Abstract
Measurement of saturation solubility of drugs in a supercritical fluid is an important parameter for the implementation of supercritical technology in pharmaceutical industry. CO2 is the most sorted substance as a supercritical fluid since it has attractive properties like easily achievable critical temperature, moderate pressure. Cancer is increasingly affecting the mankind, a proper dosage while treating would help in minimizing the drug usage. The bioavailability of the drug is mainly influenced by the drug particle size. An appropriate technology is always useful in making suitable drug particles; thus, supercritical fluid technology (SFT) is considered as promising technique for the production of micro and nanoparticles. Since, particle production process through SFT needs solubility information, appropriate solubility information is necessary. In the present work, Crizotinib (anti-cancer drug) solubility in supercritical carbon dioxide (scCO2) is measured and reported, for the first time. The obtained solubilities are at temperatures 308, 318, 328,338 K and pressures 12, 15, 18, 21, 24 to 27 MPa. The measured solubilities are ranged in terms of mole fraction from (0.483 × 10−5 to 0.791 × 10−5) at 308 K, (0.315 × 10−5 to 0.958 × 10−5) at 318 K, (0.26 × 10−5 to 1.057 × 10−5) at 328 K, (0.156 × 10−5 to 1.219 × 10−5) at 338 K. The cross over region is observed at 14.5 MPa. To expand the application of the solubility data, few important solubility models and three cubic equations of sate (cubic EoS) models along with Kwak and Mansoori mixing rules are investigated. Sublimation and salvation enthalpies of Crizotinib dissolution in scCO2 are calculated.
Similar content being viewed by others
Introduction
Traditionally, pharmaceutical particle production processes use several organic solvents for processing their products. Quite often, remnant of solvents causes serious pollution and sometimes reactions with pharmaceutical products and result in unnecessary byproducts. These issues are effectively handled using supercritical fluid technologies (SFTs). Although, there is a scope of using several substances as supercritical fluids, CO2 as supercritical fluid has gained importance for the last three decades. Commonly, all supercritical fluids (SCFs) have gas like diffusivities and liquid like densities makes them attractive for extraction processes. When CO2 pressure and temperature conditions are maintained above 7.39 MPa and 304.15 K, it will act as supercritical fluid and it is commonly abbreviated as supercritical carbon dioxide (scCO2)1,2,3,4. Due to these features, it is used as a solvent in various process applications1,2,3,4,5,6,7,8.Some of the major applications of scCO2 in process industry are pharmaceutical particle size design, food processing, textile dyeing and extraction1,2,3,4.Solubility information is necessary for the proper implementation of SFTs for particle size design process. The task of measuring solubility of important anti-cancer drugs in scCO2 is taken in this work. In recent literature, solubilities of several anti-cancer drugs in scCO2 are readily available8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26, but the Crizotinib solubility in scCO2 is not reported, therefore in this work for the first time its solubility is measured and reported. Crizotinib is used in the treatment of some kind of non-small cell lung cancer (NSCLC) that spreads to nearby tissues or to other parts of the body27.Crizotinib helps to stop the growth of tumor cells by blocking the anaplastic lymphoma kinase (ALK) protein from working; it is also termed as fusion mutation. Appropriate dosage is attained with proper drug particle size and it is very critical for the treatment of cancer. Thus, the information obtained this study is useful in preparing various size drug particles using scCO2. For practical purpose obtaining solubilities at various conditions are cumbersome hence model identification for the solubility is essential28. The data developed in this work are tested with few important solubility models and three cubic equation of state models (cubic EoS) along with Kwak and Mansoori mixing rules29.
The purpose of this study is in two levels. In the first level, Crizotinib solubility in scCO2 is determined and in the second, data obtained are correlated with existing solubility models and with three Cubic EoS models along with Kwak and Mansoori mixing rules.
Experimental
Materials
Crizotinib is supplied by Amin Pharma company (CAS Number: 877399-52-5, mass purity > 99%). CO2 (CAS Number: 124-38-9, mass purity > 99.9%) is purchased from Fadak company, Kashan (Iran). Dimethyl sulfoxide (DMSO, CAS Number: 67-68-5, mass purity > 99%) is procured from Sigma Aldrich company. Table 1 indicates the information about the chemicals utilized in this work.
Experiment details
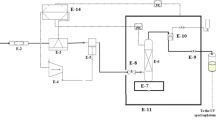
The experimental setup utilized in this study is shown in Fig. 1. The details about the solubility apparatus and experimental procedure are presented in our earlier studies (Fig. 1)30,31,32,33,34,35,36,37,38,39,40,41,42. However, a brief description is presented in this section. The method of solubility measurement utilized is classified as an isobaric-isothermal method43. All measurements were made with high precision while controlling temperatures and pressures within ± 0.1 K and ± 0.1 MPa, respectively. Solubility measurements were performed at least in triplicate for each data point. For each measurement, 1 g of Crizotinib drug was used. In line with our previous works, for this compound, the observed equilibrium was within 60 min. After equilibrium, 600 µL saturated scCO2 sample was collected via 2-status 6-way port valve in a DMSO preloaded vial. After discharging 600 µL saturated scCO2, the port valve was washed with 1 ml DMSO. Thus, the total saturation solution was 5 ml. Drug solubility in terms of mole fraction is calculated with the following formula:
where \(n_{{{\text{drug}}}}\) is number of moles of the drug, and \(n_{{{\text{CO}}_{{2}} }}\) is number of moles of CO2 in the sampling loop.
Experimental setup for solubility measurement, E1—CO2 cylinder; E-2—Filter; E-3—Refrigerator unit; E-4—Air compressor; E-5—High pressure pump; E-6—Equilibrium cell; E-7—Magnetic stirrer; E-8—Needle valve; E-9—Back-pressure valve; E-10 Six-port, two position valve; E-11—Oven; E-12—Syringe; E13—Collection vial; E-14—Control panel.
Furthermore, the above quantities are given as:
where \(C_{{\text{s}}}\) is the drug concentration in saturated sample vial in g/L. The volume of the sampling loop and vial collection are V1(L) = 600 \(\times\) 10−6 m3 and Vs(L) = 5 \(\times\) 10−3 m3, respectively. The \(M_{s}\) and \(M_{{CO_{2} }}\) are the molecular weights of the drug and CO2, respectively. Solubility is also described as
The relation between S and \(y_{2}\) is
The Crizotinib’s solubility was determined using a UV–Visible spectrophotometer (Model UNICO-4802) and DMSO solvent was used for the measurement of its solubility. Drug samples were analyzed at wave length of 270 nm.
Modeling
The Crizotinib’s solubility data measured in this study is correlated by four standard solubility models namely Chrastil, Modefied Chrastil, Mendez-Santiago and Teja and Bartle et al. Moreover, three cubic EoS models along with Kwak and Mansoori mixing rules are explored for the solubility data correlation. More details about the models considered in this work are presented in the following subsections6.
Standard solubility models
Chrastil model44
Chrastil model represents by Eq. (6) that relates solubility mole fraction in terms of solvent density, association number and temperature
where model constants are \(\kappa ,A_{1} \;{\text{and}}\;B_{1}\).
Equation (6) is alternatively represented as45
where \(\kappa ,A_{1} \;{\text{and}}\;B_{1}\) are the model parameters.
Modified Chrastil model46
Dimensionally corrected Charstil model is also known as modified Charstil model and is described in terms of solvent density, association number and temperature as
where \(\kappa^{\prime},A_{2} \;{\text{and}}\;B_{2}\) are the model parameters.
Méndez-Santiago and Teja (MT) model47
Measured data self-consistency is checked with this model and represents by Eq. (9)
where A3 and C3 are the model parameters.
Bartle et al., model48
Sublimation enthalpy of the dissolved solids in SCFs is measured with this model and stated as
where A4, B4 and C4 are the model parameters. From parameter B4, sublimation enthalpy is estimated (\(\Delta_{sub} H = - B_{4} R\)).
Equation of sate (EoS) modeling
There are several EoS models among them Redlich-Kwong (RK), Soave–Redlich–Kwong (SRK) and Peng-Robinson (PR) EoSs are commonly used in correlating solubility of solid compounds in scCO2.All these models for correlation requires adjustable interaction parameters and they are found to be temperature dependent49,50. In the year 1986 Kwak and Mansoori developed a new concept in the spirit of van der Waals mixing rules (VdWmrs), which resulted in temperature independent interaction parameters. They have demonstrated the correlating ability of RK and PR EoSs29. However, SRK EoS has not been explored, therefore in this work, the SRK EoS along with Kwak and Mansoori mixing rules are explored and finally two forms of solubility models for SRK EoS model is proposed. More details about the EoS modeling are presented in the following subsections.
RK EoS with Kwak and Mansoori mixing rules29
RK EoS in terms of compressibility factor (Z) is given by
VdWmrs for RK EoS are expressed as
Equations (12) to (15) combined with Eq. (11), will constitute the RK EoS for mixtures, consistent with the statistical-mechanical basis of the VdW mixing rules.
PR EoS with Kwak and Mansoori mixing rules29,49,50
PR EoS in terms of compressibility factor (Z) is given by
where
Equation (16) provides three independent constants in terms of a, b and c. Now following the guidelines given by Kwak and Mansoori mixing rules result in the following expressions for a, b and c
With the following interaction parameters
Equations (17) to (22) combined with Eq. (16), will constitute the PR EoS for mixtures, consistent with the statistical-mechanical basis of the VdW mixing rules.
SRK EoS with Kwak and Mansoori mixing rules38
In the following, SRK EoS is given by51
where V is the molar volume and other parameters have usual meanings. The pure component parameter a, which is a function of temperature and b, which is a constant and they are obtained from the following relations.
where m is a constant given by
where ‘ω’ is the acentric factor. In 1993, Soave proposed a new α(T) function for heavy hydrocarbons to be used with SRK EoS52
where
In order to separate thermodynamic variables from constants of the SRK EoS, we have adopted the following two ways.
When Eqs. (26), (27) and (23) are combined to get the following form for SRK EoS in terms of compressibility factor (Z)
where
This form of the SRK EoS suggests three independent constants namely a, b and c. Now following the guidelines given by Kwak and Mansoori mixing rules result in following expressions for a, b and c
With the following interaction parameters:
Equations (32) to (37) combined with Eq. (31), will constitute the SRK EoS for mixtures, consistent with the statistical-mechanical basis of the VdW mixing rules.
When Eqs. (28), (29), (30) and (23) are combined to get the following form.
where
This form of the SRK EoS suggests that four independent constants exist in the EoS namely a, b, c and d. Now following the guidelines given by Kwak and Mansoori mixing rules results in following expressions for a, b, c and d
With the following interaction parameters:
Equations (39) to (46) combined with Eq. (38), will constitute the SRK EoS for mixtures, consistent with the statistical-mechanical basis of the VdW mixing rules.
EoS model for the solubility of solids in scCO2
The mole fraction of dissolved solid drug i (solute) in Solvent scCO2 is expressed as53
where \(P_{i}^{s}\) is the sublimation pressure and other parameters have usual meanings. The saturation fugacity coefficient (\(\hat{\phi }_{i}^{s}\)) is assumed to be unity. The required expression for the solid solute fugacity coefficient in the ScCO2(\(\left( {\hat{\phi }_{i}^{{ScCO_{2} }} } \right)\)) is calculated using three cubic EoS along with Kwak and Mansoori mixing rules. They are obtained from the following basic thermodynamic relation54
Equations (49) to (52) represent the fugacity coefficients expressions used in this study;
For RK EoS
For PR EoS
where
For SRK EoS
When three parameters are considered
where
When four parameters are considered
where
For the data regression, fminsearch (MATLAB 2019) algorithm has been used. Solubility models are regressed with the following objective function55
Final results are indicated in terms of an average absolute relative deviation percentage (AARD %).
Results and discussion
The reliability of the experimental setup for the solubility measurement was reported in our previous works with naphthalene and alphatocopherol compounds. Crizotinib’s solubilities in scCO2 at various conditions are reported in Table 2. Table 2 also indicates scCO2 density obtained from NIST database56. Table 3 indicates solubility range of some of the anticancer drugs reported in the literature. For the Crizotinib the measured solubilities are observed to range from 0.156 × 10−5 to 1.219 × 10−5 in mole fraction. Figure 2 indicates solubility isotherms and at 14.5 MPa cross over region is observed. Solubility of pure Crizotinib in scCO2 the crossover point (14.5 MPa) is rather unique with respect to temperature. Below 14.5 MPa rise in temperature causes drop in solubility in scCO2 phase, while above this point the opposite effect occurs. The following two ways of thermodynamic explanation may be attributed to this phenomenon as at pressures below the crossover pressure the density of the scCO2 is more sensitive to temperature changes than at higher pressures. A temperature decrease in this region affects the solubility of the drug in two ways. The vapor pressure of the drug solid decrease while the density of the scCO2 (proportional to its solvent power) increase thus the density effect predominates and results in the solubility increase. While on the other hand at pressures above the crossover pressure a temperature increase causes an increase the vapor pressure of the drug while the density of the scCO2 decrease thus the vapor pressure effect predominates and the solubility increases57. Another way to explain crossover pressure is that at crossover pressure the partial molar configurational enthalpy equals the negative of the sublimation enthalpy58. Table 4 indicates all the standard solubility models utilized in this work. Furthermore, it also indicates all the regressed parameters along with AARD%. The correlating ability of these models are shown in Figs. 3, 4, 5 and 6. The solubility data reported in this study is considered self-consistent since all the data is aligned to a single correlation line (Fig. 5). From literature it is clear that Chrastiland modified Chrastil models are useful in calculating thermodynamic properties like heat of reaction and solvation enthalpy of the dissolution process, therefore these models are considered for the correlating the data31,33.The total enthalpy of Crizotinib’s dissolution in scCO2 is calculated from the Chrastil model parameter A1(i.e., \(\Delta H_{total} = - A_{1} R\)). The sublimation enthalpy of Crizotinibis calculated from Bartle model parameter (i.e.,\(\Delta H_{sub} = - B_{4} R\)). Sublimation and solvation are the two major steps in dissolution process and the enthalpy of solvation is calculated from the difference between \(\Delta H_{total} - \Delta H_{sub}\). A negative sign is attributed to the solvation process. Similar, this approach is used to calculate all these thermodynamic quantities for modified Chrastil and Bartle models combination, thus estimated values are presented in Table 5.
The cubic EoS model requires critical properties of Crizotinib and CO2 and these are estimated with group contribution methods based on the chemical structure54,59,60,61. Table 6 presents all the estimated critical and physical properties of the drug considered this work. The EoS model regression results are tabulated in Table 7 along with some statistical parameters. From the AARD% it is clear that existing models (RK, SRK EoSs (three parameters) and PR EoS) are poorly correlating the solubility data (Supplementary information Fig. S1, S2, S3). The Crizotinib-scCO2 system is highly nonlinear system and to correlate such behavior we may need more adjustable parameter, therefore a new form of solubility model based on Kwak and Mansoori guidelines for SRK EoS is proposed. In the new model, one extra parameter is introduced in the term ‘a’ when compared to existing three parameter SRK EoS. Since more parameters are present in the model the regression results will be better in terms of AARD%. Thus, the proposed SRK EoS model is having four parameters. Further the new EoS model is found to correlate the data better than the existing EoS models. From the AARD% it is clear that four parameter SRK EoS correlates the solubility data much better than PR EoS model. Experimental data points and four parameter SRK EoS model predictions are depicted in Fig. 7. Due to poor correlation the RK, PR and three parameter SRK EoSs results are not shown as figures. Overall, four parameter SRK EoS is able to provide satisfactory solubility correlation results. The success of four parameter SRK EoS may be attributed to its number of parameters that constitute the model.
Goodness of the models is quantified using an indicator known as Akaike Information Criterion (AIC) and corrected AIC (AICc)62,63,64. As we know, if the experimental data points are less thanforty, the AICc is employed. An expression that relates AICc with AIC, number of data points (N) and number of parameters in the model (\(Q\)), is
where AIC is \(N\;\ln \left( {{{SSE} \mathord{\left/ {\vphantom {{SSE} N}} \right. \kern-\nulldelimiterspace} N}} \right) + 2Q\) and SSE is error sum of squares. According to this indicator, the best model would have least AICc value. The summary of AIC, AICc, SSE and RMSD values for various models are presented in Table 8. From AICc values, it is clear that Chrastil and modified Chrastil models are the better models, whereas four parameter SRK EoS model is the best model among EoS models.
Conclusions
Solubilities of Crizotinib in ScCO2 at various conditions are presented at (T = 308, 318, 328 and 338 K) and (P = 12, 15, 18, 21, 24 and 27 MPa), for the first time. The measured solubilities are in the range from 0.156 × 10−5 to 1.219 × 10−5 in terms of mole fraction. The obtained data was modeled with four standard models and three EoS models combining with Kwak and Mansoori mixing rules. Chrastil and Modified Chrastil models are observed to correlate the data with least AARD% and AICc values. Among EoS models, four parameter SRK EoS model is able to correlate the data satisfactorily and on par with standard models. Finally, all the standard solubility models considered in this study are able to provide reasonable solubility results.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to confidential cases and are available from the corresponding author on reasonable request.
Abbreviations
- A 1 , B 1 :
- A 2 , B 2 :
-
Adjustable parameters for Eq. (8)
- A 3 , B 3, C 3 :
-
Adjustable parameters for Eq. (9)
- A 4 , B 4, C 4 :
-
Adjustable parameters for Eq. (10)
- AARD% :
-
Percent of average absolute relative deviation
- AIC :
-
Akaike information criterion
- AIC c :
-
Corrected AIC
- C s :
-
Concentration of drug inside collection vial in g/L
- E1- A14:
-
Apparatus symbols
- H sol :
-
Crizotinib solvation enthalpy in kJ/mol
- H vap :
-
Crizotinib vaporization enthalpy in kJ/mol
- H tot :
-
Total enthalpy of Crizotinib in kJ/mol
- kij, lij , m ij , n ij :
- \(M_{{co_{2} }} ,M_{s}\) :
-
Molar mass of CO2 and drug in g/mol
- \(n_{CO2}\) :
-
Moles of CO2, in mol
- \(n_{{d{\text{rug}}}}\) :
-
Moles of Crizotinibdrug, in mol
- N :
-
Number of experimental data for Eq. (55)
- NIST:
-
National institute of standards and technology
- OF :
-
Objective function
- P c :
-
Critical pressure, in Pa
- Q :
-
Number of parameters in the model for Eq. (55)
- \(P_{sub}\) :
-
Pure solid sublimation pressure, in MPa
- R :
-
Universal gas constant, in J/(mol∙K)
- S :
-
Solubility of Crizotinibin equilibrium state, in g/L
- SSE :
-
Sum of squares error
- scCO2 :
-
Supercritical carbon dioxide
- T :
-
Temperature
- T c :
-
Critical temperature
- v s :
-
Molar volume of drug, in m3/mol
- V 1, Vs :
-
Volume of sampling loop and collection vial, respectively, in μL
- y2, yi :
-
Solubility of solute mole fraction
- \(y_{2,i}^{{{\text{exp}}}} ,y_{2,i}^{{{\text{calc}}}}\) :
-
Experimental and calculated solubility points
- Z :
-
Compressibility factor
- \(\rho\) :
-
Density in kg/m3
- \(\rho_{1}\) :
-
Mass density of scCO2 in kg/m3
- \(\hat{\phi }_{2}^{s}\) :
-
Pure substance fugacity coefficient
- \(\hat{\phi }_{2}^{{scCO_{2} }}\) :
-
Fugacity coefficient of solute in fluid phase
- k :
- ω :
-
Acentric factor
References
Subramaniam, B., Rajewski, R. A. & Snavely, K. Pharmaceutical processing with supercritical carbon dioxide. J. Pharm. Sci. 86(8), 885–890 (1997).
Elvassore, NKI. Pharmaceutical processing with supercritical fluids. In Bertucco, AGVG, (Ed) High Pressure Process Technology: Fundamentals and Applications. pp. 612–625. (Elsevier Science, Springer, 2001)
Gupta, R. B., Chattopadhyay, P. Method of forming nanoparticles and microparticles of controllable size using supercritical fluids and ultrasound. US patent No. 20020000681 (2002).
Reverchon, E., Adami, R., Caputo, G. & De Marco, I. Spherical microparticles production by supercritical antisolvent precipitation: Interpretation of results. J. Supercrit. Fluids 47(1), 70–84 (2008).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Experimental measurements and thermodynamic modeling of Coumarin-7 solid solubility in supercritical carbon dioxide: Production of nanoparticles via RESS method. Fluid Phase Equilib. 483, 122–143 (2019).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Roshanghias, A. Solubility of pazopanib hydrochloride (PZH, anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling. J. Supercrit. Fluids 190, 105759 (2022).
Sodeifian, G., Sajadian, S. A., Ardestani, N. S. & Razmimanesh, F. Production of loratadine drug nanoparticles using ultrasonic-assisted rapid expansion of supercritical solution into aqueous solution (US-RESSAS). J. Supercrit. Fluids 147, 241–253 (2019).
Alwi, R. S. & Garlapati, C. New correlations for the solubility of anticancer drugs in supercritical carbon dioxide. Chem. Pap. 76, 1385–1399 (2022).
Zabihi, S. et al. Loxoprofen solubility in supercritical carbon dioxide: Experimental and modeling approaches. J. Chem. Eng. Data 65, 4613–4620 (2020).
Pishnamazi, M. et al. Thermodynamic modelling and experimental validation of pharmaceutical solubility in supercritical solvent. J. Mol. Liq. 319, 114120 (2020).
Guney, O. & Akgerman, A. Solubilities of 5Fluorouracil and β-estradiol in supercritical carbon dioxide. J. Chem. Eng. Data 45(6), 1049–1052 (2000).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids 146, 89–99 (2019).
Sodeifian, G., Sajadian, S. A. & Ardestani, N. S. Determination of solubility of aprepitant (an antiemetic drug for chemotherapy) in supercritical carbon dioxide: Empirical and thermodynamic models. J. Supercrit. Fluids 128, 102–111 (2017).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Hazaveie, S. M. Experimental data and thermodynamic modeling of solubility of sorafenib tosylate, as an anti-cancer drug, in supercritical carbon dioxide: Evaluation of Wong-Sandler mixing rule. J. Chem. Thermodyn. 142, 105998 (2020).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Prediction of solubility of sunitinib malate (an anti-cancer drug) in supercritical carbon dioxide (SC–CO2): Experimental correlations and thermodynamic modeling. J. Mol. Liq. 297, 111740 (2020).
Sodeifian, G. & Sajadian, S. A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 133(1), 239–252 (2018).
Sodeifian, G., Razmimanesh, F., Ardestani, N. S. & Sajadian, S. A. Experimental data and thermodynamic modeling of solubility of Azathioprine, as an immunosuppressive and anti-cancer drug, in supercritical carbon dioxide. J. Mol. Liq. 299, 112179 (2019).
Suleiman, D., Antonio Estévez, L., Pulido, J. C., García, J. E. & Mojica, C. Solubility of anti-infammatory, anti-cancer, and anti-HIV drugs in supercritical carbon dioxide. J. Chem. Eng. Data 50, 1234–1241 (2005).
Pishnamazi, M. et al. Measuring solubility of a chemotherapy-anti cancer drug (busulfan) in supercritical carbon dioxide. J. Mol. Liq. 317, 113954 (2020).
Yamini, Y. et al. Solubilities of flutamide, dutasteride, and finasteride as antiandrogenic agents, in supercritical carbon dioxide: Measurement and correlation. J. Chem. Eng. Data 55(2), 1056–1059 (2010).
Xiang, S. T., Chen, B. Q., Kankala, R. K., Wang, S. B. & Chen, A. Z. Solubility measurement and RESOLV-assisted nanonization of gambogic acid in supercritical carbon dioxide for cancer therapy. J. Supercrit. Fluids 150, 147–155 (2019).
Pishnamazi, M. et al. Experimental and thermodynamic modeling decitabine anti cancer drug solubility in supercritical carbon dioxide. Sci. Rep. 11, 1075 (2021).
Zabihi, S. et al. Thermodynamic study on solubility of brain tumor drug in supercritical solvent: Temozolomide case study. J. Mol. Liq. 321, 114926 (2021).
Hazaveie, S. M., Sodeifian, G. & Sajadian, S. A. Measurement and thermodynamic modeling of solubility of tamsulosin drug (anti cancer and anti-prostatic tumor activity) in supercritical carbon dioxide. J. Supercrit. Fluids 163, 104875 (2020).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Roshanghias, A. Solubility of pazopanib hydrochloride (PZH, anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling. J. Supercrit. Fluids 190, 105759 (2022).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Abadian, M. Solubility of dasatinib monohydrate anticancer drug in supercritical CO2: Experimental and thermodynamic modeling. J. Mol. Liq. 346, 117899 (2022).
Frampton, J. E. Crizotinib: A review of its use in the treatment of anaplastic lymphoma kinase-positive, advanced non-small cell lung cancer. Drugs 73(18), 2031–2051 (2013).
Mahesh, G. & Garlapati, C. Modelling of solubility of some parabens in supercritical carbon dioxide and new correlations. Arab. J. Sci. Eng. 47, 5533–5545 (2021).
Kwak, T. & Mansoori, G. Van der Waals mixing rules for cubic equations of state. Applications for supercritical fluid extraction modelling. Chem. Eng. Sci. 41(5), 1303–1309 (1986).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Tamura, K. Solubility of quetiapine hemifumarate (antipsychotic drug) in supercritical carbon dioxide: Experimental, modeling and hansen solubility parameter application. Fluid Phase Equilib. 537, 113003 (2021).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Ghanaat-Ghamsari, M. Measurement and modeling of clemastine fumarate (antihistamine drug) solubility in supercritical carbon dioxide. Sci. Rep. 11(1), 1–16 (2021).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Abadian, M. CO2 Utilization for determining solubility of teriflunomide (immunomodulatory agent) in supercritical carbon dioxide. Experimental investigation and thermodynamic modeling. J. Util. 58, 101931 (2022).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Measurement, correlation and thermodynamic modeling of the solubility of Ketotifen fumarate (KTF) in supercritical carbon dioxide: Evaluation of PCP-SAFT equation of state. J. Fluid Phase Equilib. 458, 102–114 (2018).
Sodeifian, G., Detakhsheshpour, R. & Sajadian, S. A. Experimental study and thermodynamic modeling of Esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids 154, 104606 (2019).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. Solubility of amlodipine besylate (calcium channel blocker drug) in supercritical carbon dioxide: Measurement and correlations. J. Chem. Eng. Data 66(2), 1119–1131 (2021).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. The solubility of sulfabenzamide (an antibacterial drug) in supercritical carbon dioxide: Evaluation of a new thermodynamic model. J. Mol. Liq. 335, 116446 (2021).
Sodeifian, G., Hazaveie, S. M., Sajadian, S. A. & Saadati Ardestani, N. Determination of the solubility of the repaglinide drug in supercritical carbon dioxide: Experimental data and thermodynamic modeling. J. Chem. Eng. Data 64(12), 5338–5348 (2019).
Sodeifian, G., Hsieh, C.-M., Derakhsheshpour, R., Chen, Y.-M. & Razmimanesh, F. Measurement and modeling of metoclopramide hydrochloride (anti-emetic drug) solubility in supercritical carbon dioxide. Arab. J. Chem. 15, 103876 (2022).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Abadian, M. Measuring and modeling the solubility of an antihypertensive drug (losartan potassium, Cozaar) in supercritical carbon dioxide. J. Mol. Liq. 331, 115745 (2021).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Panah, H. S. Solubility measurement of an antihistamine drug (Loratadine) in supercritical carbon dioxide: Assessment of qCPA and PCP-SAFT equations of state. J. Fluid Phase Equilib. 472, 147–159 (2018).
Sodeifian, G., Sajadian, S. A. & Razmimanesh, F. Solubility of an antiarrhythmic drug (amiodarone hydrochloride) in supercritical carbon dioxide: Experimental and modeling. Fluid Phase Equilib. 450, 149–159 (2017).
Sodeifian, G., Sajadian, S. A., Razmimanesh, F. & Hazaveie, S. M. Solubility of Ketoconazole (antifungal drug) in SC-CO2 for binary and ternary systems: Measurements and empirical correlations. Sci. Rep. 11(1), 1–13 (2021).
Peper, S., Fonseca, J. M. & Dohrn, R. High-pressure fluid-phase equilibria: Trends, recent developments, and systems investigated (2009–2012). Fluid Phase Equilib. 484, 126–224 (2019).
Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 86(15), 3016–3021 (1982).
Sridar, R., Bhowal, A. & Garlapati, C. A new model for the solubility of dye compounds in supercritical carbon dioxide. Thermochim. Acta 561, 91–97 (2013).
Garlapati, C. & Madras, G. Solubilities of palmitic and stearic fatty acids in supercritical carbon dioxide. J. Chem. Thermodyn. 42(2), 193–197 (2010).
Méndez-Santiago, J. & Teja, A. S. The solubility of solids in supercritical fluids. Fluid Phase Equilib. 158, 501–510 (1999).
Bartle, K. D., Clifford, A., Jafar, S. & Shilstone, G. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 20(4), 713–756 (1991).
Garlapati, C. & Madras, G. Temperature independent mixing rules to correlate the solubilities of antibiotics and anti-inflammatory drugs in SCCO2. Thermochim. Acta 496(1–2), 54–58 (2009).
Valderrama, J. O. & Alvarez, V. H. Temperature independent mixing rules to correlate the solubility of solids in supercritical carbon dioxide. J. Supercrit. Fluids 32(1–3), 37–46 (2004).
Soave, G. Equilibrium constants from a modeified Redlich-Kwong equation of state. Chem. Eng. Sci. 27, 1197–1203 (1972).
Soave, G. Improving the treatment of heavey hydrocarbons by the SRK EOS. Fluid Phase Equilib. 84, 339–342 (1993).
Alwi, R. S. & Garlapati, C. A new semi empirical model for the solubility of dyestuffs in supercritical carbon dioxide. Chem. Pap. 75(6), 2585–2595 (2021).
Reid, R. C., Prausnitz, J. M., Poling, B. E. The properties of gases and liquids. (1987).
Valderrama, J. O. & Alvarez, V. H. Correct way of representing results when modelling supercritical phase equiliria using equation of state. Can. J. Chem. Eng. 83(3), 578–581 (2008).
https://webbook.nist.gov/chemistry/01 In Institute of Standards and Technology U.S. Department of Commerce (2018).
Chimowitz, E. H. & Pennisi, K. J. Process synthesis concepts for supercritical gas extraction in the crossover region. AICHE J. 32(10), 1665–1676 (1986).
Johnston, K. P., Barry, S. E., Read, N. K. & Holcomb, T. R. Separation of isomers using retrograde crystallization from supercritical fluids. Ind. Eng. Chem. Res. 26, 2372–2377 (1987).
Fedors, R. F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 14(2), 147–154 (1974).
Immirzi, A. & Perini, B. Prediction of density in organic crystals. Acta Crystallographica Sect. A Crystal Phys. Diffr. Theor. Gen. Crystallogr. 33(1), 216–218 (1977).
Lyman, W. J., Reehl, W.F. & Rosenblatt, D. H. In Hand book of chemical property estimation methods. (McGraw-Hill, 1982)
Akaike, H. Information theory and an extension of the maximum likelihood principle. In Petrov, B. N. C. F. (Ed.) Proceedings of the second international symposium on information theory. pp 267–81. (AkademiaaiKiado, Budapest, 1973)
Burnham, K. P. & Anderson, D. R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 33(2), 261–304 (2004).
Kletting, P. & Glatting, G. Model selection for time-activity curves: The corrected Akaike information criterion and the F-test. Z. Med. Phys. 19(3), 200–206 (2009).
Acknowledgements
Authors wish toacknowledge the research deputy of University of Kashan (Grant # Pajoohaneh-1401/28) for the financial support of this valuable project.
Author information
Authors and Affiliations
Contributions
G.S.: Conceptualization, Methodology, Validation, Investigation, Supervision, Project administration, Writing-review & editing; C.G.: Methodology, Investigation, Software, Writing-original draft; A.R.: Resources. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sodeifian, G., Garlapati, C. & Roshanghias, A. Experimental solubility and modeling of Crizotinib (anti-cancer medication) in supercritical carbon dioxide. Sci Rep 12, 17494 (2022). https://doi.org/10.1038/s41598-022-22366-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-22366-y
This article is cited by
-
Solubility measurement and modeling of hydroxychloroquine sulfate (antimalarial medication) in supercritical carbon dioxide
Scientific Reports (2023)
-
Studies on solubility measurement of codeine phosphate (pain reliever drug) in supercritical carbon dioxide and modeling
Scientific Reports (2023)
-
A machine learning approach for thermodynamic modeling of the statically measured solubility of nilotinib hydrochloride monohydrate (anti-cancer drug) in supercritical CO2
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.