Abstract
One of the main steps in choosing the drug nanoparticle production processes by supercritical carbon dioxide (SC-CO2) is determining the solubility of the solid solute. For this purpose, the solubility of Ketoconazole (KTZ) in the SC-CO2, binary system, as well as in the SC-CO2-menthol (cosolvent), ternary system, was measured at 308–338 K and 12–30 MPa using the static analysis method. The KTZ solubility in the SC-CO2 ranged between 0.20 × 10–6 and 8.02 × 10–5, while drug solubility in the SC-CO2 with cosolvent varied from 1.2 × 10–5 to 1.96 × 10–4. This difference indicated the significant effect of menthol cosolvent on KTZ solubility in the SC-CO2. Moreover, KTZ solubilities in the two systems were correlated by several empirical and semiempirical models. Among them, Sodeifian et al., Bian et al., MST, and Bartle et al. models can more accurately correlate experimental data for the binary system than other used models. Also, the Sodeifian and Sajadian model well fitted the solubility data of the ternary system with AARD% = 6.45, Radj = 0.995.
Similar content being viewed by others
Introduction
Serious fungal infections can increase due to the development of the human immunodeficiency virus (HIV), anti-cancer chemotherapy, and/or the greater utilization of the immuno-suppressive treatments in transplanting the organs1. Ketoconazole (KTZ) is mainly applied as a synthetic imidazole antifungal drug to treat fungal infections in different forms (oral tablets, topical creams, and gels). It has been applied in immunocompromised patients and advanced prostatic carcinoma1,2. KTZ has very low solubility (17 µg/ml) in the water and higher penetrability; therefore, it has been considered as a class II drug in the biopharmaceutics classification system (BCS). Improving the solubility of pharmaceutical compounds is a challenging subject as it can significantly reduce the oral bioavailability and thus the therapeutic efficacy of drugs2.
Reducing particles' size and hence increasing the available surface area can enhance the solubility and bioavailability of pharmaceutical compounds with lower water solubility. Therefore, researchers have applied different processes (such as high-pressure homogenization, evaporation, milling, and sublimation) to reduce particle size in the pharmaceutical industry. Meanwhile, supercritical fluid (SCF) technology has received much attention in medicine to decrease the size of the particles, hence, increasing their dissolution rate and bioactivity. According to studies conducted in this field, the application of supercritical solution methods in particle formation has rapidly expanded. The solubility of drugs in SCFs should be experimentally measured for designing pharmaceutical processes3.
Numerous investigators have demonstrated that solute solubility in the SCFs can be substantially improved with the addition of cosolvents (polar or non-polar)4,5,6,7,8,9,10. Gurdial et al.4, measured the solubility of o- and m-hydroxy-benzoic acid in acetone-SC-CO2 and methanol-SC-CO2 binary mixtures at the temperature range of 318–328 K and the pressure range of 90–200 bar using a continual flow apparatus. Their results indicated that the addition of little amounts of the cosolvents to SC-CO2 largely enhanced o- and m-hydroxy-benzoic acid solubility. Huang et al.11 evaluated the equilibrium mole fraction of aspirin in SC-CO2 with and without acetone cosolvent. Their results showed that acetone cosolvent could cause a fivefold increase in aspirin solubility. Also, Koga et al.9 investigated influences of cosolvents (octane & ethanol) on the solubility of fatty acids, stearic acid, and stearyl alcohol in SC-CO2 using a flow-type apparatus and showed the higher effectiveness of ethanol on the solubilities of fatty acids than octane. Hosseini et al.12 determined the solubility of clozapine and lamotrigine in SC-CO2 with menthol cosolvent at the temperature range of 313–323 K and the pressure range of 123–337 bar. The applied cosolvent enhanced the solubility of both solutes in SC-CO2. Consequently, the solubility of numerous compounds has been experimentally determined in the SCFs13,14,15,16,17,18,19,20. However, the measurement of the solubility of drugs in the SCFs under diverse pressure and temperature conditions is costly and laborious21. In this regard, thermodynamic models have been developed to decrease the number of required experimental measurements. Compared to the other SCFs, SC-CO2 has been widely employed in SCF processes due to its special thermodynamic and heat transfer properties. Additionally, CO2 is non-toxic, non-flammable, cost-effective, abundant at high levels of purity, and environmentally-friendly with comparatively lower critical pressures and temperatures (7.38 MPa & 304.1 K)22,23,24,25,26,27. Several models have been applied to correlate and predict solids solubility at supercritical conditions, among which empirical and semiempirical methods28,29,30,31,32,33,34, equations of state (EoS) (including cubic and non-cubic models)35,36,37,38,39,40,41,42,43, expanded liquid model44, intelligent computational techniques (e.g. artificial neural networks (ANN) and least square support vector machine (LS-SVM) networks), and combination of grey wolf optimizer and support vector machines (GWO-SVM) networks27,45 can be mentioned. Some characteristics such as acentric factor, and molar volume, as well as the solid vapor pressures, are essential for the calculations of EoS based models. These parameters are, however, unavailable and thus must be estimated by the group contribution techniques leading to attenuated accuracy. To overcome this drawback, several researchers have applied different empirical and semiempirical models to correlate the solubility data46,47,48,49,50,51,52,53,54,55,56,57.
In this study, a static analysis procedure was employed to determine the solubility of KTZ in SC-CO2 at different temperature and pressure conditions with and without cosolvent. The solubility of KTZ in the SC-CO2 with cosolvent has not been experimentally measured so far. Moreover, drug solubility in the SC-CO2 (i.e., the binary system) was correlated by ten semi-empirical models, including Chrastil28, Sparks et al.34, Bian et al.41, Bartle et al.51, MST32, Kumar-Johnston29, Jouyban et al.33, as well as Sodeifian et al.14, models. Also, MST32, González et al.52, Soltani-Mazloumi49, and Sodeifian-Sajadian58 models were applied to fit the solubility data of KTZ in the ternary system. Finally, the ability of different models was investigated in terms of three statistical measures: AARD, %, and Radj.
Experimental
Materials
In this work, Fadak Company (Kashan: Iran) provided carbon dioxide (CAS Number 124-38-9) with the purity of 99.99%. KTZ with the purity of 99% (CAS Number 65277-42-1) was provided by Arasto Pharmaceutical Company (Tehran, Iran). The above materials were applied with no additional treatment. Also, menthol with the purity (Ph Eur) of 99.0% (CAS Number 2216-51-5) and methanol (GC) at the purity level of 99% (CAS Number 67-56-1) were purchased from Merck (German). Tables 1 presents the structures and physicochemical features of KTZ.
Experimental apparetus
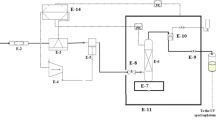
Figure 1 shows the laboratory setup used for determining solubility data of KTZ in SC-CO2 with/without cosolvent (static method). The experimental setup was completely explained in our previous paper58. It includes a carbon dioxide tank, filter, refrigerator unit, reciprocating pump equipped with air compressor for supplying driving force, solubility cells, pressure gauge, digital pressure transmitter, digital thermometer, oven, microliter valve, sample collector, flow meter, 1/8" piping, and connections. Pressure quantities were recorded at the accuracy of ± 0.1 MPa using both the pressure gauge (WIKA, Germany, Code EN 837-1) and pressure transmitter. To maintain the experimental temperature, the equilibrium cell was located in a precise oven (Froilabo Model, AE-60, France), which could retain the temperature within ± 0.1 K.
The amount of menthol and drug in saturator cell 1 (S1) and saturator cell 2 (S2) were 5 and 2 g, respectively. A magnetic stirrer (100 rpm) was applied to accelerate the equilibration and improve saturation of the particles in cells. The sintered filter was put on the top of the column to prevent the escape of menthol particles (as either powder or liquid droplets). In this research, the equilibrium time was considered 60 min (as determined by preliminary experiments). At the end of static time, 600 ± 0.6% µL of the saturated SC-CO2 was depressurized into the collection vial containing 5 ml methanol. Eventually, the loop was washed with the solvent collected in the collection vial, and the final volume of the solution was adjusted to 5 mL ± 0.6%. It should be noted that the experiments were carried out in triplicates. Consequently, the solubility of KTZ was determined by measuring the absorbance at \({\lambda }_{max}\), 220 nm (at which menthol wavelength is transparent) on the UNICO-4802 UV–Vis spectro-photometer with 1-cm pass length quartz cells. Finally, the calibration curve (with regression coefficient 0.996) was applied to obtain the medicine concentrations in the collection vial.
As presented in Tables 2 and 3, solubilities of KTZ (in the equilibrium mole fraction of the solute (y) and the grams of solute (S) per liter of SC-CO2 with/without cosolvent) were evaluated at the pressure range of 12–30 MPa and temperature range of 308–338 K. Finally, Span–Wagner equation was used to obtain the CO2 density59.
Results and discussion
Binary system
In our previous study, the reliability of the solubility setup was evaluated by determining the solubility of naphthalene and alpha-tocopherol in SC-CO2 at different pressures and temperatures and comparing them with the corresponding data in the literature60. In general, the authors systematically check and calibrate the device before testing naphthalene and alpha-tocopherol solubilities in SC-CO2.
It should be mentioned that the mole fraction and solubility (S(g/L)) of KTZ in SC-CO2 were measured at different temperature and pressure conditions (Table 2). Each experimental data was measured in triplicate to enhance the data reliability. The relative standard uncertainty of the solubility data was below 0.05. The relative standard uncertainty (Us) can be calculated by the following equation:
where \(S\left({y}_{k}\right)\) and n are the experimental standard deviation and the number of measurements of each experimental data (n = 3, in this work), respectively.
y and S (g/ L) values respectively ranged between 0.20 × 10–6 and 8.02 × 10–4, and 0.001 and 0.784. Finally, the greatest and least values of KTZ solubility were observed at (338 K, 30 MPa) and (338 K, 12 MPa), respectively.
Figure 2a shows an increase in the solubility of KTZ with pressure increment at each isotherm. An enhancement in the density also increased the solubility at the elevated pressures. Generally, SC-CO2 density and solute vapor pressure are the two key factors contributing to the solubility of the solute in SC-CO2. The solubility showed an ascending trend with increasing density and solute vapor pressure. At pressures below the crossover region, where the influence of increased solvent density on the solute solubility dominates over decreased solute vapor pressure, the solid solute exhibited higher solubility at lower temperatures rather than higher ones. At the top of the crossover region, when temperature increased, the solubility incremented more rapidly with pressure enhancement, which might be due to the competing effects of the reduction of SC-CO2 density and the increase of solute vapor pressure.
Figure 2a presents a pressure range of 19–20 MPa that was considered as the crossover pressure area for KTZ in the binary system. In general, several studies demonstrated that the solute vapor pressure and SC-CO2 density are the major parameters below and top of the crossover area26,60,61,62,63. Yamini and Moradi1 measured KTZ solubility in SC-CO2 at 12.2–35.5 MPa and 308–348 K considering the absorbance at \({\lambda }_{max}\) (220 nm). In the present work, the mole fraction of KTZ dissolved in SC-CO2 (in pressure and temperature spans of 12–30 MPa and 308–338 K) was 0.20 × 10–6 and 8.02 × 10–5. Their solubility data at this condition ranged from 0.7 × 10–6 to 8.16 × 10–5. The mean standard deviation between their experimental data and the present work was 2%. The effects of temperature and pressure on the solubility were the same for both works.
Ternary systems
Figure 2b and Table 3 report KTZ solubility in SC-CO2 with cosolvent (menthol) under different pressures and temperatures. Accordingly, solubility based on the solute mole fraction (y) ranged from 1.2 × 10–5 to 1.96 × 10–4. Each experimental data was measured three times to enhance the reliability of the solubility data. Figure 2b presents an increase in KTZ solubility with the pressure increment at all isotherms. The increase of density with rising the pressure led to the more powerful solvation ability of SC-CO2 and thus enhanced the solid solubility. The largest increment in solubility with rising pressure was observed at the highest temperature, which can be assigned to the impacts of the temperatures and pressures on the solvent density and pressure of the solute vapor10. As stated previously, temperature influences the solvating power by two challenging factors: the solvent density and pressure of the solute vapor. Therefore, an increment in temperature will decrease the solubility below the crossover pressure area and also increased the solubility above the crossover pressure area. Finally, the crossover point in the ternary system was between 13 and 15 MPa.
In the ternary system (solute-SC-CO2-cosolvent), the enhancement factor has been considered to study the cosolvent effect. This factor is the ratio of the obtained solubility of solute within the ternary system to that of the binary system. By investigating the presented results in Table 3, it can be founded out that the solubility was increased by adding menthol to SC-CO2. The cosolvent effect “e” was applied to better evaluate the solubility enhancement 7,64:
Table 3 presents the values of "\(e"\) in this study. The highest cosolvent effect was (61.2-fold) is related to the pressure of 12 MPa and a temperature of 338 K. Other researchers also reported the cosolvent effect in their studies. Hosseini et al.12, compared the solubility of clozapine and lamotrigine in SC-CO2 (with solid cosolvent (menthol)) with the cosolvent-free condition. The solubility of clozapine showed an approximate 56-fold enhancement while that of lamotrigine was increased almost 8 times. Sabet et al.65 measured acetaminophen solubility in SC-CO2 with and without menthol solid cosolvent under different temperatures and pressures. As shown by the results, menthol strongly augmented acetaminophen solubility by (8.27-fold). Gupta and Thakur66 investigated the solubility of phenytoin in SC-CO2. They concluded that solid solute solubility in SC-CO2 is only 3 µmol/mol while its solubility increased to 1302 µmol/mol (at 45 °C and at 196 bar) in SC-CO2 with menthol solid cosolvent. Notably, interactions between menthol and phenytoin resulted in a 400-fold solubility enhancement. Sodeifian and Sajadian10 determined the solubility of letrozole under different circumstances in SC-CO2 with and without menthol. Solid co-solvent could increase letrozole solubility up to 7.1 folds compared to the binary system (without solid cosolvent).
In general, the increase in the solubility of solids in ternary systems (CO2 + cosolvent) can be attributed to the increase in solvent density, dipole–dipole interactions, and also hydrogen bonding between the solute and the cosolvent67. In this case, upon adding menthol to the cell, the density of the SCF enhanced, leading to an increment in the solubility. The polarity of SC-CO2 can also be affected by the cosolvent. Menthol enhanced the solubility of KTZ in CO2 due to the presence of a hydroxyl (polar) group and a hydrocarbon group (nonpolar) in the respective structures. As a result, it can be concluded that stronger attractive polar interaction and hydrogen bonding could lead to greater solubility. Also, by comparing values of e in Table 3, it can be inferred that cosolvent effects decreased with the increment of the pressure, which is compatible with the published studies4,5,6,11.
Correlation of the binary system
The present study considered ten semiempirical equations for correlating KTZ solubility in SC-CO2, as listed in Table 4. Figure 3 depicts the outputs of the correlation at different temperatures. Then, statistical criteria were employed to investigate the abilities of semiempirical models. As a general rule, the more adjustable parameters lead to more accurate correlations. To provide a reliable accuracy criterion to compare the models with different numbers of adjustable parameters, AARD and Radj with the following equations were used68:
So that Z represents the number of the adjustable variables for each model.
where N refers to the numbers of data points in each set. Moreover, Q stands for the numbers of the independent variables in each equation. Radj can be used to compare models with different numbers of independent variables and R2 represents the correlation coefficient69.
where SSE is the error of the sum of squares and SST is the total sum of squares.
Correlation results and optimal values of the parameters are presented in Table 5. The mean-values of AARD% for Chrastil, Sparks et al., K-J, Bian et al., Bartle et al., MST, Jouyban et al., and Sodeifian et al., models were 10.01, 11.52, 09.93, 09.22, 07.55, 09.61, 15.11 and 06.94%, respectively. According to the ANOVA results, it can be concluded that Bian et al., (Radj = 0.991), MST (Radj = 0.996), and Sodeifian et al., (Radj = 0.999) models could more accurately correlate KTZ solubility (Fig. 3).
The energy term describing the temperature term coefficient in Chrastil, Sparks et al., and Bartle et al., models were considered to determine the heat of solvation (ΔHsol.), the vaporization heat of the solute (ΔHvap.), and total heat (ΔHt). The second tunable variables of Chrastil, Sparks et al., and Bartle et al., models were used to calculate ΔHt and ΔHvap, respectively. Also, ∆Hsol was calculated based on the difference between ∆Hvap and ∆Htotal. Based on Table 6, the enthalpy of KTZ dissolution in SC-CO2 and ∆Htotal were 99.32 and 101.70 kJ.mol−1, respectively. Also, ∆Hvap. was calculated by Bartle et al., as 121.80 kJ.mol−1. According to our data, solvation and vaporization processes are endothermic and exothermic, respectively. The value of ∆Hvap was bigger than ∆Htotal. Due to differences between ΔHtotal and ΔHvap values, ΔHsol values from different models were calculated − 22.48 and − 20.10 kJ.mol−1.
Correlation of the ternary system
The present research assessed the correlation of KTZ solubilities in SC-CO2 with menthol by five semiempirical models (Table 7). Menthol solubility in SC-CO2 was reported in previous work58. The statistical criteria (i.e., Radj and AARD%) were applied to examine the capability of the presented models. A genetic algorithm was also used to obtain adjustable parameters. Figure 4 and Table 8 present the compatibility of KTZ solubility data with the semiempirical results. The highest accuracy was offered by the Sodeifian and Sajadian model (AARD,% = 06.45, Radj = 0.995), followed by those of González et al. (AARD,% = 07.51, Radj = 0.991), MST (AARD,% = 08.97, Radj = 0.986) and Soltani and Mazloumi (AARD,% = 07.09, Radj = 0.992), respectively.
Conclusions
In this research, the KTZ solubility in SC-CO2 (with and without menthol) was experimentally measured at the temperature range of 308–338 K and the pressure range of 12–30 MPa using spectrophotometric analysis. The tests were carried out in triplicates to enhance the reliability of the solubility data. Moreover, the mole fractions(y) and KTZ solubility (S (g/ L)) in SC-CO2 (binary system) ranged between 0.001 and 0.784 and 0.2 × 10–6 and 8.02 × 10–5, while the mole fractions of the drug in the SC-CO2 with cosolvent (i.e., the ternary system) ranged in 1.2 × 10–5–1.96 × 10–4. Therefore, it can be concluded that the solubility increased in the presence of menthol. Several semi-empirical and empirical models were utilized for correlating experimental results of binary and ternary systems. Among them, Sodeifian et al. model managed to correlate the experimental data for the mentioned binary system at higher accuracy. In the case of the ternary system, the highest accuracy was provided by the Sodeifian and Sajadian model.
References
Yamini, Y. & Moradi, M. Measurement and correlation of antifungal drugs solubility in pure supercritical CO2 using semiempirical models. J. Chem. Thermodyn. 43, 1091–1096. https://doi.org/10.1016/j.jct.2011.02.020 (2011).
Maniruzzaman, M. et al. Development and optimization of ketoconazole oral strips by means of continuous hot-melt extrusion processing. J. Pharm. Pharmacol. 68, 890–900. https://doi.org/10.1111/jphp.12569 (2016).
Manna, L. & Banchero, M. Solubility of tolbutamide and chlorpropamide in supercritical carbon dioxide. J. Chem. Eng. Data 63, 1745–1751. https://doi.org/10.1021/acs.jced.8b00050 (2018).
Foster, N. R., Singh, H., Yun, S. J., Tomasko, D. L. & Macnaughton, S. J. Polar and nonpolar cosolvent effects on the solubility of cholesterol in supercritical fluids. Ind. Eng. Chem. Res. 32, 2849–2853. https://doi.org/10.1021/ie00023a056 (1993).
Ekart, M. P. et al. Cosolvent interactions in supercritical fluid solutions. AIChE J. 39, 235–248. https://doi.org/10.1002/aic.690390206 (1993).
Ting, S. S., Tomasko, D. L., Foster, N. R. & Macnaughton, S. J. Solubility of naproxen in supercritical carbon dioxide with and without cosolvents. Ind. Eng. Chem. Res. 32, 1471–1481. https://doi.org/10.1021/ie00019a022 (1993).
Huang, Z., Kawi, S. & Chiew, Y. Solubility of cholesterol and its esters in supercritical carbon dioxide with and without cosolvents. J. Supercrit. Fluids 30, 25–39. https://doi.org/10.1016/S0896-8446(03)00116-5 (2004).
Reddy, S. N. & Madras, G. Modeling of ternary solubilities of solids in supercritical carbon dioxide in the presence of cosolvents or cosolutes. J. Supercrit. Fluids 63, 105–114. https://doi.org/10.1016/j.supflu.2011.11.016 (2012).
Koga, Y., Iwai, Y., Hata, Y., Yamamoto, M. & Arai, Y. Influence of cosolvent on solubilities of fatty acids and higher alcohols in supercritical carbon dioxide. Fluid Phase Equilib. 125, 115–128. https://doi.org/10.1016/S0378-3812(96)03090-7 (1996).
Sodeifian, G. & Sajadian, S. A. Solubility measurement and preparation of nanoparticles of an anticancer drug (Letrozole) using rapid expansion of supercritical solutions with solid cosolvent (RESS-SC). J. Supercrit. Fluids 133, 239–252. https://doi.org/10.1016/j.supflu.2017.10.015 (2018).
Huang, Z., Lu, W. D., Kawi, S. & Chiew, Y. C. Solubility of aspirin in supercritical carbon dioxide with and without acetone. J. Chem. Eng. Data 49, 1323–1327. https://doi.org/10.1021/je0499465 (2004).
Hosseini, M. H., Alizadeh, N. & Khanchi, A. R. Effect of menthol as solid cosolvent on the solubility enhancement of clozapine and lamorigine in supercritical CO2. J. Supercrit. Fluids 55, 14–22. https://doi.org/10.1016/j.supflu.2010.09.002 (2010).
Sodeifian, G. & Sajadian, S. A. Measuring and Modeling the Solubility of Pharmaceutical Substances for the Production of Nanoparticles Using Supercritical Fluid and Ultrasound Technology. PhD thesis, 211 (2018).
Sodeifian, G., Razmimanesh, F. & Sajadian, S. A. Solubility measurement of a chemotherapeutic agent (Imatinib mesylate) in supercritical carbon dioxide: Assessment of new empirical model. J. Supercrit. Fluids 146, 89–99. https://doi.org/10.1016/j.supflu.2019.01.006 (2019).
Sodeifian, G., Ardestani, N. S., Sajadian, S. A. & Panah, H. S. Experimental measurements and thermodynamic modeling of Coumarin-7 solid solubility in supercritical carbon dioxide: Production of nanoparticles via RESS method. Fluid Phase Equilib. 483, 122–143. https://doi.org/10.1016/j.fluid.2018.11.006 (2019).
Pitchaiah, K. C. et al. Experimental measurements and correlation of the solubility of N, N-dialkylamides in supercritical carbon dioxide. J. Supercrit. Fluids 143, 162–170. https://doi.org/10.1016/j.supflu.2018.08.007 (2019).
Sodeifian, G., Ardestani, N. S. & Sajadian, S. A. Solubility measurement of a pigment (Phthalocyanine green) in supercritical carbon dioxide: Experimental correlations and thermodynamic modeling. Fluid Phase Equilib. 494, 61–73. https://doi.org/10.1016/j.fluid.2019.04.024 (2019).
Sodeifian, G., Saadati Ardestani, N., Sajadian, S. A. & Soltani Panah, H. Experimental measurements and thermodynamic modeling of Coumarin-7 solid solubility in supercritical carbon dioxide: Production of nanoparticles via RESS method. Fluid Phase Equilib. 483, 122–143. https://doi.org/10.1016/j.fluid.2018.11.006 (2019).
Sodeifian, G., Drakhsheshpoor, R. & Sajadian, S. A. Experimental study and thermodynamic modeling of Esomeprazole (proton-pump inhibitor drug for stomach acid reduction) solubility in supercritical carbon dioxide. J. Supercrit. Fluids https://doi.org/10.1016/j.supflu.2019.104606 (2019).
Sodeifian, G., Hazaveie, S. M., Sajadian, S. A. & Saadati Ardestani, N. Determination of the solubility of the repaglinide drug in supercritical carbon dioxide: Experimental data and thermodynamic modeling. J. Chem. Eng. Data 64, 5338–5348. https://doi.org/10.1021/acs.jced.9b00550 (2019).
Reddy, S. N. & Madras, G. Measurement and correlation of quaternary solubilities of dihydroxybenzene isomers in supercritical carbon dioxide. J. Supercrit. Fluids 73, 63–69. https://doi.org/10.1016/j.supflu.2012.11.003 (2013).
Shamsipur, M., Karami, A. R., Yamini, Y. & Sharghi, H. Solubilities of some 1-hydroxy-9, 10-anthraquinone derivatives in supercritical carbon dioxide. J. Supercrit. Fluids 32, 47–53. https://doi.org/10.1016/j.supflu.2004.01.006 (2004).
Sodeifian, G., Sajadian, S. A. & Ardestani, N. S. Optimization of essential oil extraction from Launaea acanthodes Boiss: Utilization of supercritical carbon dioxide and cosolvent. J. Supercrit. Fluids 116, 46–56. https://doi.org/10.1016/j.supflu.2016.05.015 (2016).
Sodeifian, G., Sajadian, S. A. & Ardestani, N. S. Experimental optimization and mathematical modeling of the supercritical fluid extraction of essential oil from Eryngium billardieri: Application of simulated annealing (SA) algorithm. J. Supercrit. Fluids 127, 146–157. https://doi.org/10.1016/j.supflu.2017.04.007 (2017).
Sodeifian, G. & Sajadian, S. A. Investigation of essential oil extraction and antioxidant activity of Echinophora platyloba DC. using supercritical carbon dioxide. J. Supercrit. Fluids 121, 52–62. https://doi.org/10.1016/j.supflu.2016.11.014 (2017).
Sodeifian, G., Sajadian, S. A. & Daneshyan, S. Preparation of Aprepitant nanoparticles (efficient drug for coping with the effects of cancer treatment) by rapid expansion of supercritical solution with solid cosolvent (RESS-SC). J. Supercrit. Fluids 140, 72–84. https://doi.org/10.1016/j.supflu.2018.06.009 (2018).
Sodeifian, G., Sajadian, S. A., Razmimanesh, F. & Ardestani, N. S. A comprehensive comparison among four different approaches for predicting the solubility of pharmaceutical solid compounds in supercritical carbon dioxide. Korean J. Chem. Eng. 35, 2097–2116. https://doi.org/10.1007/s11814-018-0125-6 (2018).
Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 86, 3016–3021. https://doi.org/10.1021/j100212a041 (1982).
Kumar, S. K. & Johnston, K. P. Modelling the solubility of solids in supercritical fluids with density as the independent variable. J. Supercrit. Fluids 1, 15–22. https://doi.org/10.1016/0896-8446(88)90005-8 (1988).
Del Valle, J. M. & Aguilera, J. M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res. 27, 1551–1553. https://doi.org/10.1021/ie00080a036 (1988).
Gordillo, M., Blanco, M., Molero, A. & De La Ossa, E. M. Solubility of the antibiotic Penicillin G in supercritical carbon dioxide. J. Supercrit. Fluids 15, 183–190. https://doi.org/10.1016/S0896-8446(99)00008-X (1999).
Mendez-Santiago, J. & Teja, A. S. Solubility of solids in supercritical fluids: Consistency of data and a new model for cosolvent systems. Ind. Eng. Chem. Res. 39, 4767–4771. https://doi.org/10.1021/ie000339u (2000).
Jouyban, A., Chan, H.-K. & Foster, N. R. Mathematical representation of solute solubility in supercritical carbon dioxide using empirical expressions. J. Supercrit. Fluids 24, 19–35. https://doi.org/10.1016/S0896-8446(02)00015-3 (2002).
Sparks, D. L., Hernandez, R. & Estévez, L. A. Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model. Chem. Eng. Sci. 63, 4292–4301. https://doi.org/10.1016/j.ces.2008.05.031 (2008).
Chapman, W. G., Gubbins, K. E., Jackson, G. & Radosz, M. SAFT: Equation-of-state solution model for associating fluids. Fluid Phase Equilib. 52, 31–38. https://doi.org/10.1016/0378-3812(89)80308-5 (1989).
Economou, I. G., Gregg, C. J. & Radosz, M. Solubilities of solid polynuclear aromatics (PNA’s) in supercritical ethylene and ethane from statistical associating fluid theory (SAFT): Toward separating PNA’s by size and structure. Ind. Eng. Chem. Res. 31, 2620–2624. https://doi.org/10.1021/ie00011a028 (1992).
McCabe, C. & Jackson, G. SAFT-VR modelling of the phase equilibrium of long-chain n-alkanes. Phys. Chem. Chem. Phys. 1, 2057–2064. https://doi.org/10.1039/A808085B (1999).
Gross, J. & Sadowski, G. Perturbed-chain SAFT: An equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 40, 1244–1260. https://doi.org/10.1021/ie0003887 (2001).
Hosseini Anvari, M. & Pazuki, G. A study on the predictive capability of the SAFT-VR equation of state for solubility of solids in supercritical CO2. J. Supercrit. Fluids 90, 73–83. https://doi.org/10.1016/j.supflu.2014.03.005 (2014).
Peng, D.-Y. & Robinson, D. B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 15, 59–64. https://doi.org/10.1021/i160057a011 (1976).
Bian, X.-Q., Zhang, Q., Du, Z.-M., Chen, J. & Jaubert, J.-N. A five-parameter empirical model for correlating the solubility of solid compounds in supercritical carbon dioxide. Fluid Phase Equilib. 411, 74–80. https://doi.org/10.1016/j.fluid.2015.12.017 (2016).
Sodeifian, G., Sajadian, S. A. & Derakhsheshpour, R. Experimental measurement and thermodynamic modeling of Lansoprazole solubility in supercritical carbon dioxide: Application of SAFT-VR EoS. Fluid Phase Equilib. 507, 112422. https://doi.org/10.1016/j.fluid.2019.112422 (2020).
Sodeifian, G., Razmimanesh, F., Sajadian, S. A. & Panah, H. S. Solubility measurement of an antihistamine drug (loratadine) in supercritical carbon dioxide: Assessment of qCPA and PCP-SAFT equations of state. Fluid Phase Equilib. 472, 147–159. https://doi.org/10.1016/j.fluid.2018.05.018 (2018).
Cabral, V., Santos, W., Muniz, E., Rubira, A. & Cardozo-Filho, L. Correlation of dye solubility in supercritical carbon dioxide. J. Supercrit. fluids 40, 163–169 (2007).
Bian, X.-Q., Zhang, Q., Zhang, L. & Chen, J. A grey wolf optimizer-based support vector machine for the solubility of aromatic compounds in supercritical carbon dioxide. Chem. Eng. Res. Des. 123, 284–294. https://doi.org/10.1016/j.cherd.2017.05.008 (2017).
Jouyban, A., Khoubnasabjafari, M. & Chan, H.-K. Modeling the entrainer effects on solubility of solutes in supercritical carbon dioxide. Chem. Pharm. Bull. 53, 290–295. https://doi.org/10.1248/cpb.53.290 (2005).
Keshmiri, K., Vatanara, A. & Yamini, Y. Development and evaluation of a new semi-empirical model for correlation of drug solubility in supercritical CO2. Fluid Phase Equilib. 363, 18–26. https://doi.org/10.1016/j.fluid.2013.11.013 (2014).
Garlapati, C. & Madras, G. New empirical expressions to correlate solubilities of solids in supercritical carbon dioxide. Thermochim. Acta 500, 123–127. https://doi.org/10.1016/j.tca.2009.12.004 (2010).
Soltani, S. & Mazloumi, S. H. A new empirical model to correlate solute solubility in supercritical carbon dioxide in presence of co-solvent. Chem. Eng. Res. Des. 125, 79–87. https://doi.org/10.1016/j.cherd.2017.07.006 (2017).
Reddy, T. A. & Garlapati, C. Dimensionless empirical model to correlate pharmaceutical compound solubility in supercritical carbon dioxide. Chem. Eng. Technol. 42, 2621–2630. https://doi.org/10.1002/ceat.201900283 (2019).
Bartle, K. D., Clifford, A. A., Jafar, S. A. & Shilstone, G. F. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide. J. Phys. Chem. Ref. Data 20, 713–756. https://doi.org/10.1063/1.555893 (1991).
González, J. C., Vieytes, M. R., Botana, A. M., Vieites, J. M. & Botana, L. M. Modified mass action law-based model to correlate the solubility of solids and liquids in entrained supercritical carbon dioxide. J. Chromatogr. A 910, 119–125. https://doi.org/10.1016/S0021-9673(00)01120-1 (2001).
Méndez-Santiago, J. & Teja, A. S. The solubility of solids in supercritical fluids. Fluid Phase Equilib. 158, 501–510. https://doi.org/10.1016/S0378-3812(99)00154-5 (1999).
Sodeifian, G., Nasri, L., Razmimanesh, F. & Abadian, M. Measuring and modeling the solubility of an antihypertensive drug (losartan potassium, Cozaar) in supercritical carbon dioxide. J. Mol. Liquids https://doi.org/10.1016/j.molliq.2021.115745 (2021).
Sodeifian, G., Garlapati, C., Razmimanesh, F. & Sodeifian, F. Solubility of amlodipine besylate (calcium channel blocker drug) in supercritical carbon dioxide: Measurement and correlations. J. Chem. Eng. Data 66, 1119–1131. https://doi.org/10.1021/acs.jced.0c00913 (2021).
Sodeifian, G., Razmimanesh, F., Ardestani, N. S. & Sajadian, S. A. Experimental data and thermodynamic modeling of solubility of Azathioprine, as an immunosuppressive and anti-cancer drug, in supercritical carbon dioxide. J. Mol. Liq. 299, 112179. https://doi.org/10.1016/j.molliq.2019.112179 (2020).
Sodeifian, G., Alwi, R. S., Razmimanesh, F. & Tamura, K. Solubility of quetiapine hemifumarate (antipsychotic drug) in supercritical carbon dioxide: Experimental, modeling and hansen solubility parameter application. Fluid Phase Equilib. https://doi.org/10.1016/j.fluid.2021.113003 (2021).
Sodeifian, G. & Sajadian, S. A. Experimental measurement of solubilities of sertraline hydrochloride in supercriticalcarbon dioxide with/without menthol: Data correlation. J. Supercrit. Fluids 149, 79–87. https://doi.org/10.1016/j.supflu.2019.03.020 (2019).
Span, R. & Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 25, 1509–1596. https://doi.org/10.1063/1.555991 (1996).
Sodeifian, G., Sajadian, S. A. & Ardestani, N. S. Determination of solubility of Aprepitant (an antiemetic drug for chemotherapy) in supercritical carbon dioxide: Empirical and thermodynamic models. J Supercrit. Fluids 128, 102–111. https://doi.org/10.1016/j.supflu.2017.05.019 (2017).
Wang, X. et al. Characterization and stability of tanshinone IIA solid dispersions with hydroxyapatite. Materials 6, 805–816 (2013).
Sodeifian, G., Sajadian, S. A. & Razmimanesh, F. Solubility of an antiarrhythmic drug (amiodarone hydrochloride) in supercritical carbon dioxide: Experimental and modeling. Fluid Phase Equilib. 450, 149–159. https://doi.org/10.1016/j.fluid.2017.07.015 (2017).
Perrotin-Brunel, H. et al. Solubility of Δ 9-tetrahydrocannabinol in supercritical carbon dioxide: Experiments and modeling. J. Supercrit. Fluids 52, 6–10. https://doi.org/10.1016/j.supflu.2009.12.001 (2010).
Thakur, R. & Gupta, R. B. Rapid expansion of supercritical solution with solid cosolvent (RESS−SC) process: Formation of griseofulvin nanoparticles. Ind. Eng. Chem. Res. 44, 7380–7387. https://doi.org/10.1021/ie050417j (2005).
Sabet, J. K., Ghotbi, C., Dorkoosh, F. & Striolo, A. Solubilities of acetaminophen in supercritical carbon dioxide with and without menthol cosolvent: Measurement and correlation. Sci. Iran. 19, 619–625 (2012).
Thakur, R. & Gupta, R. B. Formation of phenytoin nanoparticles using rapid expansion of supercritical solution with solid cosolvent (RESS-SC) process. Int. J. Pharm. 308, 190–199. https://doi.org/10.1016/j.ijpharm.2005.11.005 (2006).
Bitencourt, R. G., Palma, A. M., Coutinho, J. A., Cabral, F. A. & Meirelles, A. J. Prediction of solid solute solubility in supercritical CO2 with cosolvents using the CPA EoS. Fluid Phase Equilib. 482, 1–10. https://doi.org/10.1016/j.fluid.2018.10.020 (2019).
Jouyban, A. et al. Solubility prediction in supercritical CO2 using minimum number of experiments. J. Pharm. Sci. 91, 1287–1295. https://doi.org/10.1002/jps.10127 (2002).
Montgomery, D. C. Design and analysis of experiments (John wiley & sons, 2017).
Acknowledgements
Hereby, the researchers thank the great financial supports supplied by the research deputy of University of Kashan for supporting the present applied, beneficial, and worthwhile plan (Grant # Pajoohaneh-1398/9). Moreover, the researchers appreciate the Arasto Pharmaceutical Company.
Author information
Authors and Affiliations
Contributions
G.S.: Conceptualization, Methodology, Validation, Investigation, Supervision, Writing—review & editing. S.A.S.: Methodology, Investigation, Project administration. F.R.: Writing- original draft, Investigation, Resources. S.M.H.: Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sodeifian, G., Sajadian, S.A., Razmimanesh, F. et al. Solubility of Ketoconazole (antifungal drug) in SC-CO2 for binary and ternary systems: measurements and empirical correlations. Sci Rep 11, 7546 (2021). https://doi.org/10.1038/s41598-021-87243-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87243-6
This article is cited by
-
Studies on solubility measurement of codeine phosphate (pain reliever drug) in supercritical carbon dioxide and modeling
Scientific Reports (2023)
-
Solubility measurement and modeling of hydroxychloroquine sulfate (antimalarial medication) in supercritical carbon dioxide
Scientific Reports (2023)
-
Para-Hydroxy Benzoic Acid Coformer Enable Enhanced Solubility, Dissolution, and Antifungal Activity of Ketoconazole Cocrystals
Journal of Pharmaceutical Innovation (2023)
-
Utilization of CO2 in supercritical conditions for the synthesis of cyclic poly (N-isopropylacrylamide) via emulsion and homogeneous reactions
Scientific Reports (2022)
-
Solubility measurement and thermodynamic modeling of pantoprazole sodium sesquihydrate in supercritical carbon dioxide
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.