Abstract
When hemoglobin (Hb) is fully saturated with oxygen, the additional gain in oxygen delivery (DO2) achieved by increasing the fraction of inspired oxygen (FiO2) is often considered clinically insignificant. In this study, we evaluated the change in DO2, interrogated by mixed venous oxygen saturation (SvO2), in response to a change in FiO2 of 0.5 during cardiac surgery. When patients were hemodynamically stable, FiO2 was alternated between 0.5 and 1.0 in on-pump cardiac surgery patients (pilot study), and between 0.3 and 0.8 in off-pump coronary artery bypass grafting patients (substudy of the CARROT trial). After the patient had stabilized, a blood gas analysis was performed to measure SvO2. The observed change in SvO2 (ΔSvO2) was compared to the expected ΔSvO2 calculated using Fick’s equation. A total 106 changes in FiO2 (two changes per patient; total 53 patients; on-pump, n = 36; off-pump, n = 17) were finally analyzed. While Hb saturation remained near 100% (on-pump, 100%; off-pump, mean [SD] = 98.1% [1.5] when FiO2 was 0.3 and 99.9% [0.2] when FiO2 was 0.8), SvO2 changed significantly as FiO2 was changed (the first and second changes in on-pump, 7.7%p [3.8] and 7.6%p [3.5], respectively; off-pump, 7.9%p [4.9] and 6.2%p [3.9]; all P < 0.001). As a total, regardless of the surgery type, the observed ΔSvO2 after the FiO2 change of 0.5 was ≥ 5%p in 82 (77.4%) changes and ≥ 10%p in 31 (29.2%) changes (mean [SD], 7.5%p [3.9]). Hb concentration was not correlated with the observed ΔSvO2 (the first changes, r = − 0.06, P = 0.677; the second changes, r = − 0.21, P = 0.138). The mean (SD) residual ΔSvO2 (observed − expected ΔSvO2) was 0%p (4). Residual ΔSvO2 was more than 5%p in 14 (13.2%) changes and exceeded 10%p in 2 (1.9%) changes. Residual ΔSvO2 was greater in patients with chronic kidney disease than in those without (median [IQR], 5%p [0 to 7] vs. 0%p [− 3 to 2]; P = 0.049). DO2, interrogated by SvO2, may increase to a clinically significant degree as FiO2 is increased during cardiac surgery, and the increase of SvO2 is not related to Hb concentration. SvO2 increases more than expected in patients with chronic kidney disease. Increasing FiO2 can be used to increase DO2 during cardiac surgery.
Similar content being viewed by others
Introduction
The ultimate goal of hemodynamic management is to optimize oxygen transport and maintain adequate tissue oxygenation. Shoemaker et al. demonstrated in their early study that reduced oxygen transport was a predictor of death after major surgery for life-threatening shock1. The concept of oxygen transport optimization evolved following that study, and has become an important component of goal-directed hemodynamic management2.
Convective oxygen transport describes oxygen delivery (DO2) to peripheral tissues and organs via the circulation system, which can be managed by monitoring mixed venous oxygen saturation (SvO2)3,4. DO2 is a product of cardiac output (CO) and arterial oxygen content (CaO2)3,4, and CaO2 is a function of hemoglobin (Hb), arterial oxygen saturation (SaO2), and arterial oxygen partial pressure (PaO2), described as follows5:
where k1 (Hüfner’s constant) and k2 (Bunsen’s coefficient) are approximately 1.34 ml/g and 0.0034 ml/dl/mmHg, respectively. As can be inferred from this equation, the theoretical contribution of PaO2 to DO2 is negligible compared to the Hb concentration3,6. Consequently, it is a generally accepted idea that an increase in DO2 that can be achieved by increasing the fraction of inspired oxygen (FiO2) is minimal after Hb is saturated. This concept can lead physicians to overlook the importance of FiO2 adjustment in perioperative DO2 management.
Therefore, based on our clinical experience, we hypothesized that a significant increase in DO2 could be achieved by increasing FiO2 (and PaO2), even after Hb is fully saturated in cardiac surgery patients. To evaluate this hypothesis, we analyzed the effect of changing FiO2 on DO2 reflected as SvO2 in patients undergoing cardiac surgery.
Results
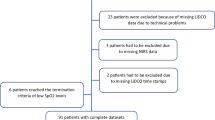
The study flow chart is presented in Fig. 1. Among the on-pump cardiac surgery patients (n = 40) enrolled in protocol 1 (see the “Methods” section), four dropped out because the blood gas results were missing (n = 2), rewarming was started during the study (n = 1) or red blood cells were transfused during the study (n = 1) (Fig. Methods1a). None of the participants (n = 17) of the CARROT trial who underwent off-pump coronary artery bypass grafting (OPCAB) dropped out from protocol 2 (Fig. 1b; see the “” section). Missing values were omitted without data imputation. The remaining 53 patients (on-pump, n = 36; OPCAB, n = 17) were included in the final analysis (Fig. 1).
The patient characteristics are described in Table 1. The mean (SD) Hb concentration was 7.7 g/dl (1.3), and the mean nasopharyngeal temperature was 29.2 °C (1.5), in on-pump patients following protocol 1. The mean Hb concentration and the nasopharyngeal temperature were 11.5 g/dl (2.1) and 35.8 °C (0.6), respectively in OPCAB patients following protocol 2. The mean cardiopulmonary bypass (CPB) flow rate was 4.1 l/min (0.5) in on-pump patients, and the mean CO measured via a pulmonary artery catheter using the thermodilution method was 3.3 l/min (0.5) in OPCAB patients. The hemodynamic variables measured at T0–T2 throughout the study are presented in Supplementary Table S1 online.
Comparison of SvO2 levels measured at different FiO2 levels
SaO2 remained relatively constant during both protocols. SaO2 was 100% in all on-pump cardiac surgery patients at every FiO2 level. In OPCAB patients, the mean (SD) SaO2 was 98.1% (1.5) when FiO2 was 0.3 and 99.9% (0.2) when FiO2 was 0.8. The pattern of PaO2 change in response to the change of FiO2 in every patient is shown in Fig. 2 and Supplementary Table S1 online.
Change of PaO2 according to that of fraction of inspired oxygen in (a) on-pump and (b) off-pump patients. PaO2, arterial oxygen partial pressure. Created with R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/.
The changes in SvO2 throughout the study period are shown in Fig. 3. SvO2 changed significantly with the change of FiO2 (and PaO2) in on-pump cardiac surgery patients (mean [SD], T0–T1 7.7%p [3.8] and T1–T2 7.6%p [3.5]; both P < 0.001) and OPCAB patients (T0–T1 7.9%p [4.9] and T1–T2 6.2%p [3.9]; both P < 0.001). Regardless of the surgery type, 82 (77.4%) changes had an observed ΔSvO2 ≥ 5%p and 31 (29.2%) had an observed ΔSvO2 ≥ 10%p (mean [SD], 7.5%p [3.9]).
Change of SvO2 according to different FiO2 levels in (a) on-pump and (b) off-pump patients. SvO2, mixed venous oxygen saturation; FiO2, fraction of inspired oxygen. Created with R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/.
ΔSvO2 according to Hb concentration
The relationship between Hb concentration and ΔSvO2 is shown in Fig. 4. The Hb concentration (within the range 5.1–15.3 g/dl) was not correlated with the observed ΔSvO2 (T0–T1, r = − 0.06, P = 0.677; T1–T2, r = − 0.21, P = 0.138).
The relationship between ΔSvO2 and hemoglobin concentration. ΔSvO2, change of mixed venous oxygen saturation. Red dots, T0–T1; blue dots, T1–T2. Created with R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/.
Comparison of the observed and expected ΔSvO2
The Bland–Altman plot for the observed and expected ΔSvO2 is presented in Fig. 5. Overall, SvO2 changed following a change in FiO2. The maximum residual ΔSvO2 (observed − expected ΔSvO2) was 12%p, and the mean (SD) residual ΔSvO2 was 0%p (4). Residual ΔSvO2 was more than 5%p in 14 (13.2%) changes. Residual ΔSvO2 exceeded 10%p in two changes.
Bland–Altman plot for the observed versus the expected ΔSvO2. Dashed lines indicate the limits of agreement (the mean ± 1.96 × the standard deviation of the residual ΔSvO2). ΔSvO2, change of mixed venous oxygen saturation. Red points, T0–T1; blue points, T1–T2. Created with R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/.
Exploratory analysis of factors associated with residual SvO2
A forest plot summarizing residual ΔSvO2 according to preoperative comorbidities is presented in Fig. 6. Patients with chronic kidney disease (n = 6) had significantly greater residual ΔSvO2 than those without chronic kidney disease (n = 47) (median [IQR], 5%p [0 to 7] vs. 0%p [− 3 to 2]; P = 0.049) (Fig. 6). However, no significant difference was observed in residual ΔSvO2 between patients with and without diabetes (n = 16 and 37, respectively; mean [SD], 2%p [5] vs. − 1%p [4]; P = 0.104), or between those with and without hypertension (n = 23 and 30, respectively; 0%p [5] vs. 0%p [4]; P = 0.933). Residual ΔSvO2 also did not differ according to whether patients had cerebrovascular disease or not (n = 6 and 47, respectively; median [IQR], − 1%p [− 1 to 1] vs. 0%p [− 4 to 3]; P = 0.967), or whether they had congestive heart failure or not (n = 13 and 40, respectively; mean [SD], 1%p [4] vs. 0%p [5]; P = 0.544).
Comparison of the residual ΔSvO2 according to comorbidities. Asterisks refer to non-parametric results. Points indicate the mean or the median, lines 95% confidence interval or interquartile range. ΔSvO2, change of mixed venous oxygen saturation. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/.
The change of Hb equivalent that increases DO2 to the same extent as ΔFiO2 of 0.5
The median (IQR) ΔHb equivalent that increases DO2 to the same extent as ΔFiO2 of 0.5 was 0.7 (0.6–0.8) g/dl. The maximum value was 1.1 g/dl. The distribution of the ΔHb equivalent values is presented as a histogram in Fig. 7. In more than 90% of changes with a change of FiO2, ΔFiO2 of 0.5 was equivalent to an ΔHb of more than 0.5 g/dl (97 changes, 91.5%), suggesting that use of a higher FiO2, at least temporarily, can achieve a similar effect as transfusion in terms of DO2.
Distribution of the ΔHb equivalent that increases oxygen delivery to the same extent as ΔFiO2 of 0.5. ΔHb, change of hemoglobin concentration. Created with R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org/.
Discussion
In this study, SvO2 increased by ≥ 5%p in more than three-quarters of FiO2 changes where FiO2 was increased from 0.3 to 0.8 or 0.5 to 1.0 during cardiac surgery, and by ≥ 10%p in more than one-quarter of FiO2 changes. While Hb remained almost fully saturated, PaO2 changed remarkably as FiO2 was changed. There was no significant association between Hb concentration and ΔSvO2. These results indicate that DO2 can increase significantly following an increase in PaO2 induced by raising FiO2 during cardiac surgery. The median ΔHb equivalent to the FiO2 change of 0.5, in terms of its ability to increase DO2, was 0.7 g/dl. In addition, SvO2 tended to increase beyond the expected value that was calculated using the Fick’s equation, in patients with chronic kidney disease.
In most patients undergoing cardiac surgery, SaO2 is maintained at nearly 100% due to supplemental oxygen therapy, unless there is a significant shunt or pulmonary morbidity. In our study, SaO2 was 100% at every FiO2 level during on-pump cardiac surgery, and more than 98% and 99% at the FiO2 levels of 0.3 and 0.8, respectively, during OPCAB. In such a situation, it is generally expected that the contribution of PaO2 to DO2 will be much smaller than that of Hb-bound oxygen3,6; thus, manipulating FiO2 would have very little influence on SvO2 (or DO2)5. Therefore, clinicians may focus only on Hb concentration and transfusion when optimizing DO2.
Several studies have shown that perioperative DO2 management is associated with complications after cardiac surgery, such as neurologic injury8,9,10,11 and renal dysfunction12,13,14. However, previous studies mostly evaluated the effect of CO and Hb concentration rather than FiO28,9,13. Hogue et al. reported that atrial fibrillation accompanied by low CO had a significant effect on the likelihood of postoperative stroke8. Bahrainwala et al. explained the link between reduced DO2 and postoperative stroke in terms of a decrease of Hb concentration alone9. Ranucci et al. also showed that severe hemodilution during CPB increases the risk of renal dysfunction, but emphasized that this can be attenuated by increasing DO2 with raising CO (pump flow)13.
Early studies by Clowes et al.15 and Shoemaker et al.1 revealed that survivors of peritonitis and shock have consistently higher DO2 and oxygen consumption (VO2) than those who died. Although our study showed that higher FiO2 significantly elevates DO2, this does not necessarily mean that the use of high FiO2 would improve clinical outcomes: there are several issues that need to be addressed. First, there is growing concern about the harmful effects of hyperoxia caused by high FiO2, although most previous clinical studies failed to demonstrate significantly poorer clinical outcomes due to hyperoxia or high FiO216,17. Second, DO2 can increase in response to transfusion or intravascular volume expansion, but we do not know whether achieving the same level of SvO2 with different modalities results in an equivalent distribution of oxygen to the organs; the distribution of oxygen supply and demand differs among organs18. Weinrich et al. failed to find a correlation between surgical site oxygen saturation and central venous oxygen saturation in patients undergoing major non-cardiac surgery19. Similar findings have been reported in patients undergoing CPB cardiac surgery, where a significant difference between SvO2 and venous oxygen saturation measured at the brain or gut was demonstrated20,21. This heterogeneity not only exists at the global level, but also at the regional level within an organ22. However, these are poorly investigated topics, so further studies on are necessary. Currently, there is no firm consensus or established guidelines regarding the optimal oxygen therapy for patients undergoing cardiac surgery, and the present study did not answer this question. We are conducting a multicenter, cluster-randomized trial (the CARROT trial; Clinicaltrials.gov, NCT03945565) to compare the effects of different levels of intraoperative FiO2 (0.3 vs. 0.8) on clinical outcomes after OPCAB, including the length of postoperative hospital stay and major organ injuries.
Recent large-scale randomized trials, such as the TRICS III23 and the TITRe224, failed to demonstrate a difference between restrictive and liberal transfusion strategies in terms of composite adverse outcomes after cardiac surgery. In these trials, only Hb concentration was tested as a trigger for red blood cell transfusion23,24,25. However, from the present study, and our previous study26 it can be inferred that there may be unknown interactions or confounders that make interpretation of the effect of transfusion on outcomes more complex. Although establishing the Hb threshold is currently the highest priority for transfusion and DO2 optimization, oxygen therapy and plasma dissolved oxygen should also be considered.
The present study had several limitations. First, only a small number of patients were included without an a priori sample size calculation. Furthermore, two heterogeneous groups of patients (on- and off-pump cardiac surgery patients) with different hemodynamic statuses and comorbidities were enrolled. The levels of FiO2 also differed between the study protocols. Moreover, we only assessed the immediate effect of a change in FiO2 on SvO2, and did not evaluate whether increasing SvO2 using a higher FiO2 ameliorates oxygenation of vital organs (which would improve clinical outcomes). We expect that the CARROT trial will answer these questions. Second, several (important) variables, such as CO, Hb concentration, and VO2, were assumed to be constant during the FiO2 changes for this analysis, which was inevitable for the calculation of expected ΔSvO2. To minimize the influence of these values, the both protocols were conducted when it was considered the most hemodynamically stable with the least surgical manipulation (see the “Methods” section). Obviously, changes in these variables were minimal during the study period (Supplementary Table S1 online), but may have affected the observed and expected ΔSvO2 values to a certain extent. Third, we only included cardiac surgery patients in this study. Thus, our results may not be applicable to patients in other settings, such as non-cardiac surgery patients and non-surgical, critically ill patients. Fourth, this study did not uncover the mechanism, or assess the clinical impact, of the phenomenon whereby a change in SvO2 caused by a change in FiO2 was larger than expected in patients with chronic kidney disease.
In conclusion, DO2, interrogated by SvO2, may be significantly elevated by increasing FiO2 during cardiac surgery. Increasing FiO2 may be considered when an increase in DO2 is necessary during cardiac surgery. However, considering the potential risk of hyperoxia, further studies evaluating the clinical effect of this practice are necessary.
Methods
Study population
This study was comprised of on-pump cardiac surgery and OPCAB parts. The part involving patients undergoing CPB cardiac surgery was a pilot study, which was approved by the Institutional Review Board of Seoul National University Hospital (IRB no., 1909-145-1067) and registered at ClinicalTrials.gov (NCT04144205). The other part, for patients undergoing OPCAB, was a substudy of the CARROT trial (IRB no., 1902-021-1008; ClinicalTrials.gov, NCT03945565).
The present study was performed in compliance with the guidelines for Good Clinical Practice and the Declaration of Helsinki. All participants recruited to this study provided written informed consent.
Protocol 1: on-pump cardiac surgery
This part of the study was a pilot study for a future multicenter, randomized trial. Forty patients who presented for elective CBP cardiac surgery between November 4, 2019 and February 11, 2020 were enrolled in this study. There was no a priori sample size calculation. The exclusion criteria were preoperative supplemental oxygen at a dose equivalent to FiO2 of > 0.5, symptomatic cerebrovascular disease, and > 50% cerebral artery stenosis.
After CPB was initiated, the ascending aorta was cross-clamped and a cardioplegic solution was infused. Body temperature was measured at the nasopharynx and bladder, and was lowered to 28–32 °C. The α-stat strategy was applied for the pH management during CPB. FiO2 is initially set to 0.6 on the CBP oxygenator as a routine practice at our institution. After asystole was obtained and body temperature had stabilized, FiO2 was sequentially changed from 0.5 to 1.0, and back to 0.5, in the first half of the patients enrolled, and from 1.0 to 0.5, and back to 1.0, in the other half. Following a 5- to 10-min equilibration period for the three sequential FiO2 levels (T0–T2, respectively), blood gas analysis was performed using arterial and mixed venous blood sampled from the radial artery and venous reservoir of the CPB machine, respectively. A point-of-care analyzer (Gem®Premier™3000; Instrumentation Laboratory, Bedford, MA, USA) was utilized for the blood gas analysis. The pump flow rate of the CPB machine was recorded as the CO. Heart rate and mean blood pressure were also measured during the FiO2 changes.
Protocol 2: off-pump coronary artery bypass grafting
This part of the study was a substudy of the CARROT trial, in which elective OPCAB patients were cluster-randomized on a monthly basis to receive FiO2 of either 0.3 or 0.8 during surgery. The length of postoperative hospital stay was the primary endpoint; other clinical outcomes will be compared in the CARROT trial. All participants taking part in the CARROT trial from November 1 to December 31, 2019 were consecutively enrolled in this substudy. Exclusion criteria for the CARROT trial included robot-assisted surgery, surgery via a thoracotomy, minimally invasive direct coronary artery bypass grafting, concomitant major surgery, any pulmonary condition requiring supplemental oxygen through any route before surgery, and preoperative use of mechanical circulatory assist devices.
After anesthesia was induced, the patients in the CARROT trial were mechanically ventilated with FiO2 of 0.3 or 0.8 during surgery based on the above-described cluster randomization (November 2019, FiO2 of 0.3; December 2019, FiO2 of 0.8). A pulmonary artery catheter (Swan-Ganz CCOmbo V 774HF75; Edwards Lifesciences, Irvine, CA, USA) was placed and connected to a continuous SvO2 and CO monitoring device (Vigilance II™; Edwards Lifesciences). The substudy protocol was performed during graft harvesting to ensure hemodynamic stability and minimal blood loss. FiO2 was changed from 0.3 to 0.8, and then back to 0.3, in patients allocated to receive FiO2 of 0.3 in the CARROT trial, while in those who received FiO2 of 0.8 it was changed from 0.8 to 0.3, and then back to 0.8 (T0–T2, respectively). FiO2 was held at each level for 5 to 10 min for stabilization, and blood gas analysis was performed at T0–T2 on arterial and mixed venous blood obtained from the radial and pulmonary arteries, respectively. No intravenous fluids were infused during the study protocol. Nasopharyngeal temperature, heart rate, and mean blood pressure were recorded during the FiO2 changes.
Statistical analysis
The primary endpoint was the observed ΔSvO2 in response to a change in FiO2. Secondary endpoint was the difference between the observed and expected ΔSvO2 values (observed − expected ΔSvO2), i.e., the residual ΔSvO2.
Forty and 17 patients were recruited for protocol 1 (a pilot study) and protocol 2 (a substudy of the CARROT trial), respectively, without a sample size calculation. The statistical analysis was performed as follows. First, the observed ΔSvO2 was compared to zero (i.e., no change) using the one-sample t-test in on-pump cardiac and OPCAB patients. The observed ΔSvO2 of each patient was calculated as the absolute difference in SvO2 values measured at T0 versus T1, and T1 versus T2, thus giving two ΔSvO2 values per patient: the Bonferroni’s correction was applied. Second, we explored the distribution of the observed ΔSvO2 according to Hb concentration on a scatterplot, regardless of the surgery type. Assuming that the Hb concentration was constant during the change of FiO2 (T0–T2), the Hb concentration measured at T0 was taken as the representative value and used in the analysis. Pearson’s correlation analysis was performed to evaluate the association of Hb concentration with the observed ΔSvO2. Third, the observed ΔSvO2 was compared to the expected ΔSvO2 using a Bland–Altman plot, and the residual ΔSvO2 was calculated. The expected ΔSvO2 was calculated using Fick’s equation7
where VO2 is oxygen consumption and CvO2 is the mixed venous oxygen content. As described earlier,
and similarly,
where PvO2 is the mixed venous oxygen partial pressure. Therefore,
We assumed that CO and VO2 remained constant from T0 to T2; hence, the following equation was established.
or
Rearranging this equation, the expected ΔSvO2 (T0–T1 and T1–T2) was calculated as follows:
where ΔSaO2, ΔPaO2, and ΔPvO2 are the absolute difference of the SaO2, PaO2, and PvO2 values measured at T0 versus T1, and T1 versus T2. Fourth, an exploratory analysis was performed to identify factors potentially associated with the degree of ΔSvO2 according to ΔPaO2. Residual ΔSvO2 was compared among patients with and without chronic kidney disease, diabetes, hypertension, cerebrovascular disease, and congestive heart failure using the independent t-test or Wilcoxon rank-sum test after checking for normality. Only the residual SvO2 calculated at T0 versus T1 was used for this exploratory analysis. Fifth, we exploratively calculated the ΔHb equivalent that could increase DO2 to the same extent as ΔFiO2 of 0.5. For this calculation, it was assumed that CO remained unchanged, so the following equation was established. The ΔHb equivalent was calculated by rearranging the equation.
\({k}_{1}\times Hb \times {SaO}_{2}\left[{high\, \,FiO}_{2}\right]+{k}_{2}\times {PaO}_{2}[{high\, \,FiO}_{2}]\)
All statistical analyses and data visualization were performed using R software (version 4.0.0; R Development Core Team, Vienna, Austria). Continuous variables are expressed as mean (SD) or median (IQR) as appropriate, and categorical variables are expressed as numbers (%). A P-value < 0.05 was considered significant.
Data availability
The data supporting this publication can be accessed by contacting the corresponding authors on reasonable request.
References
Shoemaker, W. C., Montgomery, E. S., Kaplan, E. & Elwyn, D. H. Physiologic patterns in surviving and nonsurviving shock patients. Use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Arch. Surg. 106, 630–636 (1973).
Pinsky, M. R. Hemodynamic evaluation and monitoring in the ICU. Chest 132, 2020–2029 (2007).
Kandel, G. & Aberman, A. Mixed venous oxygen saturation. Its role in the assessment of the critically ill patient. Arch. Intern. Med. 143, 1400–1402 (1983).
McLellan, S. A. & Walsh, T. S. Oxygen delivery and haemoglobin. Contin. Educ. Anaesth. Crit. Care Pain 4, 123–126 (2004).
Shepherd, S. J. & Pearse, R. M. Role of central and mixed venous oxygen saturation measurement in perioperative care. Anesthesiology 111, 649–656 (2009).
Leach, R. M. & Treacher, D. F. The pulmonary physician in critical care * 2: Oxygen delivery and consumption in the critically ill. Thorax 57, 170–177 (2002).
Hogue, C. W. Jr., Murphy, S. F., Schechtman, K. B. & Davila-Roman, V. G. Risk factors for early or delayed stroke after cardiac surgery. Circulation 100, 642–647 (1999).
Bahrainwala, Z. S. et al. Intraoperative hemoglobin levels and transfusion independently predict stroke after cardiac operations. Ann. Thorac. Surg. 91, 1113–1118 (2011).
Magruder, J. T. et al. Correlating oxygen delivery during cardiopulmonary bypass with the neurologic injury biomarker ubiquitin C-terminal hydrolase L1 (UCH-L1). J. Cardiothorac. Vasc. Anesth. 32, 2485–2492 (2018).
Murphy, G. S., Hessel, E. A. 2nd. & Groom, R. C. Optimal perfusion during cardiopulmonary bypass: An evidence-based approach. Anesth. Analg. 108, 1394–1417 (2009).
de Somer, F. et al. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: Time for a goal-directed perfusion management?. Crit. Care 15, R192 (2011).
Ranucci, M. et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann. Thorac. Surg. 80, 2213–2220 (2005).
Magruder, J. T. et al. Nadir oxygen delivery on bypass and hypotension increase acute kidney injury risk after cardiac operations. Ann. Thorac. Surg. 100, 1697–1703 (2015).
Clowes, G. H., Vucinic, M. & Weidner, M. G. Circulatory and metabolic alterations associated with survival or death in peritonitis: Clinical analysis of 25 cases. Ann. Surg. 163, 866–885 (1966).
Spoelstra-de Man, A. M., Smit, B., Oudemans-van Straaten, H. M. & Smulders, Y. M. Cardiovascular effects of hyperoxia during and after cardiac surgery. Anaesthesia 70, 1307–1319 (2015).
Heinrichs, J., Lodewyks, C., Neilson, C., Abou-Setta, A. & Grocott, H. P. The impact of hyperoxia on outcomes after cardiac surgery: A systematic review and narrative synthesis. Can. J. Anaesth. 65, 923–935 (2018).
Williams, L. R. & Leggett, R. W. Reference values for resting blood flow to organs of man. Clin. Phys. Physiol. Meas. 10, 187–217 (1989).
Weinrich, M. et al. Central venous oxygen saturation does not correlate with the venous oxygen saturation at the surgical site during abdominal surgery. Clin. Hemorheol. Microcirc. 39, 409–415 (2008).
Lindholm, L., Hansdottir, V., Lundqvist, M. & Jeppsson, A. The relationship between mixed venous and regional venous oxygen saturation during cardiopulmonary bypass. Perfusion 17, 133–139 (2002).
McDaniel, L. B. et al. Mixed venous oxygen saturation during cardiopulmonary bypass poorly predicts regional venous saturation. Anesth. Analg. 80, 466–472 (1995).
Iversen, P. O. & Nicolaysen, G. Fractals describe blood flow heterogeneity within skeletal muscle and within myocardium. Am. J. Physiol. 268, H112-116 (1995).
Mazer, C. D. et al. Restrictive or liberal red-Cell transfusion for cardiac surgery. N. Engl. J. Med. 377, 2133–2144 (2017).
Murphy, G. J. et al. Liberal or restrictive transfusion after cardiac surgery. N. Engl. J. Med. 372, 997–1008 (2015).
Mazer, C. D. et al. Six-month outcomes after restrictive or liberal transfusion for cardiac surgery. N. Engl. J. Med. 379, 1224–1233 (2018).
Nam, K. et al. Intraoperative transfusion and an increased preoperative C-reactive protein level are associated with higher mortality after off-pump coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 159, 566–567 (2020).
Fick, A. Ueber die messung des blutquantums in den herzventrikeln. Phys. Med. Ges. Wurzburg. 2, 16–28 (1870).
Acknowledgements
UPINEMED Inc. (http://upinemed.co.kr; Seoul, Korea) provided cartridges used for a point-of-care blood gas analyzer (Gem®Premier™3000, Instrumentation Laboratory, Bedford, MA, USA) to Yunseok Jeon (no. 10-2019-0490). Otherwise, the authors have no financial funding source to disclose.
Author information
Authors and Affiliations
Contributions
K.N. designed and conducted the study, analysed the data, and wrote up of the first draft; H.-B.K. analysed the data, wrote up of the first draft, and revised the paper; Y.-L.K. designed the study, wrote up of the first draft, and revised the paper; Y.H.J. recruited the patients, conducted the study, and revised the paper; J.-W.J. designed the study, analysed the data, and revised the paper; J.B. recruited the patients and wrote up of the first draft; S.L. and Y.J.C. analysed the data and revised the paper; J.-K.S. and Y.J. conceived and designed the study and revised the paper. K.N. and H.-B.K. contributed equally to this study and share the role of first author. J.-K.S. and Y.J. contributed equally to this study and share the role of corresponding author.
Corresponding authors
Ethics declarations
Competing interests
UPINEMED Inc. (http://upinemed.co.kr; Seoul, Korea) provided cartridges used for a point-of-care blood gas analyzer (Gem®Premier™3000, Instrumentation Laboratory, Bedford, MA, USA) to Yunseok Jeon (no. 10–2019-0490). Otherwise, the authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nam, K., Kim, HB., Kwak, YL. et al. Effect of changes in inspired oxygen fraction on oxygen delivery during cardiac surgery: a substudy of the CARROT trial. Sci Rep 11, 17862 (2021). https://doi.org/10.1038/s41598-021-97555-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-97555-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.