creatinine)
No significant difference was observed, in levels of urea and creatinine, among healthy control subgroups (HGI, II, III, IV, V, VI and VII). Mel-C/L nanoparticles administration (5 and 10 mg/kg in DGVI and DGVII, respectively) has significantly reduced kidney function parameters; where, levels of urea and creatinine in these subgroups were nearly the same as healthy control subgroups (Fig. 3).
Kidney function parameters (urea and creatinine) levels in different experimental groups. Data were presented as mean ± SEM, *represented a significance difference in comparison to diabetic untreated subgroup DGI and #represented a significance difference in comparison to healthy control subgroup HGI at p < 0.05 by One Way ANOVA and Tukey post hoc test (n = 6). HGI Control untreated rats, HGII Healthy rats that received free Mel (5 mg/kg), HGIII Healthy rats that received free Mel (10 mg/kg), HGIV Healthy rats that received unloaded C/L nanoparticles (5 mg/kg), HGV Healthy rats that received unloaded C/L nanoparticles (10 mg/kg), HGVI Healthy rats that received Mel-C/L nanoparticles (5 mg/kg), HGVII Healthy rats that received Mel-C/L nanoparticles (10 mg/kg), DGI Control untreated rats, DGII Diabetic rats that received free Mel (5 mg/kg), DGIII Diabetic rats that received free Mel (10 mg/kg), DGIV Diabetic rats that received unloaded C/L nanoparticles (5 mg/kg), DGV Diabetic rats that received unloaded C/L nanoparticles (10 mg/kg), DGVI Diabetic rats that received Mel-C/L nanoparticles (5 mg/kg), DGVII Diabetic rats that received Mel-C/L nanoparticles (10 mg/kg).
Lipid profile
Levels of triglycerides and cholesterol, in serum and liver tissue homogenate of diabetic untreated subgroup DGI, were higher than those of healthy control subgroup HGI; this was accompanied with an increase in low-density lipoprotein (LDL)-Cholesterol level and a decrease in high-density lipoprotein (HDL)-Cholesterol level in serum samples. Only, Mel-C/L nanoparticles succeeded in decreasing the elevated levels of cholesterol (in serum and liver) and LDL level, in a significant way, to be similar to healthy control subgroup HGI. Administration of C/L nanoparticles was not effective in normalization of lipid profile in diabetic subgroups (DGIV and DGV); the same was observed in free Mel treated subgroup DGIII (Fig. 4).
Lipid profile levels in serum and liver tissue homogenate in different experimental groups. Data were presented as mean ± SEM, *represented a significance difference in comparison to diabetic untreated subgroup DGI and #represented a significance difference in comparison to healthy control subgroup HGI at p < 0.05 by One Way ANOVA and Tukey post hoc test (n = 6). HGI Control untreated rats, HGII Healthy rats that received free Mel (5 mg/kg), HGIII Healthy rats that received free Mel (10 mg/kg), HGIV Healthy rats that received unloaded C/L nanoparticles (5 mg/kg), HGV Healthy rats that received unloaded C/L nanoparticles (10 mg/kg), HGVI Healthy rats that received Mel-C/L nanoparticles (5 mg/kg), HGVII Healthy rats that received Mel-C/L nanoparticles (10 mg/kg), DGI Control untreated rats, DGII Diabetic rats that received free Mel (5 mg/kg), DGIII Diabetic rats that received free Mel (10 mg/kg), DGIV Diabetic rats that received unloaded C/L nanoparticles (5 mg/kg), DGV Diabetic rats that received unloaded C/L nanoparticles (10 mg/kg), DGVI Diabetic rats that received Mel-C/L nanoparticles (5 mg/kg), DGVII Diabetic rats that received Mel-C/L nanoparticles (10 mg/kg).
Oxidative stress and antioxidant enzymes
Untreated diabetic subgroup, DGI, showed a vigorous increase in MDA level and a significant decrease in the antioxidant enzymes level (Gpx and SOD). Administration of free Mel, with the dose of 10 mg/kg in DGIII, has elevated the antioxidant enzyme levels and reduced MDA level more than the dose of 5 mg/kg (Fig. 5). On the contrary, 5 mg/kg of Mel-C/L nanoparticles administration (in DGVI) significantly normalized the levels of MDA, GPx and SOD to be similar to those of healthy control subgroups (HGI, II, III, IV, V, VI and VII).
Oxidative stress parameters (MDA, GPx and SOD) levels in different experimental groups. Data were presented as mean ± SEM, *represented a significance difference in comparison to diabetic untreated subgroup DGI and #represented a significance difference in comparison to healthy control subgroup HGI at p < 0.05 by One Way ANOVA and Tukey post hoc test (n = 6). HGI Control untreated rats, HGII Healthy rats that received free Mel (5 mg/kg), HGIII Healthy rats that received free Mel (10 mg/kg), HGIV Healthy rats that received unloaded C/L nanoparticles (5 mg/kg), HGV Healthy rats that received unloaded C/L nanoparticles (10 mg/kg), HGVI Healthy rats that received Mel-C/L nanoparticles (5 mg/kg), HGVII Healthy rats that received Mel-C/L nanoparticles (10 mg/kg), DGI Control untreated rats, DGII Diabetic rats that received free Mel (5 mg/kg), DGIII Diabetic rats that received free Mel (10 mg/kg), DGIV Diabetic rats that received unloaded C/L nanoparticles (5 mg/kg), DGV Diabetic rats that received unloaded C/L nanoparticles (10 mg/kg), DGVI Diabetic rats that received Mel-C/L nanoparticles (5 mg/kg), DGVII Diabetic rats that received Mel-C/L nanoparticles (10 mg/kg).
Pro-inflammatory cytokines and immunoglobulin
Pro-inflammatory cytokines (TNF-α, IL-12 and -1β) and immunoglobulin (IgA, IgE and IgG) levels were greatly elevated, in untreated diabetic subgroup DGI and diabetic subgroups treated with C/L nanoparticles (DGIV and DGV), when compared to healthy control subgroups. Although, free melatonin reduced cytokines and immunoglobulin levels (especially, the dose of 10 mg/kg), but their levels remained higher than those of healthy control subgroups. On the other hand, Mel-C/L nanoparticles (5 and 10 mg/kg) succeeded in decreasing their levels to be similar to healthy control subgroups (Fig. 6).
Pro-inflammatory cytokines (TNF-α, IL-12 and -1β) and immunoglobulin (IgA, IgE and IgG) levels in different experimental groups. Data were presented as mean ± SEM, *represented a significance difference in comparison to diabetic untreated subgroup DGI and #represented a significance difference in comparison to healthy control subgroup HGI at p < 0.05 by One Way ANOVA and Tukey post hoc test (n = 6). HGI Control untreated rats, HGII Healthy rats that received free Mel (5 mg/kg), HGIII Healthy rats that received free Mel (10 mg/kg), HGIV Healthy rats that received unloaded C/L nanoparticles (5 mg/kg), HGV Healthy rats that received unloaded C/L nanoparticles (10 mg/kg), HGVI Healthy rats that received Mel-C/L nanoparticles (5 mg/kg), HGVII Healthy rats that received Mel-C/L nanoparticles (10 mg/kg), DGI Control untreated rats, DGII Diabetic rats that received free Mel (5 mg/kg), DGIII Diabetic rats that received free Mel (10 mg/kg), DGIV Diabetic rats that received unloaded C/L nanoparticles (5 mg/kg), DGV Diabetic rats that received unloaded C/L nanoparticles (10 mg/kg), DGVI Diabetic rats that received Mel-C/L nanoparticles (5 mg/kg), DGVII Diabetic rats that received Mel-C/L nanoparticles (10 mg/kg).
Histopathologiocal and immunohistochemical examination
Healthy non-diabetic groups (HGI to HGVII) and diabetic groups treated with 10 mg/kg free Mel or Mel-C/L nanoparticles (5 or 10 mg/kg) showed: (1) pancreatic sections with average-sized pale-staining islets of Langerhans, positive immunostain for insulin and negative immunosatin for NF-κB, (2) liver sections with average central veins with average hepatocytes arranged in single cell cords, and (3) kidney sections with average glomeruli with average Bowman’s spaces and average collecting tubules.
On the other hand, untreated diabetic group (DGI) and diabetic groups treated with free Mel (5 mg/kg) or unloaded C/L nanoparticles (5 or 10 mg/kg) showed: (1) pancreatic sections with few small-sized hypocellular pale-staining islets of Langerhans with scattered apoptotic β cells and markedly edematous cytoplasm, negative immunostain for insulin, and positive immunostain for NF-κB (Fig. 7), (2) liver sections with dilated congested central veins and marked hepatocytes steatosis, and (3) kidney sections with small-sized glomeruli with widened Bowman’s spaces and proximal tubules with mildly vacuolated epithelial lining (Fig. 8).
Pancreatic sections of healthy non-diabetic groups (HGI to HGVII) and diabetic groups treated with free Mel (10 mg/kg, DGIII) or Mel-C/L nanoparticles (5 or 10 mg/kg; DGVI or DGVII) showing average-sized pale-staining islets of Langerhans (black arrow) (A,D,F,G; H&E X200), positive immunostain for insulin (yellow arrow) (H,J,L; X200) and negative immunosatin for NF-κB (M,R; X200); pancreatic sections of untreated diabetic group (DGI) and diabetic groups treated with free Mel (5 mg/kg, DGII) or unloaded C/L nanoparticles (5 or 10 mg/kg; DGIV or DGV) showing few small-sized hypocellular pale-staining islets of Langerhans with scattered apoptotic β cells and markedly edematous cytoplasm (red arrow) (B,C,E; H&E X200), negative immunostain for insulin (blue arrow) (I,K; X200), and positive immunostain for NF-B (N,O,P,Q; X200). HGI Control untreated rats, HGII Healthy rats that received free Mel (5 mg/kg), HGIII Healthy rats that received free Mel (10 mg/kg), HGIV Healthy rats that received unloaded C/L nanoparticles (5 mg/kg), HGV Healthy rats that received unloaded C/L nanoparticles (10 mg/kg), HGVI Healthy rats that received Mel-C/L nanoparticles (5 mg/kg), HGVII Healthy rats that received Mel-C/L nanoparticles (10 mg/kg), DGI Control untreated rats, DGII Diabetic rats that received free Mel (5 mg/kg), DGIII Diabetic rats that received free Mel (10 mg/kg), DGIV Diabetic rats that received unloaded C/L nanoparticles (5 mg/kg), DGV Diabetic rats that received unloaded C/L nanoparticles (10 mg/kg), DGVI Diabetic rats that received Mel-C/L nanoparticles (5 mg/kg), DGVII Diabetic rats that received Mel-C/L nanoparticles (10 mg/kg).
Haematoxylin and eosin sections showing: (A,E,F) average central veins (black arrow) with average hepatocytes arranged in single cell cords (blue arrow) in liver of non-diabetic groups (HGI to HGVII) and diabetic groups treated with free Mel (10 mg/kg, DGIII) or Mel-C/L nanoparticles (5 or 10 mg/kg; DGVI or DGVII) (X200); (B–D) dilated congested central veins (yellow arrow) with marked micro-vesicular steatosis of hepatocytes (red arrow) in peri-venular area of liver in untreated diabetic group (DGI) and diabetic groups treated with free Mel (5 mg/kg, DGII) or unloaded C/L nanoparticles (5 or 10 mg/kg; DGIV or DGV) (X200 and X400); (G,K,L) average glomeruli with average Bowman’s spaces (green arrow) and average collecting tubules (brown arrow) in kidney of non-diabetic groups (HGI to HGVII) and diabetic groups treated with free Mel (10 mg/kg, DGIII) or Mel-C/L nanoparticles (5 or 10 mg/kg; DGVI or DGVII) (X200); (H–J) small-sized glomeruli with widened Bowman’s spaces (orange arrow) and proximal tubules with mildly vacuolated epithelial lining (violet arrow) in kidney of untreated diabetic group (DGI) and diabetic groups treated with free Mel (5 mg/kg, DGII) or unloaded C/L nanoparticles (5 or 10 mg/kg; DGIV or DGV) (X200). HGI Control untreated rats, HGII Healthy rats that received free Mel (5 mg/kg), HGIII Healthy rats that received free Mel (10 mg/kg), HGIV Healthy rats that received unloaded C/L nanoparticles (5 mg/kg), HGV Healthy rats that received unloaded C/L nanoparticles (10 mg/kg), HGVI Healthy rats that received Mel-C/L nanoparticles (5 mg/kg), HGVII Healthy rats that received Mel-C/L nanoparticles (10 mg/kg), DGI Control untreated rats, DGII Diabetic rats that received free Mel (5 mg/kg), DGIII Diabetic rats that received free Mel (10 mg/kg), DGIV Diabetic rats that received unloaded C/L nanoparticles (5 mg/kg), DGV Diabetic rats that received unloaded C/L nanoparticles (10 mg/kg), DGVI Diabetic rats that received Mel-C/L nanoparticles (5 mg/kg), DGVII Diabetic rats that received Mel-C/L nanoparticles (10 mg/kg).
Discussion
Due to their capacity to provide regulated drug release at certain places, biodegradable polymer nanoparticles have become more important for drug delivery. Sustained release techniques should be more beneficial for a range of purposes because Mel has a short half-life. By encasing mel within nanoparticles, its solubility and stability can be increased, as well as the duration of its pharmacological effects. Mel has a low capacity to be absorbed from mucosal and dermal surfaces and a short half-life with quick elimination from circulation as a result of its molecular properties52. Since Mel has the capacity to affect cell membrane receptors and serve as an antioxidant molecule, a number of negative consequences may be noted after administration. As was already said, new drug delivery methods have made it possible for researchers to study Mel in an effort to solve some of its limitations for routine clinical usage in fields other than sleep medicine53.
In this study, the EE% of Mel in prepared nanoparticles was 28.4% and the DL% was 5.1%. Our results were comparable to those of Hafner et al.35 and Lopes Rocha Correa et al.38; who reported 27% and 4.8% for EE% and DL%, respectively. The zeta potential of the prepared nanoparticles was − 23 mV. TEM image showed that the nanoparticles size ranged from 75 to 98 nm; on the other hand, DLS analysis showed that Mel-C/L nanoparticles have a size of 20 nm. This difference can be attributed to: (1) DLS is a technique that determine particle size in suspension (i.e. it measures the hydration sphere diameter) and (2) TEM is a number based method while DLS is an intensity based method54,55. The results were in agreement with Hafner et al.35 and Lopes Rocha Correa et al.39 who reported that C/L nanoparticles have a size of 207.63 ± 14.61 nm and 160.43 ± 4.45 nm when loaded with Mel. L (S45) has a negative charge due to its high content of lipids, therefore, it interacts strongly with positively charged chitosan to give a stable nanoparticles56,57.
XRD was used to verify the formation of Mel-C/L nanoparticles. The XRD pattern of Mel showed four sharp peaks at 17.4, 24.4, 25.6 and 26.1°. While, C and L XRD patterns showed broad bands at around 20 and 20.1, respectively. Mel-C/L nanoparticles have XRD diffraction superimposition of L, C and Mel; where the diffraction peaks of Mel appeared with the characteristic broad peaks of both C and L. Unloaded C/L nanoparticles showed broad band in the range of 15–24. Our results were in agreement with Topal et al.58 who reported that Mel has sharp peaks at the diffraction angles of 2θ 17.8°, 24.8°, 25.2° and 26.2° that confirmed its crystalline strucutre; and Cheng et al.59 who showed that L has a large broad peak at 20.3° which indicated its amorphous characteristics.
In this study, we evaluated the in vitro antioxidant, anti-inflammatory, anticoagulant and cytotoxicity activities of the prepared nanoparticles before its use in vivo. The results indicated that Mel-C/L nanoparticles can be recommended to be used, safely, as a useful therapy against diabetes. Where, the prepared Mel-C/L nanoparticles demonstrated: (1) prolonged coagulation time (PT and PTT), (2) potential antioxidant and membrane stabilizing activities and (3) the lack of cytotoxicity up to concentration of 31.25 μg/ml.
Mel can decrease blood glucose by stimulating the insulin signaling via G protein coupled membrane receptors (MT1 and MT2) expressed on the islets of Langerhans60, and it can also regulate glucose homeostasis, leading to glucose uptake into skeletal muscle, according to the studies of Peschke et al.27, Ha et al.61 and Promsan and Lungkaphin28. Mel also has an indirect antioxidant effect by upregulating the antioxidant enzymes (SOD, GPx) and downregulating oxidant enzymes (NO synthase)28,29, resulting in free radicles production suppression that in turn reduce inflammation30,31. Mel functions as an antioxidant, anti-inflammatory, and anti-diabetic; it directly scavenges ROS like H2O2 and O2−28.
Diabetes induction, in untreated subgroup, significantly reduced body weight and elevated FBG and FTA levels in comparison to healthy control. Also, chronic hyperglycemia led to oxidative stress and inflammation in many organs; this was evident by the elevation in kidney and liver function measured parameters and the decrease in the antioxidant enzymes levels (Gpx and SOD). Levels of pro-inflammatory cytokines (TNF-α, IL-12 and -1β) and immunoglobulin (IgA, IgE and IgG) were significantly elevated in pancreatic tissue of untreated rats; these observation were confirmed by the significant expression of NF-κB in pancreatic sections. Moreover, hyperglycemia led to an elevation in levels of lipid profile (triglycerides, cholesterol and LDL-C), kidney (urea and creatinine) and liver (ALT and AST) function parameters. The histopathological examination of liver and kidney in diabetic rats proofed the biochemical results. Where, liver sections with dilated congested central veins and marked hepatocytes steatosis were observed in diabetic untreated group; in addition to, kidney sections with small-sized glomeruli with widened Bowman’s spaces and proximal tubules with mildly vacuolated epithelial lining. Pancreatic sections with few small-sized hypocellular pale-staining islets of Langerhans with scattered apoptotic β cells and markedly edematous cytoplasm, negative immunostain for insulin, and positive immunostain for NF-κB were detected in untreated diabetic group.
According to Pham-Huy et al.62, oxidative stress is hypothesised to have an impact on how diabetes-related vascular issues proceed. They added that the main cause of mortality in patients with diabetes was a rise in vascular diseases rather than a raised blood glucose level. According to Lipinski63, increased formation of free radicals in diabetes was associated with decreased levels of the antioxidant enzymes CAT, SOD, and GPx, which in turn caused tissue damage. Because of protein glycation, glucose oxidation, and increased lipid peroxidation, oxidative stress in diabetes damages enzymes and increases insulin resistance64. In mitochondria, a portion of oxygen is converted to water and the remaining oxygen is transformed into oxygen free radical (O−), a significant ROS that is transformed into other reactive species like ONOO−, OH−, and H2O2, which in turn modulates insulin signaling65. Chronic oxidative stress and inflammation have both been connected to the aetiology of diabetes mellitus. In pathological states, inflammation and oxidative stress were intimately linked, according to Ambade and Mandrekar66. Furthermore, chronic inflammation promotes cellular damage mostly through the overproduction of free radicals and the depletion of antioxidant enzymes67. By activating transcription factors including NF-κB and activator protein-1, free radical buildup promotes the production of pro-inflammatory cytokines68.
In this study, although administration of free Mel (10 mg/kg) revealed some therapeutic effects in treated groups, but lower doses of Mel-C/L nanoparticles (5 mg/kg) enhanced the kidney and liver function, improved the decrease in rats’ body weight, reduced blood glucose level, increased insulin secretion and ameliorated the disturbance in lipid profile. Moreover, a regeneration of pancreatic β cells and decreased expression of NF-κB were observed in pancreatic sections of rats treated with 5 mg/kg Mel-C/L nanoparticles. Diabetic groups treated with Mel-C/L nanoparticles (5 or 10 mg/kg) showed pancreatic sections with average-sized pale-staining islets of Langerhans, positive immunostain for insulin and negative immunosatin for NF-κB. Liver sections with average central veins with hepatocytes arranged in single cell cords and kidney sections with average glomeruli with average Bowman’s spaces (green arrow) and average collecting tubules were, also, observed in treated group. These findings indicated the in vivo anti-inflammatory and antioxidant effects of the prepared nanoparticles; moreover, nanoparticles application decreased the administrated Mel dose. Several studies demonstrated that free Mel administration faces some obstacles like Mel susceptibility to oxidation and insolubility in water69. Therefore, encapsulation of Mel in C/L nanoparticles has prevented its degradation, enhanced its therapeutic properties, and decreased the required therapeutic dose for diabetes treatment70.
Due to melatonin's limited oral bioavailability, oxidation sensitivity, and moderate aqueous environment solubility. These restrictions made it difficult to treat diabetic rats by giving them free melatonin orally. The results demonstrated that C/L nanoparticles, which were distinguished by their high stability and mucoadhesive properties, provided an excellent solution for these issues by effectively encasing and shielding melatonin until it enters the bloodstream, where it is slowly released until it reaches its site of function. Furthermore, According to the results, doses 5 and 10 mg/kg of Mel-C/L nanoparticles yielded similar results and showed efficiency in lowering blood glucose, regenerating beta cells to produce insulin, and reducing oxidative stress caused by diabetes mellitus, so the recommended 5 mg dose is chosen to limit the side effects of melatonin as much as possible.
In conclusion, it was proven that Mel-C/L nanoparticles showed remarkable anti-inflammatory, anti-coagulant, and anti-oxidant effects, in addition to its efficiency in reducing blood glucose levels of STZ-induced diabetic rats and its great ability to promote the regeneration of β-cells in the pancreas. Moreover, nanoparticles application decreased the administrated Mel dose that in turn can diminish the side effects of free Mel administration. The study showed a future hope for the treatment of diabetes by Mel-C/L nanoparticles. There were some limitations in the study that should be addressed in the future research. The study was, only, conducted on Sprague dawley rats, so the results may not be generalizable to humans. Also, the long-term effects of using Mel-C/L nanoparticles, which is a significant limitation in the context of chronic diseases such as diabetes. Moreover, the study did not compare the efficacy of Mel-C/L nanoparticles with other existing treatments for diabetes. However, the present study can be the first step in management of diabetes mellitus and its complicated symptoms by Mel-C/L nanoparticles; in addition, Mel-C/L nanoparticles succeeded in beta cells neogenesis. However, further research is required to investigate the long-term effects of using Mel-C/L nanoparticles, their efficacy in higher animals then humans, and their comparative effectiveness with other existing treatments.
Data availability
All data generated or analysed during this study are included in this published article.
Change history
15 April 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1038/s41598-024-59109-0
References
Shi, G. J. et al. Involvement of growth factors in diabetes mellitus and its complications: A general review. Biomed. Pharmacother. 101, 510–527 (2018).
Watson, C. P. N., Moulin, D., Watt-Watson, J., Gordon, A. & Eisenhoffer, J. Controlled-release oxycodone relieves neuropathic pain: A randomized controlled trial in painful diabetic neuropathy. Pain 105(1–2), 71–78 (2003).
Hayat, S. A., Patel, B., Khattar, R. S. & Malik, R. A. Diabetic cardiomyopathy: Mechanisms, diagnosis and treatment. Clin. Sci. (Lond). 107(6), 539–557 (2004).
Boudina, S. & Abel, E. D. Diabetic cardiomyopathy causes and effects. Rev. Endocr. Metab. Disord. 11(1), 31–39 (2010).
Altmann, C. & Schmidt, M. H. The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. Int. J. Mol. Sci. 19(1), 110 (2018).
Dow, C. et al. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 33(2), 141–156 (2018).
Xu, X., Chen, P., Zheng, Q., Wang, Y. & Chen, W. Effect of pioglitazone on diabetic nephropathy and expression of HIF-1α and VEGF in the renal tissues of type 2 diabetic rats. Diabetes Res. Clin. Pract. 93(1), 63–69 (2011).
Tziomalos, K. & Athyros, V. G. Diabetic nephropathy: New risk factors and improvements in diagnosis. Rev. Diabet. Stud. 12(1–2), 110–118 (2015).
Amaral, S., Oliveira, P. J. & Ramalho-Santos, J. Diabetes and the impairment of reproductive function: Possible role of mitochondria and reactive oxygen species. Curr. Diabetes Rev. 4(1), 46–54 (2008).
Alkhamees, O. A. Quercetin attenuates testicular damage and oxidative stress in streptozotocin-induced diabetic rats. Br. J. Pharmacol. Toxicol. 5(2), 88–97 (2004).
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107(9), 1058–1070 (2010).
Frese, T. et al. Pineal melatonin synthesis is decreased in type 2 diabetic Goto-Kakizaki rats. Life Sci. 85(13–14), 526–533 (2009).
Rodrigues, S. C. et al. Increased corticosterone levels contribute to glucose intolerance induced by the absence of melatonin. Feder. Am. Soc. Exp. Biol. 2013, 1161 (2013).
Sun, H. et al. Melatonin treatment improves insulin resistance and pigmentation in obese patients with acanthosis nigricans. Int. J. Endocrinol. 2018, 2304746 (2018).
Milosavljević, A. et al. Melatonin levels in human diabetic dental pulp tissue and its effects on dental pulp cells under hyperglycaemic conditions. Int. Endodon. J. 51(10), 1149–1158 (2018).
de Oliveira, A. C. et al. Combined treatment with melatonin and insulin improves glycemic control, white adipose tissue metabolism and reproductive axis of diabetic male rats. Life Sci. 199, 158–166 (2018).
Sartori, C. et al. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology 150(12), 5311–5317 (2009).
Behram, K. Y., Guntekin, U., Tosun, V., Korucuk, N. & Bozdemir, M. N. Melatonin protects against streptozotocin-induced diabetic cardiomyopathy by the phosphorylation of vascular endothelial growth factor-A (VEGF-A). Cell Mol. Biol. 64(14), 47–52 (2018).
Heo, J. I. et al. Melatonin improves insulin resistance and hepatic steatosis through attenuation of alpha-2-HS-glycoprotein. J. Pineal. Res. 65(2), e12493 (2018).
Xu, J. et al. Melatonin alleviates cognition impairment by antagonizing brain insulin resistance in aged rats fed a high-fat diet. J. Pineal. Res. 67(2), e12584 (2019).
Hardeland, R. Melatonin in Healthy Aging and Longevity. Hormones in Ageing and Longevity. 209–242 (Springer; 2017).
Esposito, E. & Cuzzocrea, S. Antiinflammatory activity of melatonin in central nervous system. Curr. Neuropharmacol. 8(3), 228–242 (2010).
Boga, J. A. et al. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal. Res. 66(1), e12534 (2019).
She, Q. et al. Impacts of circadian rhythm and melatonin on the specific activities of immune and antioxidant enzymes of the Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 89, 345–353 (2019).
Zhang, C. et al. The protective effects of melatonin on oxidative damage and the immune system of the Chinese mitten crab (Eriocheir sinensis) exposed to deltamethrin. Sci. Total Environ. 653, 1426–1434 (2019).
Garcia-Maurino, S., Pozo, D., Carrillo-Vico, A., Calvo, J. R. & Guerrero, J. M. Melatonin activates Th1 lymphocytes by increasing IL-12 production. Life Sci. 65(20), 2143–2150 (1999).
Peschke, E., Bähr, I. & Mühlbauer, E. Melatonin and pancreatic islets: Interrelationships between melatonin, insulin and glucagon. Int. J. Mol. Sci. 14(4), 6981–7015 (2013).
Promsan, S. & Lungkaphin, A. The roles of melatonin on kidney injury in obese and diabetic conditions. BioFactors 46(4), 531–549 (2020).
Farid, A. et al. Melatonin relieves diabetic complications and regenerates pancreatic beta cells by the reduction in NF-kB expression in streptozotocin induced diabetic rats. Saudi J. Biol. Sci. 29(7), 103313 (2022).
Hardeland, R. & Pandi-Perumal, S. R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. (Lond). 2, 22 (2005).
Tordjman, S. et al. Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 15(3), 434–443 (2017).
Foley, H. M. & Steel, A. E. Adverse events associated with oral administration of melatonin: A critical systematic review of clinical evidence. Complement. Ther. Med. 42, 65–81 (2019).
Hansen, M. V. et al. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: A randomized, double-blind, placebo-controlled trial. Breast Cancer Res. Treat. 145(3), 683–695 (2014).
Baandrup, L., Lindschou, J., Winkel, P., Gluud, C. & Glenthoj, B. Y. Prolonged-release melatonin versus placebo for benzodiazepine discontinuation in patients with schizophrenia or bipolar disorder: A randomised, placebo-controlled, blinded trial. World J. Biol. Psychiatry 17(7), 514–524 (2016).
Hafner, A., Lovrić, J., Voinovich, D. & Filipović-Grcić, J. Melatonin-loaded lecithin/chitosan nanoparticles: Physicochemical characterisation and permeability through Caco-2 cell monolayers. Int. J. Pharm. 381(2), 205–213 (2009).
Schaffazick, S. R., Pohlmann, A. R., de Cordova, C. A., Creczynski-Pasa, T. B. & Guterres, S. S. Protective properties of melatonin-loaded nanoparticles against lipid peroxidation. Int. J. Pharm. 289(1–2), 209–213 (2005).
Schaffazick, S. R. et al. Incorporation in polymeric nanocapsules improves the antioxidant effect of melatonin against lipid peroxidation in mice brain and liver. Eur. J. Pharm. Biopharm. 69(1), 64–71 (2008).
Lopes Rocha Correa, V. et al. Melatonin loaded lecithin-chitosan nanoparticles improved the wound healing in diabetic rats. Int. J. Biol. Macromol. 162, 1465–1475 (2020).
England, R. J. A., Homer, J. J., Knight, L. C. & Ell, S. R. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryngol. 24, 67–68 (1999).
Singla, A. K. & Chawla, M. Chitosan: Some pharmaceutical and biological aspects-an update. J. Pharm. Pharmacol. 53, 1047–1067 (2001).
Kelmann, R. G., Kuminek, G., Teixeira, H. F. & Koester, L. S. Carbamazepine parenteral nanoemulsions prepared by spontaneous emulsification process. Int. J. Pharm. 342, 231–239 (2007).
Pavelić, Z., Skalko-Basnet, N., Filipović-Grcić, J., Martinac, A. & Jalsenjak, I. Development and in vitro evaluation of a liposomal vaginal delivery system for acyclovir. J. Control. Release 106, 34–43 (2005).
Ceraulo, L. et al. Orientation and molecular contacts of melatonin confined into AOT and lecithin reversed micellar systems. Colloids Surf. A Physicochem. Eng. Aspects 316, 307–312 (2008).
Schubert, M. A., Harms, M. & Müller-Goymann, C. C. Structural investigations on lipid nanoparticles containing high amounts of lecithin. Eur. J. Pharm. Sci. 27, 226–236 (2006).
Farid, A., Haridyy, H., Ashraf, S., Ahmed, S. & Safwat, G. Co-treatment with grape seed extract and mesenchymal stem cells in vivo regenerated beta cells of islets of Langerhans in pancreas of type I-induced diabetic rats. Stem Cell Res. Ther. 13(1), 528 (2022).
Vasconcelos, A. F. et al. Sulfonation and anticoagulant activity of fungal exocellular β-(1→6)-D-glucan (lasiodiplodan). Carbohydr. Polym. 92(2), 1908–1914 (2013).
Anosike, C. A., Obidoa, O. & Ezeanyika, L. U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). Daru 20(1), 76 (2012).
Furman, B. L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 70, 5.47.1-5.47.20 (2015).
Farid, A., Alalfy, L., Madbouly, N. Bone marrow-derived mesenchymal stem cells transplantation downregulates pancreatic NF-κB and pro-inflammatory cytokine profile in rats with type I and type II-induced diabetes: A comparison study. Biologia. https://doi.org/10.1007/s11756-023-01436-0 (2023).
Waterborg, J. H. & Matthews, H. R. The lowry method for protein quantitation. Methods Mol. Biol. 1, 1–3 (1984).
Farid, A. Preparation of polyclonal anti-Schistosoma mansoni cysteine protease antibodies for early diagnosis. Appl. Microbiol. Biotechnol. 107(5–6), 1609–1619 (2023).
Boutin, J. A. & Jockers, R. Melatonin controversies, an update. J. Pineal. Res. 70(2), e12702 (2021).
Lawrence, J. Making drugs work better: Four new drug delivery methods. Lung Cancer 15, 05 (2018).
Pabisch, S., Feichtenschlager, B., Kickelbick, G. & Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 521(4), 91–97 (2012).
Souza, T. G. F., Ciminelli, V. S. T. & Mohallem, N. D. S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 733, 012039 (2016).
Sonvico, F. et al. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 324, 67–73 (2006).
Gerelli, Y. et al. Structure of self-organized multilayer nanoparticles for drug delivery. Langmuir 24, 11378–11384 (2008).
Topal, B., Çetin Altındal, D. & Gümüşderelioğlu, M. Melatonin/HPβCD complex: Microwave synthesis, integration with chitosan scaffolds and inhibitory effects on MG-63CELLS. Int. J. Pharm. 496(2), 801–811 (2015).
Cheng, W., Luo, Z., Li, L. & Fu, X. Preparation and characterization of debranched-starch/phosphatidylcholine inclusion complexes. J. Agric. Food Chem. 63(2), 634–641 (2015).
Watanabe, K., Katagiri, S. & Hattori, A. Melatonin and glucose metabolism. Glycation Stress Res. 7(1), 105–109 (2020).
Ha, E. et al. Melatonin stimulates glucose transport via insulin receptor substrate-1/phosphatidylinositol 3-kinase pathway in C2C12 murine skeletal muscle cells. J. Pineal. Res.. 41(1), 67–72 (2006).
Pham-Huy, L. A., He, H. & Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 4(2), 89–96 (2008).
Lipinski, B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes Complic. 15(4), 203–210 (2001).
Maritim, A. C., Sanders, R. A. & Watkins, J. B. 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 17(1), 24–38 (2003).
Erejuwa, O. O. Oxidative stress in diabetes mellitus: is there a role for hypoglycemic drugs and/or antioxidants. Oxid. Stress Dis. 217–246 (2012).
Ambade, A. & Mandrekar, P. Oxidative stress and inflammation: Essential partners in alcoholic liver disease. Int. J. Hepatol. 2012, 853175 (2012).
Fialkow, L., Wang, Y. & Downey, G. P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 42(2), 153–164 (2007).
Oguntibeju, O. O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 11(3), 45–63 (2019).
Kumar Yadav, S. et al. Nanomelatonin triggers superior anticancer functionality in a human malignant glioblastoma cell line. Nanotechnology 28(36), 365102 (2017).
Tursilli, R., Casolari, A., Iannuccelli, V. & Scalia, S. Enhancement of melatonin photostability by encapsulation in lipospheres. J. Pharm. Biomed. Anal. 40(4), 910–914 (2006).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
A.F. contributed to the study conception and design. Material preparation, data collection and analysis were performed by all author. The manuscript was written and approved by all author.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-024-59109-0
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alaa, H., Abdelaziz, M., Mustafa, M. et al. RETRACTED ARTICLE: Therapeutic effect of melatonin-loaded chitosan/lecithin nanoparticles on hyperglycemia and pancreatic beta cells regeneration in streptozotocin-induced diabetic rats. Sci Rep 13, 10617 (2023). https://doi.org/10.1038/s41598-023-36929-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36929-0
This article is cited by
-
Multiple nanotechnological approaches using natural compounds for diabetes management
Journal of Diabetes & Metabolic Disorders (2024)
-
Eobania vermiculata whole-body muscle extract-loaded chitosan nanoparticles enhanced skin regeneration and decreased pro-inflammatory cytokines in vivo
Journal of Nanobiotechnology (2023)
-
Anti-Cryptosporidium oocysts polyclonal antibodies for cryptosporidiosis diagnosis and protection
AMB Express (2023)
-
Melatonin loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles reduce inflammation, inhibit apoptosis and protect rat’s liver from the hazardous effects of CCL4
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.