Abstract
Mean arterial pressure to mean pulmonary arterial pressure ratio (mAP/mPAP) has been identified as a strong predictor of perioperative complications in cardiac surgery. We therefore investigated the pharmacokinetic/pharmacodynamic (PK/PD) relationship of inhaled milrinone in these patients using this ratio (R) as a PD marker. Following approval by the ethics and research committee and informed consent, we performed the following experiment. Before initiation of cardiopulmonary bypass in 28 pulmonary hypertensive patients scheduled for cardiac surgery, milrinone (5 mg) was nebulized, plasma concentrations measured (up to 10 h) and compartmental PK analysis carried out. Baseline (R0) and peak (Rmax) ratios as well as magnitude of peak response (∆Rmax-R0) were measured. During inhalation, individual area under effect-time (AUEC) and plasma concentration–time (AUC) curves were correlated. Potential relationships between PD markers and difficult separation from bypass (DSB) were explored. In this study, we observed that milrinone peak concentrations (41–189 ng ml−1) and ΔRmax-R0 (− 0.12–1.5) were obtained at the end of inhalation (10–30 min). Mean PK parameters agreed with intravenous milrinone published data after correction for the estimated inhaled dose. Paired comparisons yielded a statistically significant increase between R0 and Rmax (mean difference, 0.58: 95% CI 0.43–0.73; P < 0.001). Individual AUEC correlated with AUC (r = 0.3890, r2 = 0.1513; P = 0.045); significance increased after exclusion of non-responders (r = 4787, r2 = 0.2292; P = 0.024). Individual AUEC correlated with ∆Rmax-R0 (r = 5973, r2 = 0.3568; P = 0.001). Both ∆Rmax-R0 (P = 0.009) and CPB duration (P < 0.001) were identified as predictors of DSB. In conclusion, both magnitude of peak response of the mAP/mPAP ratio and CPB duration were associated with DSB.
Similar content being viewed by others
Introduction
Cardiopulmonary bypass (CPB) is performed during cardiac surgery in order to maintain perfusion and oxygenation to all organs, besides the heart and lungs. Hemodynamic complications associated with difficult separation from bypass (DSB)1 represent a leading cause of mortality in cardiac surgery2. Pulmonary hypertension (PH) that can lead to right ventricular dysfunction was identified as one of the most important hemodynamic predictor and risk factor for DSB3,4. Amongst other hemodynamic parameters used in cardiac surgery, the mean artery pressure (mAP) to mean pulmonary artery pressure (mPAP) ratio has proved to be a predictor of perioperative complications5,6,7,8,9,10. In addition, the successful effect of inhaled therapy is expected to be associated with an increase in mAP/mPAP ratio and normalization of right ventricular function11,12,13. The mAP/mPAP ratio (R) remains unchanged following induction of general anesthesia5 and correlates with the eccentricity index which reflects the interventricular septal deformation in response to PH14.
Intravenous milrinone is commonly used for the treatment of PH when DSB occurs at the end of cardiac surgery15,16,17,18. An important drawback of intravenous milrinone is its association with systemic hypotension19,20,21. Therefore, inhalation has been proposed as an alternative route of administration for milrinone22,23,24. Inhaled milrinone administered before CPB has also been proposed as having a protective effect during cardiac surgery11,25,26,27 and a potential to facilitate separation from CPB in patients with PH28. In a clinical trial report, mAP/mPAP ratio was used as a pharmacodynamic (PD) marker to explore the relationship between milrinone concentration and effect exposures during inhalation period29. A relationship was found, but inhaled milrinone did not prove to facilitate separation from CPB. In that study, limited blood sampling did not allow full characterization of a pharmacokinetic (PK) and PD profile and, most importantly, a good estimation of the time point corresponding to peak concentration.
This report on inhaled milrinone will present results obtained from a full-scale PK/PD study in cardiac patients undergoing CPB having two major objectives: characterization of inhaled milrinone PKs and exploration of the concentration-effect relationship. As exploratory objectives, we wanted to identify potential predictors of DSB.
Results
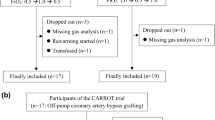
A total of 28 patients were recruited. Demographic and perioperative data are shown in Table 1. Important events and cutoff times used during data analysis are presented on a typical cardiac surgical procedure flowchart (Fig. 1). An example of PK and PD profiles obtained in a responder is shown in Fig. 2.
Pharmacokinetic study
PK sampling
Individual milrinone plasma concentration–time profiles during inhalation (A) (10–30 min) and overall from 0 to 10 h after inhalation (B) are presented in Fig. 3. One patient was scheduled to receive elective cardiac surgery but did not undergo CPB (intraoperative decision) and was only considered for PK analysis during the inhalation period. Milrinone average treatment time was 17 ± 6 min, ranging from 10 to 30 min. Mean nebulization rate was 0.086 ± 0.044 mg min−1 (0.021–0.237 mg min−1). Overall, Cmax values ranged between 41 and 189 ng ml−1 and were observed at the end of inhalation. In all 28 patients’ plasma concentrations were quantifiable up to 10 h after termination of inhalation.
Inhaled dose
Mean percentages of dose recovered from individual in vivo results combined with previously determined mean in vitro results indicated almost complete recovery (95.3% ± 10.7%) of milrinone nominal dose (5 mg) (Supplementary Table S1). In patients (n = 15), mean cumulative amount of milrinone excreted in urine over a 24-h period was 1.29 ± 0.41 mg (25.8% of the 5 mg nominal dose) while the mean estimated inhaled dose using the back-calculated approach was 1.52 ± 0.32 mg (30.5%). As corresponding values did not differ (mean difference, 0.23 mg: 95% CI − 0.06 to 0.53; P = 0.112), this back-calculated approach for the estimation of the inhaled dose was considered acceptable for milrinone and used for PK analysis in all patients (n = 28).
PK analysis
Mean PK parameter estimates obtained after fitting to data a two-compartment model (1/ŷ) with a zero-order input rate during the nebulization period are presented in Table 2. Mean terminal elimination half-life was 154 ± 17 min. The milrinone systemic exposure or AUC was found to be inversely proportional to the nebulization rate (r = 0.4728, r2 = 0.2235; P = 0.011). The non-compartmental analysis is summarized in Supplementary Table S2.
Pharmacodynamic study
PD markers
One patient was not considered for PD analysis after unsuccessful Swan-Ganz installation. For all patients, paired comparisons between R0, Rmax and Rpost-CPB yielded a statistically significant increase between R0 and Rmax (mean difference, 0.58: 95% CI 0.43 to 0.73; P < 0.001) representing a mean increase from baseline of 26.6% but not between R0 and Rpost-CPB (mean difference, 0.10: 95% CI − 0.12 to 0.33; P = 0.358) with a less substantial mean increase from baseline of 4.7% (Fig. 4A). Using a simple logistic regression, ∆Rmax-R0 was found to be directly related to the clinical endpoint DSB (P = 0.009) (Fig. 4B). When patients were categorized according to the occurrence of DSB, ∆Rmax-R0 was 0.37 (17.4%) in patients with DSB compared to 0.71 (31.3%) in patients without DSB (mean difference, 0.34: 95% CI 0.07 to 0.61; P = 0.015).
Association between R0 (n = 27), Rmax (n = 27) and Rpost-CPB (n = 25) using one-way repeated measures analysis of variance (ANOVA) (A) and association between ∆Rmax-R0 and DSB (clinical endpoint) using simple logistic regression (B). (Pulmonary artery catheter unavailable in one patient). Mean ± SD **P < 0.001. DSB difficult separation from bypass, mAP mean arterial pressure, mPAP mean pulmonary arterial pressure, R0 baseline mAP/mPAP ratio, Rmax peak mAP/mPAP ratio, Rpost-CPB post-CPB mAP/mPAP ratio, ∆Rmax-R0 magnitude of peak response.
PK/PD analysis
During the inhalation period, the relationship between AUEC and AUC was best explained by a linear regression model (r = 0.3890, r2 = 0.1513; P = 0.045) (Fig. 5A). The minimum threshold for therapeutic response in patients, i.e. the AUEC-intercept, was estimated as 1.387. Accordingly, 22 patients out of 27 were considered as responders. The exclusion of non-responders resulted in an improvement of this correlation (r = 4787, r2 = 0.2292; P = 0.024). Finally, the overall exposure to pharmacological response, AUEC, was also correlated with ∆Rmax-R0 (r = 5973, r2 = 0.3568; P = 0.001) (Fig. 5B).
Clinical endpoint
The variables retained during forward analysis are presented in Table 3.
Discussion
This is the largest report on detailed PK/PD of inhaled milrinone in cardiac surgery attempting to characterize inhaled milrinone concentration-effect relationship. When the mAP/mPAP ratio (R) was used as PD marker, magnitude of peak response before CPB (∆Rmax-R0) and CPB duration were both associated with DSB, suggesting that the former may represent a potential prognostic tool for DSB. The ∆Rmax-R0 represents the intensity of the pulmonary antihypertensive effect. The absence of a response might indicate a much more severe and irreversible pulmonary hypertension that may have prognostic value.
Given milrinone small molecular size (MW: 211.2), lipid solubility (log P: 1.17), as well as the large and well-perfused surface area provided by the lungs30, absorption process through the pulmonary route was expected to be extremely rapid (almost instantaneous)31. Indeed, many small molecules have pulmonary bioavailability approaching 100%32,33,34,35, which can be attributed to rapid pulmonary absorption and lower drug-metabolizing activity compared to the oral route36,37,38. After inhaled prochlorperazine, for example, superimposed plasma concentration–time profiles were observed after inhalation of a thermally generated aerosol or intravenous administration in both anesthetized dogs39 and humans40. Accordingly, a two-compartment model with a zero-order input was deemed adequate. In agreement with reports on mesh nebulizers41, milrinone treatment time varied greatly amongst our patients (10–30 min).
Both non-compartmental analysis and compartmental analyses yielded similar PK parameters and agreed with those reported after IV administration in congestive heart failure patients42,43 and patients undergoing cardiac surgery44,45,46, suggesting a rapid and complete pulmonary absorption of the estimated inhaled dose of milrinone in our patients. This observation was also reported by others when comparing PK with those obtained in congestive heart failure patients44 or when milrinone was administered before vs after CPB in cardiac patients47.
Time-specific single point measures of the intensity of effect represented by R0, Rmax, Rpost-CPB, as well as ∆Rmax-R0 have already been used as hemodynamic endpoints in cardiac surgery for patients with PH11. In our patients, the mean increase in Rpost-CPB was not significant at the end of CPB when compared to R0 (P = 0.358). At this time-point, it is difficult to clearly distinguish the effect attributable to milrinone residual pharmacological effect from that induced by hemodynamic changes associated with CPB weaning. It is worth pointing out that both Rmax and Rpost-CPB were opened-chest measures while R0 was determined at closed-chest. For instance, mean value for Rpost-CPB was 2.70 when measured after chest closure compared to 2.27 before chest closure. Thus, estimation of ∆Rmax-R0 mean value remains conservative and may have been higher if Rmax could have been taken under closed-chest conditions. A higher degree of significance for the net maximal effect would be expected under closed-chest conditions. It was felt that, rather than looking at separate measurements over time, a more accurate estimate of the overall effect would be obtained by integrating effect over time48 and that, especially in presence of PD fluctuations49. Therefore, AUEC was used to evaluate the net PD response. A linear relationship between milrinone systemic exposure (AUC) and the corresponding pharmacologic effect exposure (AUEC) during inhalation would represent the first step towards establishment of a potential proof of concept. Such a relationship has recently been explored in a subset of patients from a randomized controlled trial with very limited PK and PD data29 and appeared worth pursuing with a more extensive approach.
Keeping in mind that the overarching goal is to obtain a readily accessible PD marker that would adequately reflect milrinone overall effect during the inhalation period, the significant correlation observed between AUEC and ∆Rmax-R0 (single point) suggests that the overall net effect is in agreement with the magnitude of peak effect (end of inhalation). Accordingly, non-responders showed both low AUEC and low ∆Rmax-R0 values as also observed in a clinical trial29. Other studies on inhaled milrinone administered prior to CPB in cardiac surgery have also observed 18–26% of non-responders amongst their population of pulmonary hypertensive patients11,26,28. Indeed, chronic hypoxia and vascular remodeling is assumed to result in secondary and in some cases fixed pre-capillary PH, which is an independent predictor of mortality50.
Finally, as the occurrence of DSB represents the major clinical endpoint for procedures requiring CPB, several potential predictors were explored using a logistic univariate regression model. AUEC was not retained mostly because these values are not readily computed before CPB. Single point PD markers readily available prior to CPB weaning (R0, Rmax and ∆Rmax-R0) were considered because they are more pragmatic. Only the magnitude of peak response (∆Rmax-R0) and CPB duration remained in the final model. Our results are consistent with prior studies suggesting that inhaled milrinone administered prior to CPB would have a protective effect in pulmonary hypertensive patients11,26 by minimizing CPB-related inflammation27, preventing pulmonary endothelial dysfunction25 and facilitating separation from CPB28. The absence of a response might indicate a much more severe and irreversible pulmonary hypertension that may have prognostic value as suggested by a recent study51. As for CPB duration, it was already known to be a strong risk factor of DSB28. In addition, many other factors are likely to have a role in the etiology of DSB1,52.
The major limitation of this study was the impossibility of modeling each patient's whole set of concentration-effect data because PD data were often contaminated by surgical interventions. Moreover, inclusion criteria allowed a wide range of PH (sPAP 36–90 mmHg) and study population was not quite homogeneous (EuroSCORE 1.2-46.4). Milrinone dose may also have been suboptimal (taking into account the inhaled dose measured) and may require adjustments in further dose-ranging studies. In absence of rich data PK/PD analysis, our sample size may not have been sufficient. Despite this, the magnitude of peak pharmacological response (∆Rmax-R0) and CPB duration were both found to be associated with DSB.
In addition, it is known that the amount of air embolism following cardiac surgery can result in right ventricular failure which can only be quantified using transcranial Doppler52,53 which was not available at the time of the study. The amount of air is unpredictable and could explain why pre-CPB inhaled agents might not always prevent difficult separation from CPB. However, three studies using combined inhaled epoprostenol and inhaled milrinone (iE&iM), we observed that easier separation from CPB was also associated with a significant response to iE&iM treatment observed before CPB51,54 and reduced inotropic support after CPB55. In one of the study51, a higher proportion of non-responders had difficult separation from CPB and required intravenous inotropic drug support compared to responders. Use of intravenous inotropes after CPB was lower in responders to treatment (8.1% vs 27.6%; P = 0.0052). An increase of 20% in the mean arterial pressure to mPAP ratio was used to indicate a positive response to iE&iM. Another limitation of our study is the absence of a control group. A control group with intravenous milrinone would have been useful to demonstrate the hypotensive sparing effect of inhaled milrinone as supported by 4 small, randomized trials comparing inhaled versus intravenous administration24,56,57,58. The inhaled route results in a more slow release of milrinone into the systemic circulation and leads to reduced peak dose as we observed compared to intravenous administration47. This peak dose of milrinone is likely responsible for hypotension. Although a control group is rarely included in PK/PD studies, in this population other factors may influence R between the measurements of R0, Rmax and RCPB. We cannot definitively establish a causal relationship between inhaled milrinone and changes in R. Rmax-R0 may reflect a more complex responsiveness of the pulmonary circulation to inhaled milrinone. Other factors could also influence our results such as limited duration of action or insufficient number of patients.
In summary, this is the first study reporting rich PK and PD data obtained after inhalation of milrinone in cardiac surgical patients. After mesh nebulization, milrinone absorption was extremely rapid and systemic levels remained within the therapeutic range. Both peak concentrations and maximum effects were observed at the end of inhalation. Comparison of respective milrinone AUC and AUEC before CPB provided preliminary evidence of a proof of concept for the use of the mAP/mPAP ratio before CPB as a promising PD marker. The magnitude of peak pulmonary circulatory response (∆Rmax-R0) may be a predictor of DSB. Further randomized controlled studies are required to confirm these findings (NCT05450328).
Materials and methods
Patients
After approval by the institutional research ethics committee (ICM 06-888; August 5, 2008) in accordance with the Enoncé de politique des trois conseils (EPTC2) and the Declaration of Helsinki, and with permission from Health Canada (non-objection letter, ref. 108851; November 2, 2006), the study was registered in ClinicalTrials.gov (ref: NCT01725776). Written informed consent was obtained from 28 patients having preoperative PH and scheduled for elective cardiac surgery under CPB. Patients were considered having PH if either one of the following conditions was met before surgery: systolic pulmonary artery pressure (sPAP) > 35 mmHg or mPAP > 25 mmHg59. Patients with hemodynamic instability prior to surgery were excluded. Procedures were classified as coronary revascularization, valvular surgery or complex, defined as a combination of two or more different procedures. The EuroSCORE II was calculated for each patient60.
Surgical procedure
Patients were premedicated with 1–2 mg lorazepam orally 1 h before surgery and received 0.1 mg kg−1 morphine intramuscularly before entering the operating room where midazolam was given (0.01–0.05 mg kg−1 intravenously) as needed for patient comfort. Usual monitoring was installed, including a 5-lead electrocardiogram, pulse oximeter, peripheral venous line, radial arterial line, 3-lm catheter, and fast-response thermodilution pulmonary artery catheter. Anesthesia was induced with 1 μg kg−1 sufentanil and 0.04 mg kg−1 midazolam, and muscle relaxation achieved with 0.1 mg kg−1 pancuronium. After tracheal intubation, anesthesia was maintained with 1 μg kg−1 h−1 sufentanil and 0.04 mg kg−1 h−1 midazolam. Intravenous fluids (0.9% normal saline) were administered (7 cc kg−1 h−1) during surgery and titrated according to blood pressure and central venous pressure. A transesophageal echocardiography (TEE) omniplane probe was inserted. Institution of CPB was performed using ascending aortic cannulation and bi-caval or double stage cannulation of the right atrium. Intermittent (4:1) blood cardioplegia was administered during CPB; induction and temperatures ranged from 15 to 29 °C. For coronary revascularizations, systemic temperature was allowed to drift to 34 °C, valvular surgeries and complex procedures to 32–34 °C. Weaning from CPB was undertaken after rewarming to a systemic temperature > 36 °C.
Drug administration
After induction of anesthesia, a TEE exam was conducted. Then, a 5 mg dose (50–80 µg kg−1) of milrinone (Milrinone Lactate 1 mg ml−1 (base); Pharmaceutical Partners of Canada Inc., Richmond Hill, ON, CAN) was administered by inhalation before initiation of CPB, using a mesh nebulizer (Aeroneb Professional Nebulizer System; Aerogen Ltd., Galway, Ireland). The dosage was based on previous clinical trials11,29. The nebulizer was attached to the inspiratory limb of the ventilator Y-connector near the endotracheal tube. Milrinone solution was placed into the nebulizer cup and inhalation was continued until aerosol production was deemed complete after gentle tapping of the device.
Pharmacokinetic study
PK sampling
Serial arterial blood draws (5 ml) were obtained before inhalation (blank; 0 min), during inhalation (2, 5, 10, 15 min) and after the end of inhalation (0, 3, 6, 9, 15, 30, 60, 90, 120, 180, 240, 360, 480, 600 min). Two samples were also obtained after initiation of CPB (2 min) and after weaning from CPB (2 min). Blood samples were kept on ice for a short period of time and centrifuged. Plasma was immediately flash-frozen on dry ice and stored at − 80 °C. Milrinone plasma concentrations were determined by high performance liquid chromatography (HPLC) using tandem mass spectrometry detection61. The lower limit of quantification (LLOQ) was 0.3125 ng ml−1 with mean intra-assay (n = 6) and inter-assay (n = 10) precisions < 12%, expressed as coefficients of variation (CV%).
Inhaled dose
In the case of milrinone, the molecule being almost exclusively (> 95%) excreted unchanged or conjugated in urine, measurement of total urinary excretion allows for a realistic approximation of the inhaled dose42,62. For fifteen patients, complete 24 h-urine collections were therefore used for external validation. Total (conjugated and unconjugated) urinary concentrations of milrinone were measured by HPLC using ultraviolet detection63. In vivo experiments were also carried out by measuring the exhaled dose and the residual dose in the nebulizer cup. The total dose recovered was estimated by summing individual recoveries determined in vivo (including urinary excretion) and mean recovery previously obtained in vitro for components that could not be disconnected during cardiac surgery (i.e., nebulizer T-piece, Y-connector and endotracheal tube)64. Since complete 24-h urine collection is often difficult to ascertain in a clinical setting, a back-calculated approach for the estimation of the inhaled dose was used by subtracting the total dose recovered (individual in vivo and in vitro mean values) from the nominal dose administered (5 mg). This back-calculated value was then compared with the cumulative amount of milrinone recovered in urine for the same patient and considered for PK analysis.
PK analysis
Milrinone absorption process through pulmonary route is extremely rapid after inhalation35. A two-compartment model with zero-order input rate during nebulization and elimination from the central compartment was fitted to individual milrinone plasma concentration–time profiles, after standard verification of its adequacy using the Akaike information criterion. Point estimates and PK parameters were optimized for individual data using a standard minimization method (Gauss–Newton, Levenberg and Hartley) and a weighting function of 1/ŷ (where ŷ is the predicted concentration) was applied. Parameters including peak concentration (Cmax), peak time (Tmax), coefficients of bi-exponential equation describing disposition curve (A, B), fast distribution and elimination rate constants (α, β), total body clearance and apparent volume of distribution expressed as a function of bioavailability (Cl/F, V/F) were determined using WinNonlin® Version 5.3 software (PK Model 10, Pharsight Corp., Mountain View, CA, USA). Relationship between milrinone systemic exposure and nebulization rate was also explored.
For most routes of administration, the dose given to a patient is assumed to be completely delivered. This is often not the case for the pulmonary route and even less for the inhaled dose which represents the fraction of the nominal dose that ultimately reaches the distal end of the endotracheal tube. In the context of cardiac surgery (in vivo setting), milrinone inhaled dose could not be directly measured and was estimated using a back-calculated approach based on combined in vivo and in vitro data accounting for quantifiable and non-quantifiable losses within the respiratory apparatus, respectively. Since milrinone is almost completely excreted unchanged, urinary data (complete 24-h urine collection in a subset of 15 patients included herein) served as an external validation (Supplementary Table S1). According to this approach, mean total dose recovery was estimated as 95.3% of the 5 mg nominal dose, which included the inhaled dose, exhaled dose, residual and wasted doses within the nebulizer and delivery system. For these reasons, individual back-calculated inhaled doses were estimated and used for PK analyses.
Pharmacodynamic study
PD markers
Hemodynamic parameters including mAP and mPAP were continuously monitored and data recorded at 1- and 15-min intervals during the pre- and post-CPB period, respectively. The mAP/mPAP ratio (R) was later calculated and used as our PD marker mostly on the basis of sounded evidence for its prognostic value as the best predictor of perioperative complications in cardiac surgery5,6,7,8,9,10,14. Previous studies11,12 and case report13 described how increases in the ratio following administration of inhaled agents in patients are associated with improvement of the right ventricular function. The mAP/mPAP ratio was also correlated with the eccentricity index (which reflects the intraventricular deformation resulting from PH14) and identified as a potential PD marker29. A normal value for mAP/mPAP ratio is generally expected to be greater than 4; thus, lower values are good indicators of the severity of PH. Thus, in patients under general anesthesia and in absence of surgical stress, the mAP/mPAP ratio should change proportionally to any alteration of PH. Surgical interventions, whenever possible, were avoided during the inhalation period. For each patient, closed-chest baseline mAP/mPAP ratio (R0) was determined from measures collected within 10 min immediately before inhalation (both mAP and mPAP had to be stable by visual inspection for at least 3 min). As baseline values are of paramount importance for PD noncompartmental analysis, R0 values were rigorously determined by using the average value obtained from two independent experimenters. This approach for baseline characterization was carried out during the pre-inhalation period and before any intervention (TEE, legs raising, skin incision, or other surgical procedures).
Both open-chest peak mAP/mPAP ratio (Rmax) and post-CPB mAP/mPAP ratio (Rpost-CPB) were also considered as single point PD markers. Another PD marker frequently used in our clinical setting, that is the magnitude of peak response (∆Rmax-R0), was also calculated. A one-way repeated measures analysis of variance (ANOVA) (SigmaPlot™ Version 11.2, Systat Software Inc., San Jose, CA, USA) was used to compare R0, Rmax and Rpost-CPB. Lastly, the relationship between these PD markers and DSB (clinical endpoint) was also explored.
PK/PD analysis
Milrinone concentration–response relationship was analyzed by correlating patients’ respective area under the plasma concentration–time curve (AUC) and area under the response-time curve (AUEC) calculated using the linear trapezoidal rule. For the calculation of AUEC, both positive and negative fluctuations from the predetermined baseline response (R0; reference value) were taken into account during integration. Summation of all positive and negative partial AUEC yielded a net AUEC (NCA Model 220, Pharsight Corp., Mountain View, CA, USA). The AUC-AUEC relationship was investigated during the inhalation period (from 0 min until the end of inhalation). First, correlation was evaluated using all patients. The AUEC-intercept given by linear regression was considered to be the minimum threshold for response and considered as cut-off for determining responders. Then, correlation was re-evaluated in responders only. Finally, correlation between AUEC and ∆Rmax-R0 was verified, and consistency of results confirmed.
Clinical exploratory endpoint
The occurrence of DSB is considered as an important clinical endpoint in cardiac surgery. Two definitions were used to stratify the severity in weaning from CPB and were exclusively based on the type of support used from the end of CPB until the end of the surgery1. Easy separation from bypass was defined as either no support needed or only one vasoactive (norepinephrine, phenylephrine, vasopressin) or inotropic (dobutamine, milrinone, epinephrine) agent being used. Difficult separation from bypass (DSB) was defined as the requirement for at least both vasoactive and inotropic agents or also defined as ≥ 1 failure of the first weaning attempt or the requirement for an intra-aortic balloon pump or a ventricular assist device to leave the operating room. As a secondary exploratory endpoint, we explored a plausible relationship between response to inhaled milrinone (selected single point PD drivers) and DSB. Because PH was identified as one of the most important hemodynamic predictor and risk factor for DSB3,4, a positive response to inhaled milrinone in attempt to control PH was considered a potential predictor of DSB. Since the exploratory objective was to identify potential prognostic variables for DSB, variable selection was also based on clinical relevance that is prior knowledge of the pathophysiology related to CPB and factors susceptible to impact on its outcome. Logistic regression was carried out to identify factors independently associated with DSB. Several potential predictors were explored (EuroSCORE II, R0, Rmax, ∆Rmax-R0 and CPB duration). Simple and multiple logistic regressions were performed with stepwise selection (SigmaPlot™ Version 11.2, Systat Software Inc., San Jose, CA, USA) were used to develop a multivariate predictor of DSB.
Data availability
All data will be available on reasonable request to the corresponding author.
Abbreviations
- α:
-
Distribution rate constant
- β:
-
Elimination rate constant
- ANOVA:
-
Analysis of variance
- AUC:
-
Plasma concentration–time curve
- AUEC:
-
Area under the response–time curve
- Cl:
-
Total body clearance
- Cmax:
-
Peak plasma concentration
- CPB:
-
Cardiopulmonary bypass
- DSB:
-
Difficult separation from bypass
- EuroSCORE II:
-
European system for cardiac operative risk evaluation
- F:
-
Bioavailability
- mPAP:
-
Mean pulmonary artery pressure
- PD:
-
Pharmacodynamic
- PH:
-
Pulmonary hypertension
- PK:
-
Pharmacokinetic
- R:
-
Ratio
- R0 :
-
Closed-chest baseline mAP/mPAP ratio
- Rmax :
-
Open-chest peak mAP/mPAP ratio
- Rpost-CPB :
-
Post-CPB mAP/mPAP ratio
- ∆Rmax-R0 :
-
Magnitude of peak response of the mAP/mPAP ratio
- Tmax:
-
Peak time
- Vc :
-
Apparent volume of distribution of central compartment
- Vss :
-
Apparent volume of distribution at steady-state
References
Denault, A. Y., Tardif, J. C., Mazer, C. D. & Lambert, J. Difficult and complex separation from cardiopulmonary bypass in high-risk cardiac surgical patients: A multicenter study. J. Cardiothorac. Vasc. Anesth. 26, 608–616 (2012).
Reich, D. L. et al. Intraoperative hemodynamic predictors of mortality, stroke, and myocardial infarction after coronary artery bypass surgery. Anesth. Analg. 89, 814–822 (1999).
Tuman, K. J., McCarthy, R. J., March, R. J., Najafi, H. & Ivankovich, A. D. Morbidity and duration of ICU stay after cardiac surgery: A model for preoperative risk assessment. Chest 102, 36–44 (1992).
Malouf, J. F. et al. Severe pulmonary hypertension in patients with severe aortic valve stenosis: Clinical profile and prognostic implications. J. Am. Coll. Cardiol. 40, 789–795 (2002).
Robitaille, A. et al. Importance of relative pulmonary hypertension in cardiac surgery: The mean systemic-to-pulmonary artery pressure ratio. J. Cardiothorac. Vasc. Anesth. 20, 331–339 (2006).
Bianco, J. C. et al. Is patient-prosthesis mismatch a perioperative predictor of long-term mortality after aortic valve replacement?. J. Cardiothorac. Vasc. Anesth. 27, 647–653 (2013).
Rebel, A. et al. Systemic-to-pulmonary artery pressure ratio as a predictor of patient outcome following liver transplantation. World J. Hepatol. 8, 1384–1391 (2016).
Bianco, J. C. et al. Heart transplantation in patients >60 years: Importance of relative pulmonary hypertension and right ventricular failure on midterm survival. J. Cardiothorac. Vasc. Anesth. 32, 32–40 (2018).
Schubert, S. A. et al. Pulmonary-systemic pressure ratio correlates with morbidity in cardiac valve surgery. J. Cardiothorac. Vasc. Anesth. 33, 677–682 (2019).
Bianco, J. C. et al. Acute kidney injury after heart transplant: The importance of pulmonary hypertension. J. Cardiothorac. Vasc. Anesth. 35, 2052–2062 (2021).
Denault, A., Haddad, F., Lamarche, Y., Nguyen, A. & Varin, F. Pilot randomized controlled trial of inhaled milrinone in high-risk cardiac surgical patients. Surg. Curr. Res. 4, 192 (2014).
Laflamme, M. et al. Preliminary experience with combined inhaled milrinone and prostacyclin in cardiac surgical patients with pulmonary hypertension. J. Cardiothorac. Vasc. Anesth. 29, 38–45 (2015).
St-Pierre, P., Deschamps, A., Cartier, R., Basmadjian, A. J. & Denault, A. Y. Inhaled milrinone and epoprostenol in a patient with severe pulmonary hypertension, right ventricular failure, and reduced baseline brain saturation value from a left atrial myxoma. J. Cardiothorac. Vasc. Anesth. 28, 723–729 (2014).
Haddad, F. et al. Septal curvature is marker of hemodynamic, anatomical, and electromechanical ventricular interdependence in patients with pulmonary arterial hypertension. Echocardiography 31, 699–707 (2014).
Doolan, L. A., Jones, E. F., Kalman, J., Buxton, B. F. & Tonkin, A. M. A placebo-controlled trial verifying the efficacy of milrinone in weaning high-risk patients from cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 11, 37–41 (1997).
Solina, A. et al. A comparison of inhaled nitric oxide and milrinone for the treatment of pulmonary hypertension in adult cardiac surgery patients. J. Cardiothorac. Vasc. Anesth. 14, 12–17 (2000).
Feneck, R. O., Sherry, K. M., Withington, P. S. & Oduro-Dominah, A. Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 15, 306–315 (2001).
Solina, A. R. et al. Dose response to nitric oxide in adult cardiac surgery patients. J. Clin. Anesth. 13, 281–286 (2001).
Jaski, B. E., Fifer, M. A., Wright, R. F., Braunwald, E. & Colucci, W. S. Positive inotropic and vasodilator actions of milrinone in patients with severe congestive heart failure: Dose-response relationships and comparison to nitroprusside. J. Clin. Invest. 75, 643–649 (1985).
Cuffe, M. S. et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA 287, 1541–1547 (2002).
Couture, P., Denault, A. Y., Pellerin, M. & Tardif, J. C. Milrinone enhances systolic, but not diastolic function during coronary artery bypass grafting surgery. Can. J. Anesth. 54, 509–522 (2007).
Haraldsson, A., Kieler-Jensen, N. & Ricksten, S. E. The additive pulmonary vasodilatory effects of inhaled prostacyclin and inhaled milrinone in postcardiac surgical patients with pulmonary hypertension. Anesth. Analg. 93, 1439–1445 (2001).
Sablotzki, A., Starzmann, W., Scheubel, R., Grond, S. & Czeslick, E. G. Selective pulmonary vasodilation with inhaled aerosolized milrinone in heart transplant candidates. Can. J. Anesth. 52, 1076–1082 (2005).
Wang, H., Gong, M., Zhou, B. & Dai, A. Comparison of inhaled and intravenous milrinone in patients with pulmonary hypertension undergoing mitral valve surgery. Adv. Ther. 26, 462–468 (2009).
Lamarche, Y. et al. Inhaled but not intravenous milrinone prevents pulmonary endothelial dysfunction after cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 130, 83–92 (2005).
Hegazy, N. & Elhenawy, A. Comparison of hemodynamic effects of inhaled milrinone and inhaled prostacyclin after adult cardiac surgery. J. Appl. Sci. Res. 6, 38–44 (2010).
Gong, M., Lin, X. Z., Lu, G. T. & Zheng, L. J. Preoperative inhalation of milrinone attenuates inflammation in patients undergoing cardiac surgery with cardiopulmonary bypass. Med. Princ. Pract. 21, 30–35 (2012).
Lamarche, Y. et al. Preliminary experience with inhaled milrinone in cardiac surgery. Eur. J. Cardiothorac. Surg. 31, 1081–1087 (2007).
Denault, A. Y. et al. A multicentre randomized-controlled trial of inhaled milrinone in high-risk cardiac surgical patients. Can. J. Anesth. 63, 1140–1153 (2016).
Weibel, E. R. Morphometry of the Human Lung (Academic Press, 1963).
Patton, J. S. & Byron, P. R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 6, 67–74 (2007).
Patton, J. S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 19, 3–36 (1996).
Brown, R. A. Jr. & Schanker, L. S. Absorption of aerosolized drugs from the rat lung. Drug. Metab. Dispos. 11, 355–360 (1983).
Schanker, L. S., Mitchell, E. W. & Brown, R. A. Jr. Species comparison of drug absorption from the lung after aerosol inhalation or intratracheal injection. Drug Metab. Dispos. 14, 79–88 (1986).
Gavra, P., Denault, A. Y., Theoret, Y., Perrault, L. P. & Varin, F. Pharmacokinetics and pharmacodynamics of nebulized and intratracheal milrinone in a swine model of hypercapnia pulmonary hypertension. J. Cardiothorac. Vasc. Anesth. 32, 2130–2138 (2018).
Keith, I. M., Olson, E. B. Jr., Wilson, N. M. & Jefcoate, C. R. Immunological identification and effects of 3-methylcholanthrene and phenobarbital on rat pulmonary cytochrome P-450. Cancer Res. 47, 1878–1882 (1987).
Ji, C. M. Pulmonary cytochrome P-450 monooxygenase system and Clara cell differentiation in rats. Am. J. Physiol. 269, L394–L402 (1995).
Tronde, A. et al. Pulmonary absorption rate and bioavailability of drugs in vivo in rats: Structure–absorption relationships and physicochemical profiling of inhaled drugs. J. Pharm. Sci. 92, 1216–1233 (2003).
Avram, M. J. et al. Recirculatory pharmacokinetic model of the uptake, distribution, and bioavailability of prochlorperazine administered as a thermally generated aerosol in a single breath to dogs. Drug Metab. Dispos. 35, 262–267 (2007).
Rabinowitz, J. D. et al. Ultra-fast absorption of amorphous pure drug aerosols via deep lung inhalation. J. Pharm. Sci. 95, 2438–2451 (2006).
Dhand, R. Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir. Care 47, 1406–1416 (2002).
Stroshane, R. M., Koss, R. F., Biddlecome, C. E., Luczkowec, C. & Edelson, J. Oral and intravenous pharmacokinetics of milrinone in human volunteers. J. Pharm. Sci. 73, 1438–1441 (1984).
Edelson, J. et al. Pharmacokinetics of the bipyridines amrinone and milrinone. Circulation 73, 145–152 (1986).
Das, P. A. et al. Disposition of milrinone in patients after cardiac surgery. Br. J. Anaesth. 72, 426–429 (1994).
De Hert, S. G. et al. Comparison of two different loading doses of milrinone for weaning from cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 9, 264–271 (1995).
Butterworth, J. F. 4th., Hines, R. L., Royster, R. L. & James, R. L. A pharmacokinetic and pharmacodynamic evaluation of milrinone in adults undergoing cardiac surgery. Anesth. Analg. 81, 783–792 (1995).
Bailey, J. M., Levy, J. H., Kikura, M., Szlam, F. & Hug, C. C. Jr. Pharmacokinetics of intravenous milrinone in patients undergoing cardiac surgery. Anesthesiology 81, 616–622 (1994).
Kwon, Y. Handbook of Essential Pharmacokinetics, Pharmacodynamics and Drug Metabolism for Industrial Scientists (Springer, 2001).
Krzyzanski, W. & Jusko, W. J. Integrated functions for four basic models of indirect pharmacodynamic response. J. Pharm. Sci. 87, 67–72 (1998).
Simonneau, G. et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 62, D34–D41 (2013).
Elmi-Sarabi, M. et al. Pulmonary vasodilator response of combined inhaled epoprostenol and inhaled milrinone in cardiac surgical patients. Anesth. Analg. 136, 282–294 (2023).
Azzam, M. A. et al. A proposed algorithm for combining transcranial Doppler ultrasound monitoring with cerebral and somatic oximetry: A case report. Can. J. Anesth. 68, 130–136 (2021).
Denault, A. et al. Postoperative right ventricular dysfunction-Integrating right heart profiles beyond long-axis function. J. Thorac. Cardiovasc. Surg. 159, e315–e317 (2020).
Elmi-Sarabi, M. et al. Inhaled epoprostenol and milrinone effect on right ventricular pressure waveform monitoring. Can. J. Cardiol. https://doi.org/10.1016/j.cjca.2022.12.007 (2022).
Hu, X. et al. Routine intraoperative inhaled milrinone and iloprost reduces inotrope use in patients undergoing cardiac surgery: A retrospective cohort pilot study. Anesth. Analg. 131, 527–536 (2020).
Kim, T. Y., Lee, S. & Jeong, C. Intraoperative efficacy of brief inhalation of milrinone on elevated pulmonary arterial pressure in patients undergoing mitral valve surgery due to mitral regurgitation. Exp. Clin. Cardiol. 20, 3369–3378 (2014).
Elbaser, I. I. A. & El Aleem El Derie, A. A. Does inhaled milrinone facilitate weaning from cardiopulmonary bypass in children with congenital heart diseases complicated with pulmonary arterial hypertension?. Turk. J. Anaesthesiol. Reanim. 48, 127–133 (2020).
Jorairahmadi, S., Javaherforooshzadeh, F., Babazadeh, M., Gholizadeh, B. & Bakhtiari, N. Comparison of nebulized versus intravenous milrinone on reducing pulmonary arterial pressure in patients with pulmonary hypertension candidate for open-cardiac surgery: A double-blind randomized clinical trial. Anesthesiol. Pain Med. 12, e122994 (2022).
Moraes, D. & Loscalzo, J. Pulmonary hypertension: Newer concepts in diagnosis and management. Clin. Cardiol. 20, 676–682 (1997).
Nashef, S. A. EuroSCORE II. Eur. J. Cardiothorac. Surg. 41, 734–744 (2012).
Gavra, P. et al. A specific and sensitive HPLC-MS/MS micromethod for milrinone plasma levels determination after inhalation in cardiac patients. Ther. Drug Monit. 36, 663–668 (2014).
Larsson, R., Liedholm, H., Andersson, K. E., Keane, M. A. & Henry, G. Pharmacokinetics and effects on blood pressure of a single oral dose of milrinone in healthy subjects and in patients with renal impairment. Eur. J. Clin. Pharmacol. 29, 549–553 (1986).
Gavra, P., Nguyen, A. Q., Beauregard, N., Denault, A. Y. & Varin, F. High-performance liquid chromatography assay using ultraviolet detection for urinary quantification of milrinone concentrations in cardiac surgery patients undergoing cardiopulmonary bypass. Biomed. Chromatogr. 28, 1084–1089 (2014).
Nguyen, A. Q., Denault, A. Y., Theoret, Y., Perrault, L. P. & Varin, F. Inhaled milrinone in cardiac surgical patients: A pilot randomized controlled trial of jet vs mesh nebulization. Sci. Rep. 10, 2069 (2020).
Acknowledgements
The authors would like to thank Johanne Couture for her technical assistance during milrinone bioanalytical assays and the Montreal Heart Institute research team (Sophie Robichaud, Micheline Roy, Diane Leclerc, Geneviève Morin) for their help with patient recruitment and blood sampling. The authors acknowledge the thesis “Effet préventif de la milrinone inhalée chez les patients avec hypertension pulmonaire subissant une chirurgie cardiaque sous circulation extracorporelle: une approche pharmacométrique” by Anne Quynh-Nhu Nguyen. In the thesis, part of this work including some of the preliminary data were mentioned. However, by the time the thesis was published, some analyses in the current study were not completed and therefore not published.
Funding
This work was supported in part by the Groupe de Recherche Universitaire sur le Médicament/Fonds de Recherche du Québec—Santé and by the Montreal Heart Institute Foundation. A.Q.N.N.’s postgraduate studentship was initially supported by Projet Validation de l’Innovation et du Capital Intellectuel of Université de Montréal and later by Frederick Banting and Charles Best Doctoral Research Award from the Canadian Institutes of Health Research. A.Y.D.’s clinical research scholar was supported by Fonds de Recherche du Québec—Santé.
Author information
Authors and Affiliations
Contributions
A.Q.N.N., A.Y.D., Y.T., F.V.: Contribution to conception and design, acquisition of data, and analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. All authors gave final approval of the version to be published. All authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Competing interests
André Y. Denault is a consultant (2020) for CAE Healthcare; speaker (2017) and consultant (2020) for Masimo; and speaker (2019) for Edwards Lifesciences. He received a research grant (equipment) (2019) from Edwards Lifesciences.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, A.QN., Denault, A.Y., Théoret, Y. et al. Inhaled milrinone in cardiac surgical patients: pharmacokinetic and pharmacodynamic exploration. Sci Rep 13, 3557 (2023). https://doi.org/10.1038/s41598-023-29945-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-29945-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.