Abstract
A supercritical fluid, such as supercritical carbon dioxide (scCO2) is increasingly used for the micronization of pharmaceuticals in the recent past. The role of scCO2 as a green solvent in supercritical fluid (SCF) process is decided by the solubility information of the pharmaceutical compound in scCO2. The commonly used SCF processes are the rapid expansion of supercritical solution (RESS) and supercritical antisolvent precipitation (SAS). To implement micronization process, solubility of pharmaceuticals in scCO2 is required. Present study is aimed at both measuring and modeling of solubilities of hydroxychloroquine sulfate (HCQS) in scCO2. Experiments were conducted at various conditions (P = 12 to 27 MPa and T = 308 to 338 K), for the first time. The measured solubilities were found to be ranging between (0.0304 × 10–4 and 0.1459 × 10–4) at 308 K, (0.0627 × 10–4 and 0.3158 × 10–4) at 318 K, (0.0982 × 10–4 and 0.4351 × 10–4) at 328 K, (0.1398 × 10–4 and 0.5515 × 10–4) at 338 K. To expand the usage of the data, various models were tested. For the modelling task existing models (Chrastil, reformulated Chrastil, Méndez-Santiago and Teja (MST), Bartle et al., Reddy-Garlapati, Sodeifian et al., models) and new set of solvate complex models were considered. Among the all models investigated Reddy-Garlapati and new solvate complex models are able to fit the data with the least error. Finally, the total and solvation enthalpies of HCQS in scCO2 were calculated with the help of model constants obtained from Chrastil, reformulated Chrastil and Bartle et al., models.
Similar content being viewed by others
Introduction
There has been greater attention in the recent past about the application of supercritical carbon dioxide in micronization of pharmaceuticals1,2,3,4,5. The drug administration is decided by the size of the particle. As we know, intravenous drug delivery requires particles size ranging from 0.1 to 0.3 μm, inhalation delivery requires 1–5 μm and oral delivery requires 0.1–100 μm and the smaller the size of the particles, greater chance of a drug being absorbed by the human body, which helps in reducing the drug dosage1. Conventional particle reduction techniques result in products that are in the particulate range, for example jet mills provides product particles in the range 5–45 μm, hammer mill provides product particles in the range 25–600 μm, on the other hand supercritical fluids (SCFs) technology provides product particles in the range 0.1–600 μm1. But, to apply SCFs technology, solubility data of a specific drug in the desired SCF is required for the selection and design of suitable SCF process that reduces the particle size, thus the solubility will determine the operating condition of the process6,7,8. Present work is focused on the both solubility measurement and modelling of hydroxychloroquine sulfate (HCQS) in scCO2. This drug was originally developed in United States (US) to counter malaria in the year 19499,10. HCQS is considered as better alternate to chloroquine, due to less toxicity. It is also used for the treatment of Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE)9,11 disease. A recent study conducted by Pishnamazi et al., on the solubility of chloroquinein scCO2 has inspired us to take up this task12. Since 1950, chloroquine has been in use to treat malaria, however, hydroxychloroquine, a chloroquine analogue has a better safety profile due to a hydroxyl group on the side chain and is used in the treatment of connective tissue disorders13. We believe this study helps to implement SCF technology to get the desired drug size particles of HCQS and which may help to reduce the drug dosage in treatment. To expand the use of solubility data, modelling is performed with literature models and new solvate complex models.
This work is carried out in two steps. In the first, HCQS solubility in scCO2 solvent is determined and in the second, data measured in the first stage are correlated with literature models. The models employed are solid–liquid equilibrium, Chrastil, reformulated Chrastil, Méndez-Santiago and Teja (MST), Bartle et al., Reddy-Garlapati, Sodeifian et al., models and three forms of new solvate complex models.
Experimental
Materials
HCQS was supplied by TEMAD Co., Active Pharmaceuticals in gradients, Mashhad, Iran (CAS Number: 747-36-4, mass purity > 99%). CO2 (CAS Number: 124-38-9, mass purity > 99.9%) was purchased from Fadak company, Kashan, Iran. All the relevant details are tabulated in Table 1.
Solubility measurement details
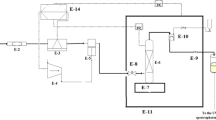
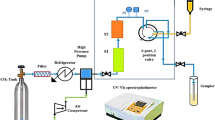
SCF-solubility of the drugs was experimentally measured via two broad classes of saturated solution-based methods, where solubility measurement can be done either (1) statically or (2) dynamically14. In the present work, a UV–vis spectrophotometer was utilized to statically examine the equilibrium solubility data of HCQS in a setup presented in Fig. 1. This experimental setup has already been validated in our previous work with alpha-tocopherol and naphthalene15. The solubilities were measured with the help of an equilibrium cell. Thermodynamically, the method employed may be regarded as an isobaric-isothermal method14. All the measurements are taken by keeping system temperatures and pressures at the desired value with high precision (i.e., ± 0.1 K and ± 0.1 MPa). Complete details about the equipment and measurement procedures are presented elsewhere15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35. However, the description of the equipment and the methodology employed in establishing solubility data are briefly presented in this section. The scCO2 is pumped to the equilibrium cell and drug sample and scCO2 are thoroughly mixed and allowed to attain equilibrium. It is observed that the equilibrium is attained in 60 min. To ensure equilibrium solubility, the experiments are performed with fresh samples at various time intervals. For a specified temperature and pressure in each experiment, the drug sample is contacted with scCO2 and stirred thoroughly in an equilibrium cell until a specific time (5 min, 10 min, 20 min, 30 min, 40 min, 50 min and 60 min) and the solubility readings are recorded. It is observed that the solubility is independent of time after 30 min. However, for solubility measurement, the samples are collected after 1 h. For each sampling a 600 µL volume saturated sample is collected via a collection valve in a deionized water preloaded sample vial. After discharging of each sample, the sampling valve was cleaned with 1 ml of deionized water. Drug sample solubility is estimated with the following formula:
where \(y_{\scriptsize 2}\) is solubility of the drug in scCO2, \({n}_{\text{drug}}\) and \({n}_{{\text{CO}}_{2}}\) are number of moles of the drug and CO2 in the sampling loop, respectively.
Experimental setup for solubility measurement, E1- CO2 cylinder; E2- Filter; E3- Refrigerator unit; E4- Air compressor; E5- High pressure pump; E6- Equilibrium cell; E7- Magnetic stirrer; E8- Needle valve; E9- Back-pressure valve; E10- Six-port, two position valve; E11- Oven; E12- Syringe; E13- Collection vial; and E14- Control panel.
The following formulas are used in data conversion
where \(C_{s}\) is concentration of the drug in g/L. V1 = 600 × 10–6 L and Vs = 5 × 10–3 L are sampling loop and vial volumes, respectively. \(M_{s}\) and \(M_{{CO_{2} }}\) are drug and CO2 molecular weights, respectively. ρ1 is density of scCO2 at each experimental condition, as presented in Table 2.
Solubility is also described as
The solubility and mole fraction relation is described as
The HCQS’s solubility is quantified in the UV–Visible spectrophotometer (Model UNICO-4802) with the help of deionized water \((conductivity \le 5\;\upmu {\text{S}}\;{\text{m}}^{ - 1} )\) as collection solvent with the wave length of 220 nm at UV spectrum.
Modelling
Although there are several approaches in modelling solubility data, solid–gas equilibrium (known as equation of state (EoS) approach), solid–liquid equilibrium (SLE, also known as expanded liquid equilibrium approach) and empirical modelling are commonly used in literature for the data correlation36,37,38,39,40,41,42,43,44,45,46,47,48,49,50. For EoS approach critical properties of both solvent (scCO2) and solute (HCQS) are required, whereas the SLE approach requires only melting temperature and melting enthalpy of the solute, but empirical modelling doesn’t need any such information. HCQS is a typical compound and it is not possible to estimate its critical properties due to the presence of H2SO4 in its structure. Due to this fact EoS modelling is not persuaded. On the other hand, experimental melting temperature and melting enthalpies of HCQS are available; due to this reason expanded liquid modelling is explored. For empirical modelling the density of scCO2, and system temperature and pressure are required; since they are readily available, it is also persuaded here. For empirical modelling, six commonly used solubility models are considered and those models have a varying number of parameters in their equations ranging from three to seven. The modelling purpose of some empirical models is to check the self-consistency of the measured data and to estimation some of the thermodynamic information of the dissolution process. In general, solubility of solids in SCFs is visualized in terms of solvate complex formation; therefore, a new set of solvate complex models is proposed for the better data fitting/correlation. More details about all the models considered in this work are presented in the following subsections.
Solid–liquid equilibrium (SLE) models
In this approach the HCQS solute is assumed to be infinitesimally dissolved in the scCO2 solvent. At equilibrium, the drug fugacity in solid phase is equal to that of fugacity of drug in scCO2 phase. From this criterion, the following solubility expression is proposed51,52,53,54,55,56,57.
From the literature, there are several models for this approach. However, Wilson activity coefficient model is considered for the data regression58.
We know from thermodynamics, \({\raise0.7ex\hbox{${f_{2}^{S} }$} \!\mathord{\left/ {\vphantom {{f_{2}^{S} } {f_{2}^{L} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${f_{2}^{L} }$}}\) ratio is
Simplified expression for \({\raise0.7ex\hbox{${f_{2}^{S} }$} \!\mathord{\left/ {\vphantom {{f_{2}^{S} } {f_{2}^{L} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${f_{2}^{L} }$}}\) is27
The required \(\gamma_{2}^{\infty }\) is obtained from Wilson model and the relevant expressions are as follows:
where
On combining Eqs. (10)–(15), we get
\(\lambda_{21}^{^{\prime}}\) and \(\lambda_{12}^{^{\prime}}\) are the energies of interactions, where subscripts 1 and 2 denote solvent and solute, respectively.
Combining all the equations, finally, resulted in SLE model, i.e., Eq. (18)
Commonly used empirical models
Chrastil's model59
In the year 1981, Josef Chrastil presented a model for the solubility of solids/liquids in SCF. The main basis of the model is a solvate complex formulation based on a simple reaction. According to Chrastil, solubility is explained by \(c_{2} = c_{2} (\kappa ,\rho_{1} ,T)\), where \(\kappa\) is association number, \(\rho_{1}\) is solvent density (scCO2) and T is temperature. Chrastil proposed Eq. (19)
An alternative from of Eq. (19) is Eq. (20)60
where y2 is solute solubility in mole fraction.
Reformulated Chrastil's model61
In the year 2009, Garlpati and Madras has reformulated Chrastil's model and it is expressed by Eq. (21), where solubility is explained by \(y_{2} = y_{2} \left( {\kappa^{\prime},\rho_{1} ,T} \right)\), in which \(\kappa^{\prime}\) is association number, \(\rho_{1}\) is solvent density (scCO2) and T is temperature.
in Eq. (21), R denotes universal constant of ideal gas, \(M_{ScF}\) is molecular weight of solvent, \(f^{ * }\) is reference pressure and \(A_{2} \;\) and \(\;B_{2}\) are the Reformulated model constants.
Méndez-Santiago and Teja (MST) model62
The MST model is used to check the consistency of the data. All the data falls around a single straight line when \(T\ln \left( {y_{2} P} \right) - C_{3} T\) versus \(\rho_{1}\) is established. MST model is expressed as Eq. (22)
where A3 to C3 are the model constants.
Bartle et al., model63
The solute sublimation enthalpy is calculated with Bartle et al., model and it is stated as
where A4 to C4 are the model constants. Using the constant \(B_{4}\), sublimation enthalpy is calculated (i.e., \(\Delta_{sub} H = - B_{4} R\)).
Reddy-Garlapati model64
It is the model developed based on the degree of freedom analysis based on drug compounds. According to this model, solubility, \(y_{2} = y_{2} \left( {T_{r} ,P_{r} } \right)\), is expressed as Eq. (24), where \(T_{r}\) and \(P_{r}\) are reduced temperature and pressures, respectively.
where \(A_{5}\) to \(F_{5}\) are the model constants.
Sodeifian et al., model65
It is another recently proposed empirical model. According to this model, solubility,\(y_{2} = y_{2} \left( {T,P,\rho_{1} } \right)\), can be calculated by
where \(A_{6}\) to \(F_{6}\) are the model constants.
New solvate complex models66
The solubility is visualized via solvate complex formation. The following simple reversible reaction is considered for the formation of solvate complex \(AB_{\kappa }\), in which ‘A’ is designated as solute and the letter ‘B’ is designated as solvent in SCF mixture.
At equilibrium
where the subscript ‘Solid’ is designated as solid phase and subscript ‘SP’ is designated as solvent phase and superscript ‘\(*\)’ is reference state.
For each species the fugacity’s expressions are
At equilibrium in vapour phase only two things exit: one is solvent and the other is solvate complex thus
where \(y_{B} , y_{{AB_{\kappa } }}\) are respective mole fractions.
If we assume the standard state of species ‘A’ is pure under same system conditions. Then
Fugacity of species ‘A’ can be written as
From thermodynamics, equilibrium constant is function of salvation enthalpy as Eq. (37)
Sublimation pressure can be expressed as Eq. (38)
Substituting Eqs. (35)–(38) into Eq. (27) along with two simplifications gives Eq. (39)
One assumption that \({{v_{A} P} \mathord{\left/ {\vphantom {{v_{A} P} {RT}}} \right. \kern-0pt} {RT}}\) is expressed as \({{Zv_{A} \rho_{1} } \mathord{\left/ {\vphantom {{Zv_{A} \rho_{1} } M}} \right. \kern-0pt} M}\) where \(\rho_{1}\) is the density of the supercritical phase and the second assumption is refereed to negligible term \({{v_{A} P_{A}^{Sub} } \mathord{\left/ {\vphantom {{v_{A} P_{A}^{Sub} } {RT}}} \right. \kern-0pt} {RT}}\)(~ 10–8–10–9) [since the sublimation pressures are very low and their order is about (~ 10–3–10–4) and the drug molar volume is also about the order (~ 10–4)]67,68,69,70.
where \(L = \left( {{{\Delta H_{s} } \mathord{\left/ {\vphantom {{\Delta H_{s} } R}} \right. \kern-0pt} R} - \beta^{\prime}} \right)\), \(M = \left( { - {{ZV_{A} } \mathord{\left/ {\vphantom {{ZV_{A} } M}} \right. \kern-0pt} M}} \right)\) and \(N = \ln \left( {\frac{{\left( {\varphi_{A}^{ * } } \right)\left( {{{\hat{\varphi }_{{AB_{\kappa } }} } \mathord{\left/ {\vphantom {{\hat{\varphi }_{{AB_{\kappa } }} } {\varphi_{{AB_{\kappa } }}^{ * } }}} \right. \kern-0pt} {\varphi_{{AB_{\kappa } }}^{ * } }}} \right)}}{{\left( {{{\hat{\varphi }_{B} } \mathord{\left/ {\vphantom {{\hat{\varphi }_{B} } {\varphi_{B}^{ * } }}} \right. \kern-0pt} {\varphi_{B}^{ * } }}} \right)^{\kappa } }}} \right) + \ln P^{ * } + q_{s} - \alpha^{\prime}\)
Equation (39) is rearranged as Eq. (40)
Further, on rearranging Eq. (40), we get Eq. (41)
Applying binomial expansion to the left side term gives Eq. (41)
We know solubility, \(y_{2}\), is related to cluster mole fraction \(y_{{AB_{\kappa } }}\) as Eq. (43)68,69
Thus the solubility is expressed as Eq. (44)
\(\kappa^{\prime\prime}\) is a function of reduced density, given by Eq. (45)71
Combining Eqs. (44) and (45) results in Eq. (46)
To reduce the number constants in Eq. (46), we have a choice to choose \(\kappa\) as a linear function of reduced density or as constant. Thus, we get the following two reduced solubility expressions.
and
In literature, related solubility expression is proposed by Rajasekhar and Madras67 as
The correlating abilities and evaluation of the new models (Eqs. 46–48) and existing model (Eq. 49) are also carried out in this work.
The data fitting to the models are performed by an objective function Eq. (50)72
The obtained deviations are indicated in terms of an average absolute relative deviation percentage (AARD%).
The entire regression task was done using (MATLAB 2019a®) version software, also this can be performed by nonlinear regression methods with the same results84,85.
Results and discussion
The experimental device used for the measurement of HCQS solubility in scCO2 is accurate and reliable. It was successfully tested to reproduce the solubilities of naphthalene in scCO2 and alpha-tocopherol in scCO2 systems and the same was reported in our earlier work15. The solubility values reported for alpha-tocopherol in our previous work, are the average of three replicate measurements with relative standard deviations lower than 5.7%15. The solubility values of naphthalene reported in our previous work are also the average of three replicate measurements with relative standard deviations lower than 6.5%15. Table 2 shows the measured solubilities of HCQS in scCO2 at various conditions and the density of the scCO2, obtained from the NIST data base73. From solubility data, it is clear that present system (HCQS+scCO2), does not exhibit the usual retrograde phenomenon. Affecting solvent density and solute power, the temperature was found to impose a dual impact on solubility in scCO2 depending on how the solute vapor pressure and solvent density are balanced. In this respect, increasing the solution temperature may enhance the solute vapor pressure, thereby contributing to stronger solvating power of SCF. At the same time, a rise of temperature may lower the scCO2 density which is known to depreciate the overall solvating power of the fluid. The mole fraction versus pressure isotherms on Fig. 2 suggest an enhancement in the solubility of drug upon elevating the temperature. This proves the dominant role of the solute vapor pressure in determining the solubility behavior irrespective of the pressure. Reports by other researchers confirm the results of the present work regarding the effect of temperature on the solubility in scCO274,75,76. From Table 2 and Fig. 2, it is clear that when temperature is raised from 308 to 338 K, there is a clear indication of a rise in solubility from 0.0304 × 10−4 to 0.1398 × 10−4 at 12 MPa (i.e., 4.6 folds’ increase) and at 27 MPa from 0.1459 × 10−4 to 0.5515 × 10−4 (i.e., 3.8 folds increase). At the same time, at 12 MPa, the density of scCO2 changes from 769 to 338 kg m−3 at 308 and 338 K, respectively. Similarly, the density of scCO2 at 27 MPa changes from 914 to 783 kg m−3 at 308 and 338 K, respectively; which means that there is decrease in density at 12 MPa (low pressure) (i.e., 338/769 = 0.4395) and there is increase in density to some extent at 27 MPa (higher pressure) (i.e., 783/914 = 0.8567). Thus, the solubility behavior of HCQS in scCO2 is highly nonlinear. This kind of high nonlinearity behavior has been observed with amlodipine besylate-scCO2 in the recent past28. This kind of high nonlinearity behavior can’t be captured with simple models. Thus models having more adjustable constants are required to fit the data and this would augment the justification for the need of development of new models.
All the regression results are summarized in Table 3 and presented in Figs. 3, 4, 5, 6, 7, 8 and 9. Solid–liquid model requires enthalpy of fusion, molar volume and melting point of the solute. Enthalpy of fusion is estimated with the help Jain et al.77, and molar volume of the solute is estimated with the help Immirzi and Perini method78. The calculated molar enthalpy and molar volumes are 65,208 J mol−1 and 3.306 × 10–4 m3 mol−1, respectively. The melting temperature is obtained from the material safety data sheet as 240 °C. From Chrastil's model constant (\(B_{1}\)), total enthalpy is calculated (\(B_{1}\) × R). From Bartle’s model constant (\(B_{4}\)), sublimation enthalpy is calculated (\(\Delta_{sub} H = - B_{4} R\)). From the magnitude difference between total and sublimation enthalpies, solvation enthalpy is calculated. Similarly, from reformulated Chrastil's model constant (\(B_{2}\)) and Bartle’s model constant (\(B_{4}\)), combination solvation enthalpy is calculated. All the calculated quantities are shown in Table 4. The regression ability of commonly used three parameter models are found to be inferior when compared to Sodeifian et al., and Reddy–Garlapati models. This may be due to a smaller number of parameters in the models; on the other hand, the correlating ability of the solvate complex models is quite good. However, among all empirical models, Reddy-Garlapati model is the best model. The newly proposed solvate complex models have more adjustable constants, and thus their predictions are also good. From the solvate complex model’s constants, it is interesting to note the behavior of association number. When association number is treated as a linear function of reduced density, the obtained average association number for the solubility data is \(\kappa^{\prime\prime}_{ave}\) = 2.21, which is lesser than that of the conventional Chrastil's model \(\kappa\) = 3.89. But, when association number is treated as quadratic function of reduced density, the obtained average association number for the solubility data is \(\kappa^{\prime\prime}_{ave}\) = 3.78, and it is matching well with association number of Chrastil’s model. From those results and from literature arguments, we can infer that association number is quadratic function of \(\rho_{r}\) so that is apparent than other forms and its correlations are reliable71,79. Thus, association number as quadratic function of \(\rho_{r}\) is recommended for the data interpolation.
HCQS solubility in scCO2 versus ρ1. Symbols are experimental data points. Solid lines are calculated from SLE model (Eq. 18).
HCQS solubility in scCO2 versus ρ1. Symbols are experimental data points. Solid lines are calculated from Chrastil's model (Eq. 20).
HCQS solubility in scCO2 versus ρ1. Symbols are experimental data points. Solid lines are calculated from SLE model (Eq. 21).
Self-consistency plot based on MST model (Eq. 22).
ln(y2 P/Pref) versus (ρ1 − ρref). Symbols are experimental data points. Solid lines are calculated from Bartle et al., model (Eq. 23).
Further, a comparative analysis is done to determine the best model for HCQS-scCO2 system. Since, a varying number of parameters are involved in the equations considered in the work, Akaike Information Criterion (AIC) and corrected AIC (AICc)80,81,82,83 are used to identify the best model. AIC alone is used when data points are more than forty; on the other hand, AICc is used when data points are less than forty. In the present there are only twenty-four solubility data points in our hands; hence, AICc is used for identifying the best model. AICc is defined as Eq. (52)
where N is solubility data points, Q is number of model constants, and SSE is error sum of squares. From the least AICc value, the best model is identified. The lower the AICc value the greater the accuracy of the model, and it is independent of the number of parameter. All the AICc values are reported in Table 5. From results, Reddy-Garlapati and the new solvate complex models are observed to be the better models.
Conclusion
Solubilities of solid HCQS in scCO2 solvent were measured at various conditions ranging from P = 12 to 27 MPa and T = 308 to 338 K. The measured data’s range is from 0.0304 × 10–4 to 0.5515 × 10−4 in terms of mole fraction. Three forms of solvate complex models explored in this study are reasonable in estimating solubility and among the three the best model is observed to have AICc and AARD values − 595.4 and 10.08% respectively. Among empirical models Reddy-Garlapati model is observed to fit the data quite well and with the corresponding AICc and AARD values − 604.1 and 10.04%, respectively.
Data availability
On request the data may be obtained from the corresponding author.
Abbreviations
- A 1, B 1 :
- A 2, B 2 :
-
Constants of Eq. (21)
- A 3, B 3 , C 3 :
-
Constants of Eq. (22)
- A 4, B 4 , C 4 :
-
Constants of Eq. (23)
- A 5, B 5 , C 5, D 5, E 5, F 5 :
-
Constants of Eq. (24)
- A 6, B 6 , C 6, D 6, E 6, F 6 :
-
Constants of Eq. (25)
- AARD%:
-
Average absolute relative deviation percentage
- AIC:
-
Akaike Information Criterion
- AICc :
-
Corrected AIC
- Cs :
-
Drug in sample in vial (g/L)
- E1 to E14:
-
Symbols used in experimental setup
- Hsol :
-
Solvation enthalpy (kJ/mol)
- Hsub :
-
Sublimation enthalpy (kJ/mol)
- Htotal :
-
Total enthalpy (kJ/mol)
- \({\text M}_{\text {CO}_2}\), MS :
-
Molar mass of CO2 and drug (g/mol)
- \({n}_{CO_2}\) :
-
Moles of carbon dioxide
- \({n}_{\mathrm{drug}}\) :
-
Moles of drug
- N :
-
Number of experimental data points
- NIST:
-
National Institute of Standards and Technology
- OF:
-
Objective function
- Q :
-
Number of parameters of a model
- P:
-
System pressure
- Pc, Pr :
-
Critical pressure, Reduced pressure
- Ps :
-
Sublimation pressure (Pa)
- R :
-
Universal constant of ideal gas, 8.314 J/mol K
- RMSD:
-
Root mean square deviation
- S:
-
Solubility (g/L)
- SSE:
-
Sum of squares error
- scCO2 :
-
Supercritical carbon dioxide
- T:
-
System temperature (K)
- Tc :
-
Critical temperature (K)
- \(v_{1} ,v_{2} ,v_{s}\) :
-
Molar volume of solvent, solute and solute (m3/mol)
- V 1 , Vs :
-
Sampling loop and collection vial volumes (μL)
- \(y_{2}\) :
-
Drug solute solubility in mole fraction
- \(\alpha ,\alpha^{\prime}\) :
- \(\beta ,\beta^{\prime}\) :
- \(\rho ,\rho_{r}\) :
-
Density, reduced density
- \(\kappa,\kappa^{\prime},\kappa^{\prime\prime},\kappa^{\prime\prime\prime}\) :
-
Association numbers
- \(\gamma_{2}^{\infty }\) :
-
Infinitesimally dilute state activity coefficient of solute
- \(\lambda^{\prime}_{12} ,\lambda^{\prime}_{21}\) :
-
Energies of interaction
References
Tabernero, A., del Valle, E. M. M. & Galán, M. A. Supercritical fluids for pharmaceutical particle engineering: Methods, basic fundamentals and modelling. Chem. Eng. Process 60, 9–25. https://doi.org/10.1016/j.cep.2012.06.004 (2012).
Subramaniam, B., Rajewski, R. A. & Snavely, K. Pharmaceutical processing with supercritical carbon dioxide. J. Pharm. Sci. 86(8), 885–890. https://doi.org/10.1021/js9700661 (1997).
Elvassore, N. K. I. Pharmaceutical processing with supercritical fluids. In High Pressure Process Technology: Fundamentals and Applications (ed. Bertucco, A. G. V. G.) 612–625 (Elsevier Science, 2001).
Gupta, R. B., Chattopadhyay, P. Method of forming nanoparticles and microparticles of controllable size using supercritical fluids and ultrasound. US patent No. 20020000681 (2002).
Reverchon, E., Adami, R., Caputo, G. & De Marco, I. Spherical microparticles production by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 47(1), 70–84. https://doi.org/10.1016/j.supflu.2008.06.002 (2008).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Experimental measurements and thermodynamic modeling of Coumarin-7 solid solubility in supercritical carbon dioxide: Production of nanoparticles via RESS method. Fluid Phase Equilib. 483, 122–143. https://doi.org/10.1016/j.fluid.2018.11.006 (2019).
Sodeifian, G., Sajadian, S. A., Ardestani, N. S. & Razmimanesh, F. Production of loratadine drug nanoparticles using ultrasonic-assisted rapid expansion of supercritical solution into aqueous solution (US-RESSAS). J. Supercrit. Fluids 147, 241–253. https://doi.org/10.1016/j.fluid.2018.05.018 (2019).
Mukhopadhyay, M. Partial molar volume reduction of solvent for solute crystallization using carbon dioxide as antisolvent. J. Supercrit. Fluids 25(3), 213–223. https://doi.org/10.1016/S0896-8446(02)00147-X (2003).
Dongala, T. et al. In vitro different biological pH conditions of hydroxychloroquine sulfate tablets is available for the treatment of COVID-19. Front. Mol. Biosci. 7, 613393. https://doi.org/10.3389/fmolb.2020.613393 (2021).
de Margerie, V. et al. Continuous manufacture of hydroxychloroquine sulfate drug products via hot melt extrusion technology to meet increased demand during a global pandemic: From bench to pilot scale. Int. J. Pharm. 605, 120818. https://doi.org/10.1016/j.ijpharm.2021.120818 (2021).
Bodur, S., Erarpat, S., Günkara, O. T. & Bakırdere, S. Accurate and sensitive determination of hydroxychloroquine sulfate used on COVID-19 patients in human urine, serum and saliva samples by GC-MS. J. Pharm. Anal. 11(3), 278–283. https://doi.org/10.1016/j.jpha.2021.01.006 (2021).
Pishnamazi, M. et al. Chloroquine (antimalaria medication with anti SARS-CoV activity) solubility in supercritical carbon dioxide. J. Mol. Liq. 322, 114539. https://doi.org/10.1016/j.molliq.2020.114539 (2021).
Doyno, C., Sobieraj, D. M. & Baker, W. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin. Toxicol. 59(1), 12–23. https://doi.org/10.1080/15563650.2020.1817479 (2020).
Peper, S., Fonseca, J. M. & Dohrn, R. High-pressure fluid-phase equilibria: Trends, recent developments, and systems investigated (2009–2012). Fluid Phase Equilib. 484, 126–224. https://doi.org/10.1016/J.FLUID.2018.10.007 (2019).
Sodeifian, G., Sajadian, S. A. & Ardestani, N. S. Determination of solubility of Aprepitant (an antiemetic drug for chemotherapy) in supercritical carbon dioxide: Empirical and thermodynamic models. J. Supercrit. Fluids 128, 102–111. https://doi.org/10.1016/j.supflu.2017.05.019 (2017).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Hazaveie, S. M. Experimental data and thermodynamic modeling of solubility of Sorafenib tosylate, as an anti-cancer drug, in supercritical carbon dioxide: Evaluation of Wong–Sandler mixing rule. J. Chem. Thermodyn. 142, 105998. https://doi.org/10.1016/j.jct.2019.105998 (2020).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Prediction of solubility of sunitinib malate (an anti-cancer drug) in supercritical carbon dioxide (SC–CO2): Experimental correlations and thermodynamic modeling. J. Mol. Liq. 297, 111740. https://doi.org/10.1016/j.molliq.2019.111740 (2020).
Sodeifian, G. & Sajadian, S. A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 133(1), 239–252. https://doi.org/10.1016/j.supflu.2017.10.015 (2018).
Sodeifian, G., Razmimanesh, F., Ardestani, N. S. & Sajadian, S. A. Experimental data and thermodynamic modeling of solubility of Azathioprine, as an immunosuppressive and anti-cancer drug, in supercritical carbon dioxide. J. Mol. Liq. 299, 112179. https://doi.org/10.1016/j.molliq.2019.112179 (2019).
Hazaveie, S. M., Sodeifian, G. & Sajadian, S. A. Measurement and thermodynamic modeling of solubility of Tamsulosin drug (anti cancer and anti-prostatic tumor activity) in supercritical carbon dioxide. J. Supercrit. Fluids 163, 104875. https://doi.org/10.1016/j.supflu.2020.104875 (2020).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Roshanghias, A. Solubility of pazopanib hydrochloride (PZH, anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling. J. Supercrit. Fluids 190, 105759. https://doi.org/10.1016/j.supflu.2022.105759 (2022).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Abadian, M. Solubility of Dasatinib monohydrate (anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling. J. Mol. Liq. 346, 117899. https://doi.org/10.1016/j.molliq.2021.117899 (2022).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Tamura, K. Solubility of quetiapine hemifumarate (antipsychotic drug) in supercritical carbon dioxide: Experimental, modeling and hansen solubility parameter application. Fluid Phase Equilib. 537, 113003. https://doi.org/10.1016/j.fluid.2021.113003 (2021).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Ghanaat-Ghamsari, M. Measurement and modeling of clemastine fumarate (antihistamine drug) solubility in supercritical carbon dioxide. Sci. Rep. 11(1), 1–16. https://doi.org/10.1038/s41598-021-03596-y (2021).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Abadian, M. CO2 utilization for determining solubility of teriflunomide (immunomodulatory agent) in supercritical carbon dioxide: Experimental investigation and thermodynamic modeling. J. CO2 Util. 58, 101931. https://doi.org/10.1016/j.jcou.2022.101931 (2022).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Measurement, correlation and thermodynamic modeling of the solubility of Ketotifen fumarate (KTF) in supercritical carbon dioxide: Evaluation of PCP-SAFT equation of state. J. Fluid Phase Equilib. 458, 102–114. https://doi.org/10.1016/j.fluid.2017.11.016 (2018).
Sodeifian, G., Detakhsheshpour, R. & Sajadian, S. A. Experimental study and thermodynamic modeling of Esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids 154, 104606. https://doi.org/10.1016/j.supflu.2019.104606 (2019).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. Solubility of amlodipine besylate (calcium channel blocker drug) in supercritical carbon dioxide: Measurement and correlations. J. Chem. Eng. Data. 66(2), 1119–1131. https://doi.org/10.1021/acs.jced.0c00913 (2021).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. The solubility of Sulfabenzamide (an antibacterial drug) in supercritical carbon dioxide: Evaluation of a new thermodynamic model. J. Mol. Liq. 335, 116446. https://doi.org/10.1016/j.molliq.2021.116446 (2021).
Sodeifian, G., Hazaveie, S. M., Sajadian, S. A. & Saadati Ardestani, N. Determination of the solubility of the repaglinide drug in supercritical carbon dioxide: Experimental data and thermodynamic modeling. J. Chem. Eng. Data 64(12), 5338–5348. https://doi.org/10.1021/acs.jced.9b00550 (2019).
Sodeifian, G., Hsieh, C.-M., Derakhsheshpour, R., Chen, Y.-M. & Razmimanesh, F. Measurement and modeling of metoclopramide hydrochloride (anti-emetic drug) solubility in supercritical carbon dioxide. Arab. J. Chem. https://doi.org/10.1016/j.arabjc.2022.103876 (2022).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Abadian, M. Measuring and modeling the solubility of an antihypertensive drug (losartan potassium, Cozaar) in supercritical carbon dioxide. J. Mol. Liq. 331, 115745. https://doi.org/10.1016/j.molliq.2021.115745 (2021).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Panah, H. S. Solubility measurement of an antihistamine drug (Loratadine) in supercritical carbon dioxide: Assessment of qCPA and PCP-SAFT equations of state. Fluid Phase Equilib. 472, 147–159. https://doi.org/10.1016/j.fluid.2018.05.018 (2018).
Sodeifian, G., Sajadian, S. A. & Razmimanesh, F. Solubility of an antiarrhythmic drug (amiodarone hydrochloride) in supercritical carbon dioxide: Experimental and modeling. Fluid Phase Equilib. 450, 149–159. https://doi.org/10.1016/j.fluid.2017.07.015 (2017).
Sodeifian, G., Sajadian, S. A., Razmimanesh, F. & Hazaveie, S. M. Solubility of Ketoconazole (antifungal drug) in SC-CO2 for binary and ternary systems: Measurements and empirical correlations. Sci. Rep. 11(1), 1–13. https://doi.org/10.1038/s41598-021-87243-6 (2021).
Sodeifian, G., Alwi, R. S. & Razmimanesh, F. Solubility of Pholcodine (antitussive drug) in supercritical carbon dioxide: Experimental data and thermodynamic modeling. J. Fluid Phase Equilib. 566, 113396. https://doi.org/10.1016/j.fluid.2022.113396 (2022).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Nateghi, H. Solubility measurement and thermodynamic modeling of pantoprazole sodium sesquihydrate in supercritical carbon dioxide. Sci. Rep. 12, 7758. https://doi.org/10.1038/s41598-022-11887-1 (2022).
Sodeifian, G., Hazaveie, S. M. & Sodeifian, F. Determination of Galantamine solubility (an anti alzheimer drug) in supercritical carbon dioxide (CO2): Experimental correlation and thermodynamic modeling. J. Mol. Liq. 330, 115695. https://doi.org/10.1016/j.molliq.2021.115695 (2021).
Sodeifian, G., Ardestani, N. S., Razmimanesh, F. & Sajadian, S. A. Experimental and thermodynamic analysis of supercritical CO2-solubility of minoxidil as an antihypertensive drug. Fluid Phase Equilib. 522, 112745. https://doi.org/10.1016/j.fluid.2020.112745 (2020).
Sodeifian, G., Garlapati, C., Hazaveie, S. M. & Sodeifian, F. Solubility of 2,4,7-triamino-6-phenylpteridine (triamterene, diuretic drug) in supercritical carbon dioxide: Experimental data and modeling. J. Chem. Eng. Data 65(9), 4406–4416. https://doi.org/10.1021/acs.jced.0c00268 (2020).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A., Golmohammadi, M. R. & Fazlali, A. Solubility of sodium valproate in supercritical carbon dioxide: Experimental study and thermodynamic modeling. J. Chem. Eng. Data 65(4), 1747–1760. https://doi.org/10.1021/acs.jced.9b01069 (2020).
Sodeifian, G., Sajadian, S. A. & Derakhsheshpour, R. Experimental measurement and thermodynamic modeling of Lansoprazole solubility in supercritical carbon dioxide: Application of SAFT-VR EoS. Fluid Phase Equilib. 507, 112422. https://doi.org/10.1016/j.fluid.2019.112422 (2020).
Sodeifian, G., Hazaveie, S. M., Sajadian, S. A. & Razmimanesh, F. Experimental investigation and modeling of the solubility of oxcarbazepine (an anti convulsant agent) in supercritical carbon dioxide. Fluid Phase Equilib. 493, 160–173. https://doi.org/10.1016/j.fluid.2019.04.013 (2019).
Sodeifian, G. & Sajadian, S. A. Experimental measurement of solubilities of sertraline hydrochloride in supercritical carbon dioxide with/without menthol: Data correlation. J. Supercrit. Fluids 149, 79–87. https://doi.org/10.1016/j.supflu.2019.03.020 (2019).
Sodeifian, G., Usefi, M. M. B., Razmimanesh, F. & Roshanghias, A. Determination of the solubility of rivaroxaban (anticoagulant drug, for the treatment and prevention of blood clotting) in supercritical carbon dioxide: Experimental data correlations. Arab. J. Chem. 16(1), 104421. https://doi.org/10.1016/j.arabjc.2022.104421 (2023).
Abadian, M., Sodeifian, G., Razmimanesh, F. & Mahmoudabadi, S. Z. Experimental measurement and thermodynamic modeling of solubility of Riluzole drug (neuroprotective agent) in supercritical carbon dioxide. Fluid Phase Equilib. 567, 113711. https://doi.org/10.1016/j.fluid.2022.113711 (2023).
Sodeifian, G., Garlapati, C. & Roshanghias, A. Experimental solubility and modelling of crizotinib (anti cancer medication) in supercritical carbon dioxide. Sci. Rep. 12, 17494. https://doi.org/10.1038/s41598-022-22366-y (2022).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Sodeifian, F. Solubility of prazosin hydrochloride (alpha blocker antihypertensive drug) in supercritical CO2: Experimental and thermodynamic modeling. J Mol Liq 362, 119689. https://doi.org/10.1016/j.molliq.2022.119689 (2022).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Nooshabadi, M. A. Solubility of ibrutinib in supercritical carbon dioxide (Sc-CO2: Data correlation and thermodynamic analysis. J. Chem. Thermodyn. 182, 107050. https://doi.org/10.1016/j.jct.2023.107050 (2023).
Sodeifian, G., Hsieh, C.-M., Tabibzadeh, A., Wang, H.-C. & Nooshabadi, M. A. solubility of palbocilib in supercritical carbon dioxide from experimental measurement and Peng–Robinson equation of state. Sci. Rep. 13, 2172. https://doi.org/10.1038/s41598-023-29228-1 (2023).
Mahesh, G. & Garlapati, C. Modelling of solubility of some parabens in supercritical carbon dioxide and new correlations. Arab. J. Sci. Eng. https://doi.org/10.1007/s13369-021-05500-2 (2021).
Alwi, R. S. & Tamura, K. Measurement and correlation of derivatized anthraquinone solubility in supercritical carbon dioxide. J. Chem. Eng. Data 60(10), 3046–3052. https://doi.org/10.1021/acs.jced.5b00480 (2015).
Alwi, R. S., Tanaka, T. & Tamura, K. Measurement and correlation of solubility of anthraquinone dyestuffs in supercritical carbon dioxide. J. Chem. Thermodyn. 74, 119–125. https://doi.org/10.1016/j.jct.2014.01.015 (2014).
Kramer, A. & Thodos, G. Solubility of 1-hexadecanol and palmitic acid in supercritical carbon dioxide. J. Chem. Eng. Data 33, 230–234. https://doi.org/10.1021/je00053a002 (1988).
Iwai, Y., Koga, Y., Fukuda, T. & Arai, Y. Correlation of solubilities of high boiling components in supercritical carbon dioxide using a solution model. J. Chem. Eng. Jpn. 25, 757–760. https://doi.org/10.1252/jcej.25.757 (1992).
Alwi, R. S., Garlapati, C. & Tamura, K. Solubility of anthraquinone derivatives in supercrirical carbon dioxide: New correlations. Molecules 26(2), 460. https://doi.org/10.3390/molecules26020460 (2021).
Kramer, A. & Tdodos, G. Adaptation of the Flory–Huggins theory for modeling supercritical solubilities of solids. Ind. Eng. Chem. Res. 27, 1506–1510. https://doi.org/10.1021/ie00080a026 (1988).
Nasri, L., Bensetiti, Z. & Bensaad, S. Modeling of the solubility in supercritical carbon dioxide of some solid solute isomers using the expanded liquid theory. J. Sci. Technol. 3(2), 39–44 (2018).
Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 86(15), 3016–3021. https://doi.org/10.1021/j100212a041 (1982).
Sridar, R., Bhowal, A. & Garlapati, C. A new model for the solubility of dye compounds in supercritical carbon dioxide. Thermochim. Acta 561, 91–97. https://doi.org/10.1016/j.tca.2013.03.029 (2013).
Garlapati, C. & Madras, G. Solubilities of palmitic and stearic fatty acids in supercritical carbon dioxide. J. Chem. Thermodyn. 42(2), 193–197. https://doi.org/10.1016/j.jct.2009.08.001 (2010).
Méndez-Santiago, J. & Teja, A. S. The solubility of solids in supercritical fluids. Fluid Phase Equilib. 158, 501–510. https://doi.org/10.1016/S0378-3812(99)00154-5 (1999).
Bartle, K. D., Clifford, A., Jafar, S. & Shilstone, G. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 20(4), 713–756. https://doi.org/10.1063/1.555893 (1991).
Reddy, T. A. & Garlapati, C. Dimensionless empirical model to correlate pharmaceutical compound solubility in supercritical carbondioxide. Chem. Eng. Technol. 42(12), 2621–2630. https://doi.org/10.1002/ceat.201900283 (2019).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids 146, 89–99. https://doi.org/10.1016/j.supflu.2019.01.006 (2019).
Anita, N. & Garlapati, C. A simple model to correlate solubility of thermolabile solids in supercritical fluids. AIP Conf. Proc. 2446, 180002. https://doi.org/10.1063/5.0108042 (2022).
Rajasekhar, Ch. & Madras, G. An association model for the solubilities of pharmaceuticals in supercritical carbon dioxide. Thermochim. Acta 507–508, 99–105. https://doi.org/10.1016/j.tca.2010.05.006 (2010).
Li, Q., Zhong, C., Zhang, Z., Liu, Y. & Zhou, Q. An equilibrium model for the correlation of the solubility of solids in supercritical fluids with cosolvent. Sep. Sci. Technol. 38, 1705–1719. https://doi.org/10.1081/SS-120019404 (2003).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Nateghi, H. Experimental solubility and thermodynamic modeling of empagliflozin in supercritical carbon dioxide. Sci. Rep. 12, 9008. https://doi.org/10.1038/s41598-022-12769-2 (2022).
Reddy, S. N. & Madras, G. An association and Wilson activity coefficient model for solubilities of aromatic solid pollutants in supercritical carbon dioxide. Thermochim. Acta 541, 49–56. https://doi.org/10.1002/ceat.201900283 (2012).
Spark, D. L., Hernandez, R. & Estevez, L. A. Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model. Chem. Eng. Sci. 63, 4292–4301. https://doi.org/10.1016/j.ces.2008.05.031 (2008).
Valderrama, J. O. & Alvarez, V. H. Correct way of representing results when modelling supercritical phase equiliria using equation of state. Can. J. Chem. Eng. 83(3), 578–581. https://doi.org/10.1002/cjce.5450830323 (2008).
National Institute of Standards and Technology U.S. Department of commerce, NIST Chemistry WebBook in 2018 October. https://webbook.nist.gov/chemistry/ (05 December 2022).
Montequi, I., Alonso, E., Martín, A. & Cocero, M. J. Solubility of diisopropoxititanium bis (acetylacetonate) in supercritical carbon dioxide. J. Chem. Eng. Data 53, 204–206. https://doi.org/10.1021/je700503c (2007).
Reddy, S. N. & Madras, G. Solubilities of benzene derivatives in supercritical carbon dioxide. J. Chem. Eng. Data 56, 1695–1699. https://doi.org/10.1021/je100863p (2011).
Wang, H., Sang, J., Guo, L., Zhu, J. & Jin, J. Solubility of polyacrylamide in supercritical carbon dioxide. J. Chem. Eng. Data 62, 341–347. https://doi.org/10.1021/acs.jced.6b00677 (2016).
Jain, A., Yang, G. & Yalkowsky, S. H. Estimation of melting points of organic compounds. Ind. Eng. Chem. Res. 43, 7618–7621. https://doi.org/10.1021/ie049378m (2004).
Immirzi, A. & Perini, B. Prediction of density in organic crystals. Acta Crystall. A Crystallogr. 33, 216–218. https://doi.org/10.1107/S0567739477000448 (1977).
Adachi, Y. & Lu, B.C.-Y. Supercritical fluid extraction with carbon dioxide and ethylene. Fluid Phase Equilib. 14, 147–156. https://doi.org/10.1016/0378-3812(83)80120-4 (1983).
Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the Second International Symposium on Information Theory (ed. Petrov, B. N. C. F.) 267–281(AkademiaaiKiado, 1973).
Burnham, K. P. & Anderson, D. R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33(2), 261–304. https://doi.org/10.1177/0049124104268644 (2004).
Kletting, P. & Glatting, G. Model selection for time-activity curves: The corrected Akaike information criterion and the F-test. Z. Med. Phys. 19(3), 200–206. https://doi.org/10.1016/j.zemedi.2009.05.003 (2009).
Anita, N. & Garlapati, C. A statistical analysis of solubility models employed in supercritical carbon dioxide. AIP Conf. Proc. 2446, 180003. https://doi.org/10.1063/5.0108200 (2022).
Haghtalab, A. & Sodeifian, G. Determination of the discrete relaxation spectrum for polybutadiene and polystyrene by a non-linear regression method. Iran. Polym. J. 11, 107–113 (2002).
Sodeifian, G. & Haghtalab, A. Discrete relaxation spectrum and K-BKZ constitutive equation for PVC, NBR and their blends. Appl. Rheol. 14, 180–189. https://doi.org/10.1515/arh-2004-0010 (2004).
Acknowledgements
We wish to record our gratitude to University of Kashan (Grant # Pajoohaneh-1401/24) for the financial support.
Author information
Authors and Affiliations
Contributions
G.S. Conceptualization, Methodology, Validation, Investigation, Supervision, Project administration, Writing-review and editing; C.G. Methodology, Investigation, Software, Writing-original draft; M.A.N. Validation, Measurement, Resources; F.R. Investigation, Validation. A.T. Measurement.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sodeifian, G., Garlapati, C., Arbab Nooshabadi, M. et al. Solubility measurement and modeling of hydroxychloroquine sulfate (antimalarial medication) in supercritical carbon dioxide. Sci Rep 13, 8112 (2023). https://doi.org/10.1038/s41598-023-34900-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34900-7
This article is cited by
-
Studies on solubility measurement of codeine phosphate (pain reliever drug) in supercritical carbon dioxide and modeling
Scientific Reports (2023)
-
A machine learning approach for thermodynamic modeling of the statically measured solubility of nilotinib hydrochloride monohydrate (anti-cancer drug) in supercritical CO2
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.