Abstract
High blood glucose in diabetic patients often causes cardiovascular diseases (CVDs) that threats to human life. Curcumin (Cur) is known as an antioxidant agent, possesses anti-inflammatory activity, and prevents CVDs. However, the clinical application of curcumin was limited due to its low bioavailability. This study aimed to investigate the ameliorative effects of chitosan-encapsulated curcumin (CEC) on heart and kidney damages in streptozotocin-induced type-1 diabetes C57BL/6 mice model. The results showed that Cur- and CEC-treatments downregulated the blood sugar and total cholesterol level as well as enhanced insulin secretion. However, blood pressure, triglycerides content, and very low-density lipoprotein-cholesterol content were not changed. Histochemistry analysis revealed that both curcumin and chitosan-encapsulated curcumin ameliorated cell hypertrophy and nucleus enlargement in the left ventricular of heart and reduced fibrosis in the kidney, especially after the chitosan-encapsulated curcumin treatment. Our study suggested that chitosan can effectively enhance the protective effect of curcumin on the heart and kidney damages in type-1 diabetes mice model.
Similar content being viewed by others
Introduction
Diabetes is a chronic disease resulting from either failure of insulin secretion, insulin action, or both. The defect of insulin mechanism leads to hyperglycemia or high blood glucose1. The Non-Communicable Diseases (NCD) Risk Factor Collaboration in 2016 reported that the prevalence of adults with diabetes increased from 108 million (1980) to 422 million (2014) in the world2. Additionally, according to the International Diabetes Federation (IDF) Atlas guideline report, the number of diabetes is expected to rise to 629 million in 20451. Type-1 diabetes is characterized by autoimmune-mediated pancreatic β-cell results in the deficiency of insulin, whereas type-2 diabetes is peripheral insulin resistance. However, both forms of diabetes is related to elevated inflammation, oxidative stress, cardiovascular diseases (CVDs), and chronic kidney diseases3.
Diabetes is also a major risk factor for health-system costs, mortality, and morbidity2,4,5,6. The previous study reported that the mortality prevalence increased in type-1 diabetes7. Moreover, it was also associated with heart failure and cardiomyopathy. The risk of heart failure was independently associated with diabetes and it was increased more than 5-folds in woman and 2-folds in men. Also, the prevalence of heart failure in diabetes is 4-times higher than the general population4. Type-1 diabetes is also leads to kidney diseases, such as ischemic damage, diabetic nephropathy, and other renal diseases, and it was significantly associated with a reduction of life quality5,8,9. Based on these conditions, type-1 diabetes is one of the main global health problems.
Insulin therapy is the most common treatment for type-1 diabetes. However, it does not always provide the metabolic regulation necessary to obviate the disease associated-complications. Therefore, in modern countries, type-1 diabetes treatment was improved by the use of insulin analogs and mechanical technologies, such as insulin pump and continuous blood glucose monitor10,11. However, at least 75% of diabetes patients live in low- to middle-income countries and they cannot provide necessary drugs for their treatment12. Additionally, some drugs for type-1 diabetes treatment accompanied by side effects for long-term use, such as stroke and severe gastrointestinal diseases13. Therefore, the new approaches have emerged for diabetes treatment including a supplement with natural resources, such as fruits, vegetables, and fish oil or through function foods with their content as pharmaceutical or nutraceutical agents, such as polyphenol, flavonoids, alkaloids, and pigments14.
Curcumin is an active ingredient which can be extracted from turmeric (Curcuma longa). Both turmeric and curcumin have been widely used as food ingredients and also used for disease treatments. Turmeric has been used as a coloring agent in many food industries and medicinal applications such as treatment of inflammations. Curcumins have been emerged as favorable functional food by its potent antioxidant activity and observed in experimental models for some diseases, such as hepatic and pancreatic diseases, arteriosclerosis, diabetic, and colitis disease model15,16,17,18. Apart from this, the direct application of curcumin was limited due to several factors. The relatively low bioavailability and poor aqueous solubility of curcumin have been reported as major problems. Therefore, various mechanisms have been formulated to enhance curcumin utilization, such as emulsion, encapsulation, and nanoparticle forms19. Encapsulations have been used to enhance the bioavailability of poorly soluble drugs. Curcumin was encapsulated by polymers have been reported, including chitosan, polyethylene glycol-poly(ethyleneimine), and oil body encapsulations19,20,21. Chitosan has been reported as an encapsulation agent due to its favorable properties, such as biocompatible, biodegradable, and high drugs loading efficiency. Curcumin encapsulated in chitosan shown the high encapsulation efficiency and small size of the nanoparticles as well as increased bioavailability21,22,23,24. Therefore, this study aimed to investigate the ameliorative effects of curcumin encapsulated by chitosan (CEC) on heart and kidney damages in streptozotocin-induced type-1 diabetes mice model.
Results
Effects of CEC on body weight
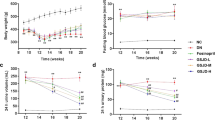
There were no any significant differences between the initial body weight of mice. The initial body weight of mice no significant differences between all groups. At the week-18 (18 W) of mice’s age, the body weight showed significant difference between the Control (Ctrl) group (25 ± 1.66 g) with another groups (Fig. 1). Treatment with curcumin (Cur) and chitosan-encapsulated curcumin (CEC) for 4 weeks (22 W) suppressed the body weight loss with the body weight of Cur- (21 ± 0.97 g) and CEC-treated (21 ± 1.16 g) groups, whereas these groups were higher than DM group (20 ± 1.29 g).
Effect of curcumin and CEC on body weight (BW) after treated for 4 weeks. Data are shown as mean ± S.D. (n = 6). Significant difference: *P < 0.05 vs. Ctrl group as analyzed by Duncan multiple range tests. Ctrl, control group; DM, diabetes without any treatment; DM + Cur, diabetes treated with curcumin (150 mg/kg); DM + CEC, diabetes treated with chitosan-encapsulated curcumin (150 mg/kg).
Effect of CEC on fasting serum glucose and insulin
The results showed that the initial fasting blood glucose level was not significantly difference between all groups. However, after intraperitoneally injected with STZ (150 mg/kg), the results showed that the glucose concentration of the STZ injected-groups significant higher when compared to control (Ctrl) group in both 17-week-old and 18-weeks-old (Fig. 2). After treated for 4 weeks (22-weeks-ols) with CEC (DM + CEC, 348 ± 85.21 mg/dL) the glucose concentration was significantly decreased when compared to the untreated-diabetes group (DM, 490 ± 31.98 mg/dL). Whereas, curcumin-treated (DM + Cur) group (363 ± 85.65 mg/dL) showed decreased glucose level, however not significantly different from DM group.
Fasting serum glucose concentration: (a) initial glucose level before STZ injection; (b,c) glucose level after STZ injection at 17 W and 18 W, respectively; (d) Glucose level after treatment for 4 weeks with curcumin and CEC. Data are shown as mean ± S.D. (n = 6). Significant difference: *P < 0.05 vs. Ctrl group and #P < 0.05 vs. DM group as analyzed by Duncan multiple range tests.
Figure 3 shown that the fasting insulin level of untreated-diabetes (DM) group (13 ± 7.36 pmol/L) significantly lower than control (Ctrl) group (35 ± 14.02 pmol/L). However, treated for 4 weeks by CEC (DM + CEC) group (29 ± 7.14 pmol/L) significantly increased the insulin level when compared to untreated-diabetes (DM) group. Curcumin-treated (DM + Cur) group (28 ± 10.27 pmol/L) also increased the insulin level, however not significantly difference when compared to the DM group.
Effect of CEC on lipid properties
Beside blood glucose and insulin levels, we also observed the total cholesterol (TC), triglycerides (TG), and very low-density lipoprotein-cholesterol (VLDL) to determine the serum lipid properties. At week-18 (18 W), the TC level of all of the diabetes groups significantly higher than the control (Ctrl) group (Fig. 4). After treated with Cur or CEC for 4 weeks, the TC levels were not showed any significant deference when compared to the Ctrl group (87 ± 6.22 mg/dL). Whereas, untreated-diabetes (DM) group (139 ± 8.13 mg/dL) was significantly higher than the Ctrl group. There were no significantly difference between TC level of both Cur and CEC, whereas DM + CEC group (102 ± 36.71 mg/dL) possessed TC level lower than DM + Cur group. For the triglycerides (TG) and VLDL levels, the results showed that there was no significantly difference for all groups (Table 1).
Effect of CEC on hemodynamic of mice
The basic measures of cardiovascular function (hemodynamic), such as systolic blood pressure (SBP), mean blood pressure (MBP), and diastolic blood pressure (DBP) was observed after 4 week’s treatment (Table 2). The results showed that there is no significantly difference in SBP, MBP, and DBP levels for all groups.
Effect of CEC on aorta, atrium, and left ventricle of mice histopathology
Masson’s trichrome staining was used to evaluate the aorta and right atrium function, and hematoxylin and eosin (H&E) staining for left atrium and left ventricle. Additionally, WGA-FITC staining also was used to evaluate the myocardial area of left ventricle. According to this staining, there were no any change in collagen composition in the right atrium and does not observed any proliferation of endothelial cells in the aorta blood vessels (Fig. 5A). Additionally, there was no change in cell size in the left atrium (Fig. 5B). However, DM mice showed a nuclear enlargement in the left ventricle (Fig. 6A,B). Treatment with curcumin (DM + Cur) and CEC (DM + CEC) successfully improved the hypertrophy and nucleus size. The myocardial area of DM group (364 ± 74.44 μm2) was significantly higher than the Ctrl group (248 ± 40.42 μm2) (Fig. 6C). After treated with Cur (DM + Cur, 286 ± 43.83 μm2) and CEC (DM + CEC, 246 ± 25.66 μm2), the myocardial area was significantly decreased when compared to the DM group. Moreover, the myocardial area of DM + CEC group significantly lower than the DM + Cur group.
Effect of CEC on the left ventricle of after treatment for 4 weeks. (A) H&E stain of the left ventricle, Magnification: 400X; (B) Fluorescence microscopy, greys are wheat-germ agglutinin labeled with FITC (WGA-FITC) and blues are the nuclei, Magnification: 400X; (B) Analysis of myocardial area in the left ventricle. Data are shown as mean ± S.D. (n = 6). Significant difference: *P < 0.05 vs. Ctrl group, #P < 0.05 vs. DM group and +P < 0.05 vs. DM + Cur group as analyzed by Duncan multiple range tests.
Effect of CEC on kidney properties
Masson’s trichrome staining was used to evaluate the kidney histopathology (Fig. 7A). The fibrosis of kidney was significantly increased in DM group (5.60 ± 2.09%) when compared to the control (Ctrl) group (1.88 ± 1.13%) (Fig. 7B). After treated with CEC (DM + CEC, 2.46 ± 0.84%) for 4 weeks, the fibrosis area significantly decreased when compared to the DM group. Moreover, the CEC-treated group also significantly lower fibrosis area when compared to curcumin-treated (DM + Cur, 4.44 ± 1.28%) group. The body urea nitrogen (BUN) was measured to evaluate the kidney function. The results showed that there was no any significant difference in BUN level between all groups. However, the BUN level of DM + Cur (27 ± 4.63 mg/dL) and DM + CEC (25 ± 7.57 mg/dL) groups were lower than the DM group (32 ± 4.97 mg/dL).
Effect of CEC on the cortex and medulla in the kidney after treatment for 4 weeks. (A) Masson’s trichrome stain; the nuclei are black to brownish black, the cytoplasm is brick red, collagen and mucus bluish green, magnification: 400X; (B) Analysis of fibrosis cortex and medulla in the kidney. Data are shown as mean ± S.D. (n = 6). Significant difference: *P < 0.05 vs. Ctrl group and #P < 0.05 vs. DM group, and +P < 0.05 vs. DM + Cur group as analyzed by Duncan multiple range tests.
Discussion
Type-1 diabetes has traditionally been associated with weight loss of the patient. Dehydration and muscle breakdown might cause a rapid weight loss in a type-1 diabetes patient25. In Fig. 1, the DM mice showed low body weight when compared to all of treatment groups. Both Cur- and CEC-treated mice suppressed the body weight loss. In week-7 (7 W), the mice showed a low glucose level (Fig. 2a). However, the glucose level increased when compared to non-diabetes (control) group after injected by STZ and it was characterized as a diabetes condition (Fig. 2b,c). High blood glucose (hyperglycemia) and low insulin level in blood are also associated with type-1 diabetes condition. Whereas, the high fasting blood glucose (FPG ≥ 126 mg/dL) characterized as diabetes26. Although, the fasting blood glucose remains in diabetes condition was significantly decreased after treated with curcumin and chitosan-encapsulated curcumin. (Fig. 2d).
The low insulin level showed in DM group mice (Fig. 3). The insulin level after treated with curcumin and chitosan-encapsulated curcumin was increased. The previous study reported that oral administration of curcumin reversed the hypoinsulinemia and glucose intolerance as well as enhanced the regenerate functional pancreatic islets in STZ-induced Swiss diabetic mice27. Additionally, the insulin level of the CEC group was higher than the curcumin-treated group.
The untreated-diabetes mice showed a high total cholesterol level (Fig. 4). After treated with curcumin and chitosan-encapsulated curcumin successfully decreased the total cholesterol (TC) level. The average level of TC in the CEC group was lower than the curcumin group. Whereas, no effect on triglycerides and very low-density lipoprotein-cholesterol level (Table 1). The previous study reported that high insulin level was not only effected to blood glucose level but also associated with cholesterol regulation including its synthesis and absorption28. According to our results, the chitosan polymer successfully enhanced the clinical utilities of curcumin in chitosan-encapsulated curcumin form. Additionally, the previous study also reported that nanocurcumin decreased HbA1c and LDL in type-2 diabetes29.
Dysfunction and failure organs, such as heart, kidney, nerves, and blood vessel are associated with chronic hyperglycemia. Both of type-1 and type-2 diabetes were associated with cardiovascular diseases and caused disability and death among the patients6,30. The hemodynamic diagnosis was used the evaluate the heart performance after treatments. The hemodynamic data showed that the blood pressure both systolic (SBP) and diastolic (DBP), there is no change for all groups after treatment (Table 2). The results showed that their levels remain normal and they are 113–160 mmHg, 81–110 mmHg for SBP and DBP, respectively31.
The Masson’s trichrome and hematoxylin-eosin (H&E) stains were used to evaluate the heart and kidney damage caused by diabetes. Based on the H&E staining of the aorta, right, and left atrium of heart, there were no any changed on their function, such as proliferation, hypertrophy, enlargement of the cell, and the collagen structure (Fig. 5). However, hypertrophy and enlargement of the cell were showed by untreated-diabetes mice group on the left ventricle of heart based on the H&E and WGA-FITC staining (Fig. 6). After treated with both curcumin and CEC, the cell size was successfully improved. Additionally, the myocardial area of left ventricle was also improved after treated with both curcumin and CEC. The CEC-treated group showed a low myocardial area when compared than the curcumin-treated group. The previous study reported that cardiomyopathy and heart failure are associated with diabetes condition. A cluster of features including decreased diastolic compliance, myocyte hypertrophy, and interstitial fibrosis are associated with cardiomyopathy in diabetes32.
Figure 7A shown the Masson’s trichrome staining of the kidney; and then after analysis, we found that the percentage of fibrosis area of untreated-diabetes group was higher than other groups another group (Fig. 7B). The fibrosis area decreased after treated with both curcumin or chitosan-encapsulated curcumin. Whereas, the fibrosis area of CEC-treated group lower than Cur-treated group. Fibrosis is a characteristic of chronic kidney disease33. Diabetes was associated with renal fibrosis and is a major health problem34. Additionally, we also determined the body urea nitrogen (BUN). The result showed that there was no statistical difference between each group. However, BUN level decreased after treated with curcumin and CEC. The previous study reported that an increased risk of incident diabetes is associated with high BUN and is associated with failure of kidney function35. In this study, we have demonstrated that curcumin and CEC showed ameliorative effect on heart and kidney in STZ-induced diabetic mice. Whereas, CEC possessed more effectively ameliorative effects on myocardial area of left ventricle and fibrosis area of cortex and medulla in kidney.
Type-1 diabetes is characterized by failure of insulin secretion by pancreatic β-cell and resulting in insulin deficiency3. Type-1 diabetes in mice model can be induced by intraperitoneal injection of a high dose of streptozotocin36,37. Hyperglycemia condition can exert feedback on molecular regulation by the generation of advanced glycation end products (AGEs). Collagen molecules were cross-linked with glycosylated protein, so that collagen cannot be degraded and as resulting in increases fibrosis38. The increase of oxidative stress is also associated with cardiomyopathy and leading to DNA damage and increase in AGEs receptor39. Therefore, using an antioxidant drug have been shown the beneficial effects of diabetes patient with cardiovascular diseases40. Curcumin has been reported to possess strong antioxidant activity and anti-inflammation, including in the case of type-1 diabetes41,42. Additionally, curcumin also has been demonstrated its ameliorative effects on the renal injury and diabetes-associated liver disorders in rodent model43,44. In vitro study, curcumin have been reported for its ameliorative effects on leukocytes infiltration by inhibiting intracellular adhesion molecule-1 expression45. Curcumin also inhibited pancreatic leukocytes infiltration in diabetic mice study by reducing proinflammatory cytokines and nuclear factor (NF)-κB activation46.
However, the limited application of curcumin such as poor aqueous solubility and low bioavailability have been reported. The previous study reported that nanocurcumin has been showed promising therapeutic enhancement more than curcumin alone47. Encapsulation by natural polymers has been used to enhance the bioavailability of poor solubility compounds, such as using chitosan. Chitosan polymer has been reported as an encapsulation agent by its favorable properties, such as biocompatible, biodegradable, and high drugs loading efficiency19,21. We used ethyl-acetate and ethanol for the chitosan solvent during the encapsulation process. According to the previous study, ethyl acetate and ethanol showed less toxicity. Ethyl acetate showed low toxicity in mice model with the lethal dose 50 (LD50) of orally administrated was higher than 2.0 g/kg/day and ethanol was about 8.3 g/kg48,49. Additionally, we also used freeze-drying process to obtain the CEC powder and the previous study reported that very less ethanol residue after freeze-drying process50. According to these conditions, we hypothesized that chitosan solvents used in the encapsulation process are safe for oral administration, especially in this model.
Previous study reported that the curcumin ameliorated diabetes models by attenuates tumor necrosis factor (TNF)-α and enhanced enzymatic antioxidant activity. This study also reported that curcumin administration protected pancreatic islet damage in STZ-induced diabetic mice27. Curcumin also upregulated nuclear factor erythroid-2-related factor-2 (Nrf-2) and reduced oxidative stress levels in skeletal muscle of high-fat diet-induced oxidative stress and glucose intolerance51. Additionally, curcumin reduced segmental sclerosis of glomerular and tubular structure as well as macrophages infiltration in kidneys of diabetic rats by inhibiting nuclear factor-kappa B (NF-𝜅B) activation52. Curcumin also improved histological abnormalities and fibrosis of diabetic kidney by inhibiting JNK/NF-kB activation53 and also regulates lysosomal enzymes activities (i.e. N-acetyl-β-d-glucosaminidase, β-d-glucuronidase) in spleen, liver, and heart of diabetic rats54.
Our previous study demonstrated that curcumin was encapsulated by chitosan and silica (SCNP) showed the small particle size (61.7 nm) with an almost spherical shape. This study also showed the effectively anti-oxidative activity on SCNP when compared to curcumin alone55. Additionally, the previous studies also reported that curcumin encapsulated in chitosan shown the nano size and high encapsulation efficiency in their forms as well as successfully carried the curcumin in nanoparticle matrix21,22,23,56. And also, chitosan-encapsulated curcumin increased solubility and bioavailability when compared to curcumin alone24,57. Based on our results, we found that both curcumin and chitosan-encapsulated curcumin suppressed the heart and kidney damage in type-1 diabetes mice model. Whereas, the chitosan-encapsulated curcumin more effectively to ameliorate the damages.
Conclusion
Overall, type-1 diabetes successfully induced by intraperitoneal injection of a high dose of streptozotocin. Both curcumin and chitosan-encapsulated curcumin successfully reduced the blood glucose and total cholesterol level as well as ameliorated the insulin level. Whereas, chitosan-encapsulated curcumin possessed more effective effects when compared to the curcumin-treated group, especially in the case of suppressed blood glucose level and increased the insulin level. Chitosan-encapsulated curcumin also more effective ameliorated the myocardial area in the left ventricle and fibrosis area in the kidney. Chitosan successfully enhanced the clinical application of curcumin to suppress the heart and kidney damages based on their morphological evaluations when compared to treated by curcumin alone in type-1 diabetes mice was induced by streptozotocin.
Methods
Materials and reagents
Curcumin (NutriPhy) was purchased from Chr. Hansen (Hørsholm, Denmark). This extract contained more than 95% of high-purity curcumin. Chitosan (Chitoligo-C, molecular weight = 120 kDa) was purchased from Lytone Enterprise, Inc. (Taipei, Taiwan) and corn oil was purchased from Yuan Shun Food Co., Ltd. (Yunlin, Taiwan). Formaldehyde solution and streptozotocin were purchased from Sigma-Aldrich (Missouri, USA). The fluorescein wheat germ agglutinin (WGA) was purchased from Vector Laboratories Inc. (California, USA).
Chitosan-encapsulated curcumin (CEC) preparation
The curcumin (500 mg) was dissolved in 20 mL of ethyl acetate and 20 mL of ethanol. The curcumin solution was mixed with chitosan solution (100 mg of chitosan in 300 mL of distilled water) by a peristaltic pump (0.2 mL/min) and continued with sonication at 20 kHz for 10 min as described by the previous study58. The filtrate and residue were separated by using filter paper. The filtrate was evaporated by using vacuum evaporator at 45 °C and dried by a free-drying process to obtained chitosan-encapsulated curcumin (CEC) powder.
Animal experiment
Six-week-old male C57BL/6 mice were purchased from the Experimental Animal Center of the National Experimental Research Institute (Yilan, Taiwan). The Institutional Animal Care and Use Committee (IACUC Approval No. 101024) of the National Taiwan Ocean University reviewed and approved all protocols. All experiments were performed in accordance with relevant guidelines and regulations. Briefly, the mice were fed a standard chow-fed diet (Laboratory Animal Diets MF-18) and water ad libitum. Mice were housed in a room maintained at 25 ± 2 °C under a 12 h day/night cycle throughout the experiment. Mice were acclimatized for 1 week. After the acclimatization phase, the mice were randomly divided into 4 groups with six mice per group (n = 6). A group was no injected by streptozotocin (STZ) (Control group, Ctrl) and 3 groups were injected by STZ (Diabetes groups, DM) (Fig. 8). Type-1 diabetes was induced by intraperitoneal injection of STZ (150 mg/kg body weight)36. The fasting blood glucose of all groups was checked at week-7 (7W) before STZ injection to induce diabetes. The fasting blood glucose also was checked at week-17 (17W) and week-18 (18W) to confirm the diabetes condition. The two groups of diabetic mice were treated by daily oral gavage either with curcumin (DM + Cur, 150 mg/kg) or chitosan-encapsulated curcumin (DM + CEC, 150 mg/kg) and the control (Ctrl) and DM group were also daily orally by corn oil according to previous study59,60. The mice were sacrificed on the week-22 (22W) of mice’s age and fasted for 12 h prior to sacrifice. Mice were euthanized by CO2 exposure in an empty chamber. The whole blood and organs (heart and kidney) were collected for future analysis.
Blood sample collection
The blood serum collection method was adapted from previous methods61. Briefly, the whole blood of mice was collected using a sterile syringe to collection tubes and allow for clotted for 1 h in room temperature, then centrifuged at 1,000 × g and 4 °C for 15 min to collect the blood serum. The serum was directly used or stored at −80 °C for future analysis.
Blood pressure measurement
The blood pressure was measured by using the Blood Pressure Monitor (Muromachi MK-2000ST, Japan). This tool was used to measure the blood pressure of small animal such as mice. It possible to measure blood pressure without preheating animals provided that room temperature is at least 23 °C. The measurement was conducted according to protocol manufacture protocol.
Fasting serum blood glucose, insulin, and lipid properties analysis
The blood glucose, total cholesterol (TC), triglycerides (TG), very-low density lipoprotein-cholesterol (VLDL-C), and body urea nitrogen (BUN) were measured by using blood biochemical analyzer (Biochemistry Analyzer, Kodak Ektachem DT60II, USA). The insulin level was measured by a commercial enzyme-linked immunosorbent assay (ELISA) kit which purchased from Mercodia AB (Cat. No. 10-1247-01, Uppsala, Sweden). All the analyses were performed according to the manufacturer’s protocols.
Histopathology analysis
The organs were collected at the day of sacrifice. The heart and kidney were fixed in 10% formalin solution. The cortex and medulla in the kidney were using to evaluation kidney histopathology. Then, the organs were embedded with paraffin and cut to slices (5 µm), then stained by hematoxylin-eosin (H&E) and Masson’s trichrome staining62. The stained-organs lesion was observed by using upright bright-field system microscopes (Optical Microscope, Nikon Eclipse 80i, USA and TissueFAXS system, TissueGnostics, Austria). The quantification of kidney fibrosis was done by using image analysis software (Image-Pro Plus 6.0, USA). In the case of heart staining, the organ was arrested by using St. Thomas’ Hospital cardioplegic solution number 2 (NaCl 110.0 mM, NaHCO3 10.0 mM, KCl 16.0 mM, MgCl2 16.0 mM, CaCl2 1.2 mM) and delivered to 4 °C prior to fixation to analyze myocardial area using wheat germ agglutinin (WGA) as described by the previous method63. The myocardial area of the left ventricle was also evaluated by using FITC-labeled WGA and observed by using a fluorescence microscope (Confocal Spectral Microscope, Leica TCS SP5, Germany).
Statistical analysis
All data were expressed as mean ± standard deviation (S.D.). Multiple comparisons of different groups were analyzed by Duncan’s test the value of P < 0.05 significant level using SPSS 22.0 program.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Aynalem, S. B. & Zeleke, A. J. Prevalence of Diabetes Mellitus and Its Risk Factors among Individuals Aged 15 Years and Above in Mizan-Aman Town, Southwest Ethiopia, 2016: A Cross Sectional Study. International Journal of Endocrinology 2018, 1–7, https://doi.org/10.1155/2018/9317987 (2018).
NCD-RisC. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. The Lancet 387, 1513–1530, https://doi.org/10.1016/s0140-6736(16)00618-8 (2016).
Wang, P. et al. Diabetes mellitus—advances and challenges in human β-cell proliferation. Nature Reviews Endocrinology 11, 201, https://doi.org/10.1038/nrendo.2015.9 (2015).
Rosano, G. M. C., Seferovic, P. & Vitale, C. Heart Failure in Patients with Diabetes Mellitus. Cardiac Failure Review 03, https://doi.org/10.15420/cfr.2016:20:2 (2017).
McFarlane, P., Gilbert, R. E., MacCallum, L. & Senior, P. Chronic Kidney Disease in Diabetes. Canadian Journal of Diabetes 37, S129–S136, https://doi.org/10.1016/j.jcjd.2013.01.037 (2013).
Shah, A. D. et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. The Lancet Diabetes & Endocrinology 3, 105–113, https://doi.org/10.1016/s2213-8587(14)70219-0 (2015).
Mameli, C. et al. Explaining the increased mortality in type 1 diabetes. World Journal of Diabetes 6, https://doi.org/10.4239/wjd.v6.i7.889 (2015).
Piscitelli, P. et al. Predictors of chronic kidney disease in type 1 diabetes: a longitudinal study from the AMD Annals initiative. Scientific Reports 7, https://doi.org/10.1038/s41598-017-03551-w (2017).
Woodrow, G., Brownjohn, A. M. & Turney, J. H. Acute renal failure in patients with type 1 diabetes mellitus. Postgraduate Medical Journal 70, 192–194, https://doi.org/10.1136/pgmj.70.821.192 (1994).
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. The Lancet 383, 69–82, https://doi.org/10.1016/s0140-6736(13)60591-7 (2014).
Hirsch, I. B. Realistic Expectations and Practical Use of Continuous Glucose Monitoring for the Endocrinologist. The. Journal of Clinical Endocrinology & Metabolism 94, 2232–2238, https://doi.org/10.1210/jc.2008-2625 (2009).
Jankun, J., Al-Senaidy, A. & Skrzypczak-Jankun, E. Can drinking black tea fight diabetes: literature review and theoretical indication. Central European Journal of Immunology 37, 167–172 (2012).
Huang, E. S., Strate, L. L., Ho, W. W., Lee, S. S. & Chan, A. T. Long-Term Use of Aspirin and the Risk of Gastrointestinal Bleeding. The American Journal of Medicine 124, 426–433, https://doi.org/10.1016/j.amjmed.2010.12.022 (2011).
Alkhatib, A. et al. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 9, https://doi.org/10.3390/nu9121310 (2017).
Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). The Journal of Alternative and Complementary Medicine 9, 161–168, https://doi.org/10.1089/107555303321223035 (2003).
Kao, N.-J., Hu, J.-Y., Wu, C.-S. & Kong, Z.-L. Curcumin represses the activity of inhibitor-κB kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. International Immunopharmacology 38, 1–7, https://doi.org/10.1016/j.intimp.2016.05.015 (2016).
Hatcher, H., Planalp, R., Cho, J., Torti, F. M. & Torti, S. V. Curcumin: From ancient medicine to current clinical trials. Cellular and Molecular Life Sciences 65, 1631–1652, https://doi.org/10.1007/s00018-008-7452-4 (2008).
Pan, M.-H. et al. Attenuation by Tetrahydrocurcumin of Adiposity and Hepatic Steatosis in Mice with High-Fat-Diet-Induced Obesity. Journal of Agricultural and Food Chemistry 66, 12685–12695, https://doi.org/10.1021/acs.jafc.8b04624 (2018).
Prasad, S., Tyagi, A. K. & Aggarwal, B. B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: the Golden Pigment from Golden Spice. Cancer Research and Treatment 46, 2–18, https://doi.org/10.4143/crt.2014.46.1.2 (2014).
Kumar, R. & Siril, P. F. Enhancing the Solubility of Fenofibrate by Nanocrystal Formation and Encapsulation. AAPS PharmSciTech 19, 284–292, https://doi.org/10.1208/s12249-017-0840-z (2017).
Shanmugam, R. et al. Formulation and characterization of chitosan encapsulated phytoconstituents of curcumin and rutin nanoparticles. International Journal of Biological Macromolecules 104, 1807–1812, https://doi.org/10.1016/j.ijbiomac.2017.06.112 (2017).
Yadav, A., Lomash, V., Samim, M. & Flora, S. J. S. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chemico-Biological Interactions 199, 49–61, https://doi.org/10.1016/j.cbi.2012.05.011 (2012).
Chuah, L. H., Billa, N., Roberts, C. J., Burley, J. C. & Manickam, S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharmaceutical Development and Technology 18, 591–599, https://doi.org/10.3109/10837450.2011.640688 (2011).
Peng, S. et al. Improving curcumin solubility and bioavailability by encapsulation in saponin-coated curcumin nanoparticles prepared using a simple pH-driven loading method. Food & Function 9, 1829–1839, https://doi.org/10.1039/c7fo01814b (2018).
Chiang, J. L., Kirkman, M. S., Laffel, L. M. B. & Peters, A. L. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care 37, 2034–2054, https://doi.org/10.2337/dc14-1140 (2014).
American Diabetes Association, A. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 32, S62–S67, https://doi.org/10.2337/dc09-S062 (2009).
El-Azab, M. F., Attia, F. M. & El-Mowafy, A. M. Novel role of curcumin combined with bone marrow transplantation in reversing experimental diabetes: Effects on pancreatic islet regeneration, oxidative stress, and inflammatory cytokines. European Journal of Pharmacology 658, 41–48, https://doi.org/10.1016/j.ejphar.2011.02.010 (2011).
Pihlajamäki, J., Gylling, H., Miettinen, T. A. & Laakso, M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. Journal of Lipid Research 45, 507–512, https://doi.org/10.1194/jlr.M300368-JLR200 (2004).
Rahimi, H. R. et al. The effect of nano-curcumin on HbA1c, fasting blood glucose, and lipid profile in diabetic subjects: a randomized clinical trial. Avicenna J Phytomed 6, 567–577 (2016).
Wang, C. C. L., Hess, C. N., Hiatt, W. R. & Goldfine, A. B. Clinical Update: Cardiovascular Disease in Diabetes Mellitus. Circulation 133, 2459–2502, https://doi.org/10.1161/circulationaha.116.022194 (2016).
Milani-Nejad, N. & Janssen, P. M. L. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacology & Therapeutics 141, 235–249, https://doi.org/10.1016/j.pharmthera.2013.10.007 (2014).
Wang, J., Song, Y., Wang, Q., Kralik, P. M. & Epstein, P. N. Causes and Characteristics of Diabetic Cardiomyopathy. The Review of Diabetic. Studies 3, 108–108, https://doi.org/10.1900/rds.2006.3.108 (2006).
Duffield, J. S. Cellular and molecular mechanisms in kidney fibrosis. Journal of Clinical Investigation 124, 2299–2306, https://doi.org/10.1172/jci72267 (2014).
Kanasaki, K., Taduri, G. & Koya, D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Frontiers in Endocrinology 4, https://doi.org/10.3389/fendo.2013.00007 (2013).
Xie, Y. et al. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney International 93, 741–752, https://doi.org/10.1016/j.kint.2017.08.033 (2018).
Tsai, P.-H., Liu, J.-J., Chiu, W.-C., Pai, M.-H. & Yeh, S.-L. Effects of dietary glutamine on adhesion molecule expression and oxidative stress in mice with streptozotocin-induced type 1 diabetes. Clinical Nutrition 30, 124–129, https://doi.org/10.1016/j.clnu.2010.07.005 (2011).
Deeds, M. C. et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Laboratory Animals 45, 131–140, https://doi.org/10.1258/la.2010.010090 (2011).
Williams, L. J., Nye, B. G. & Wende, A. R. Diabetes-Related Cardiac Dysfunction. Endocrinology and Metabolism 32, https://doi.org/10.3803/EnM.2017.32.2.171 (2017).
Wellen, K. E. & Hotamisligil, G. S. Inflammation, stress, and diabetes. Journal of Clinical Investigation 115, 1111–1119, https://doi.org/10.1172/jci25102 (2005).
Xu, Z. & Kong, X.-Q. Bixin ameliorates high fat diet-induced cardiac injury in mice through inflammation and oxidative stress suppression. Biomedicine & Pharmacotherapy 89, 991–1004, https://doi.org/10.1016/j.biopha.2017.02.052 (2017).
Menon, V. P. & Sudheer, A. R. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease Advances in Experimental Medicine and Biology Ch. Chapter 3, 105–125 (2007).
Meng, B., Li, J. & Cao, H. Antioxidant and Antiinflammatory Activities of Curcumin on Diabetes Mellitus and its Complications. Current Pharmaceutical Design 19, 2101–2113, https://doi.org/10.2174/1381612811319110011 (2013).
Okada, K. et al. Curcumin and Especially Tetrahydrocurcumin Ameliorate Oxidative Stress-Induced Renal Injury in Mice. The Journal of Nutrition 131, 2090–2095, https://doi.org/10.1093/jn/131.8.2090 (2001).
Zhang, D.-W., Fu, M., Gao, S.-H. & Liu, J.-L. Curcumin and Diabetes: A Systematic Review. Evidence-Based Complementary and Alternative Medicine 2013, 1–16, https://doi.org/10.1155/2013/636053 (2013).
Youn, G. S., Kwon, D.-J., Ju, S. M., Choi, S. Y. & Park, J. Curcumin ameliorates TNF-α-induced ICAM-1 expression and subsequent THP-1 adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. BMB Reports 46, 410–415, https://doi.org/10.5483/BMBRep.2013.46.8.014 (2013).
Castro, C. N. et al. Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes. Clinical & Experimental Immunology 177, 149–160, https://doi.org/10.1111/cei.12322 (2014).
Flora, G., Gupta, D. & Tiwari, A. Nanocurcumin: A Promising Therapeutic Advancement over Native Curcumin. Critical Reviews in Therapeutic Drug Carrier Systems 30, 331–368, https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2013007236 (2013).
Kim, S.-H. et al. Acute oral toxicity of the ethyl acetate fraction ofOrostachys japonicusin mice. Pharmaceutical Biology 52, 1345–1350, https://doi.org/10.3109/13880209.2014.892142 (2014).
Bartsch, W., Sponer, G., Dietmann, K. & Fuchs, G. Acute toxicity of various solvents in the mouse and rat. LD50 of ethanol, diethylacetamide, dimethylformamide, dimethylsulfoxide, glycerine, N-methylpyrrolidone, polyethylene glycol 400, 1,2-propanediol and Tween 20. Arzneimittelforschung 26, 1581–1583 (1976).
Takada, A., Nail, S. L. & Yonese, M. Influence of Ethanol on Physical State of Freeze-Dried Mannitol. Pharmaceutical Research 26, 1112–1120, https://doi.org/10.1007/s11095-009-9829-y (2009).
He, H.-J. et al. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World Journa l of Diabetes 3, https://doi.org/10.4239/wjd.v3.i5.94 (2012).
Soetikno, V. et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutrition & Metabolism 8, https://doi.org/10.1186/1743-7075-8-35 (2011).
Pan, Y. et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. British Journal of Pharmacology 166, 1169–1182, https://doi.org/10.1111/j.1476-5381.2012.01854.x (2012).
Chougala, M. B., Bhaskar, J. J., Rajan, M. G. R. & Salimath, P. V. Effect of curcumin and quercetin on lysosomal enzyme activities in streptozotocin-induced diabetic rats. Clinical Nutrition 31, 749–755, https://doi.org/10.1016/j.clnu.2012.02.003 (2012).
Kong, Z.-L., Kuo, H.-P., Johnson, A., Wu, L.-C. & Chang, K. L. B. Curcumin-Loaded Mesoporous Silica Nanoparticles Markedly Enhanced Cytotoxicity in Hepatocellular Carcinoma Cells. International Journal of Molecular Sciences 20, https://doi.org/10.3390/ijms20122918 (2019).
Anitha, A. et al. Curcumin-Loaded N,O-Carboxymethyl Chitosan Nanoparticles for Cancer Drug Delivery. Journal of Biomaterials Science, Polymer Edition ahead-of-print, 1–20, https://doi.org/10.1163/092050611x581534 (2012).
Peng, S. et al. Enhancement of Curcumin Bioavailability by Encapsulation in Sophorolipid-Coated Nanoparticles: An in Vitro and in Vivo Study. Journal of Agricultural and Food Chemistry 66, 1488–1497, https://doi.org/10.1021/acs.jafc.7b05478 (2018).
Esmaeilzadeh-Gharedaghi, E. et al. Effects of processing parameters on particle size of ultrasound prepared chitosan nanoparticles: An Artificial Neural Networks Study. Pharmaceutical Development and Technology 17, 638–647, https://doi.org/10.3109/10837450.2012.696269 (2012).
Farhangkhoee, H., Khan, Z. A., Chen, S. & Chakrabarti, S. Differential effects of curcumin on vasoactive factors in the diabetic rat heart. Nutrition & Metabolism 3, https://doi.org/10.1186/1743-7075-3-27 (2006).
Aggarwal, M. L., Chacko, K. M. & Kuruvilla, B. T. Systematic and comprehensive investigation of the toxicity of curcuminoid-essential oil complex: A bioavailable turmeric formulation. Molecular Medicine Reports 13, 592–604, https://doi.org/10.3892/mmr.2015.4579 (2016).
Yu, Z. et al. Differences between Human Plasma and Serum Metabolite Profiles. PLoS ONE 6, https://doi.org/10.1371/journal.pone.0021230 (2011).
Goldner, J. A modification of the masson trichrome technique for routine laboratory purposes. Am J Pathol 14, 237–243 (1938).
Jynge, P. et al. The St. Thomas’ Hospital cardioplegic solution: a characterization in two species. (Distributed by the Almqvist & Wiksell Periodical Co., 1981).
Acknowledgements
The sponsor had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review or approval of the manuscript and the decision to submit the manuscript for publication. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Z.-L.K., conception and approved the submitted version; H.-I.Y., conception; Y.-L.Y., analysis and interpretation of data; S.S., wrote the main manuscript text and approved the submitted version; C.-S.L., approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sudirman, S., Lai, CS., Yan, YL. et al. Histological evidence of chitosan-encapsulated curcumin suppresses heart and kidney damages on streptozotocin-induced type-1 diabetes in mice model. Sci Rep 9, 15233 (2019). https://doi.org/10.1038/s41598-019-51821-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51821-6
This article is cited by
-
Isofraxidin exerts anti-diabetic, antilipidemic, and antioxidant effects and protects renal tissues via inhibition of NF-ĸB in Streptozotocin-induced diabetic rats
Molecular & Cellular Toxicology (2022)
-
Effect of curcumin nanoparticles on streptozotocin-induced male Wistar rat model of Alzheimer’s disease
Metabolic Brain Disease (2022)
-
Food as medicine: targeting the uraemic phenotype in chronic kidney disease
Nature Reviews Nephrology (2021)
-
Combating COVID-19 with tissue engineering: a review
Emergent Materials (2021)
-
Nanocurcumin improved glucose metabolism in streptozotocin-induced diabetic rats: a comparison study with Gliclazide
Environmental Science and Pollution Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.