Abstract
There is increasing genetic evidence for the role of microglia in neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and motor neuron disease. Therefore, there is a need to generate authentic in vitro models to study human microglial physiology. Various methods have been developed using human induced Pluripotent Stem Cells (iPSC) to generate microglia, however, systematic approaches to identify which media components are actually essential for functional microglia are mostly lacking. Here, we systematically assess medium components, coatings, and growth factors required for iPSC differentiation to microglia. Using single-cell RNA sequencing, qPCR, and functional assays, with validation across two labs, we have identified several medium components from previous protocols that are redundant and do not contribute to microglial identity. We provide an optimised, defined medium which produces both transcriptionally and functionally relevant microglia for modelling microglial physiology in neuroinflammation and for drug discovery.
Similar content being viewed by others
Introduction
Microglia are the resident macrophages of the brain parenchyma, responsible for a broad range of homeostatic functions, including clearance of apoptotic cells and misfolded proteins, and synaptic pruning during neurodevelopment1. Microglia are associated with a number of neurodegenerative disorders, notably Alzheimer’s Disease (AD)2,3,4, Parkinson’s disease (PD)5, and motor neuron disease/amyotrophic lateral sclerosis (ALS)6, with many of the genes with highest genetic risk score being expressed in microglia, including; TREM2, CD33, APOE, LRRK2, and C9orf72.
Studies of primary microglia have been mostly restricted to rodent models, as access to fresh primary human microglia is severely limited. Furthermore, microglia are highly sensitive to their environment, so the removal and culturing of microglia away from their original homeostatic environment is likely to result in immune activation, and they will therefore differ from their in vivo state, such that they may not recapitulate the disease cellular physiology in vitro7.
As an alternative to rodent and primary microglia, several labs have developed methods for generating human microglia from induced Pluripotent Stem Cells (iPSC)8,9,10,11,12,13,14,15,16,17,18(Table 1). These protocols all aim to roughly recapitulate the sequence of events in the developing embryo. This approach potentially facilitates the study of the effect of disease genotype on microglial phenotype, as iPSCs provide a limitless resource, faithfully reproducing the donor’s genetic background, which can also be readily gene-edited as required. However, issues with iPSC-based methodologies exist, including: variable reproducibility of protocols between different labs; variation across different cell lines; unchallenged adoption of medium components from other protocols; and use of undefined medium components. The multiple iPSC microglial protocols that have been published (including by our laboratory10), use different combinations of growth factors and media supplements including IL-34, TGF-β1, M-CSF, GM-CSF, CD200, CX3CR1, supplements B27 and N-2, and different substrates, in an attempt to recapitulate primary microglia in vitro. Several studies require further differentiation steps via co-culture, integration into organoids or xenotransplantation into mice, thus limiting accessibility of these models12,16. Relevant here, the medium developed by our lab10 was optimised for co-culture with iPSC-cortical neurons, rather than monoculture. Systematic comparison of the different components has been minimal, to identify which, if any, are redundant or provide minimal benefit to producing iPSC-microglia in monoculture.
Here, we set out to systematically compare and identify how different growth factor, medium component and substrate combinations affect resultant iPSC-microglial phenotypes, transcriptomes, and survival in monoculture, in an attempt to identify a medium which can best recapitulate human microglia in vitro without the need for co-culture or xenotransplantation. We validated the results across two laboratories, using the same iPSC, basal medium, growth factors, and reagent providers. Building from the previously published microglial differentiation protocol by Haenseler et al.10, which starts with iPSC differentiation to mesoderm via embryoid bodies, hemogenic endothelium induction, then bulk myeloid differentiation to primitive macrophage/microglia precursors, in a highly scalable pipeline. However, Haenseler et al. optimised the final iPSC-microglia maturation medium for co-culture of iPSC-microglia with iPSC-neurons. Here, we directly compared the addition and removal of key components from this and other published protocols, and assessed their ability to recapitulate human microglia in monoculture in vitro. We identify substantial variation between previously published protocols for generating iPSC-microglia and present an optimised media composition which is reproducible across multiple genetic backgrounds, improves microglial retention and transcriptionally most closely corresponds to primary microglia. This iPSC-microglia maturation protocol can be applied to study microglia-associated disease settings and can be scaled for drug and phenotypic screening.
Methods
Consent for use of human material
All iPSC were obtained with informed consent from all subjects, donors and/or their legal guardian(s) and with the approval of all the relevant institutions. All methods and experimental protocols were carried out in accordance within University of Oxford and Wellcome Sanger Institute guidelines and regulations. All experimental protocols were approved by the Ethics Committee of the University of Oxford and Wellcome Sanger Institute. IPSC lines were generated previously as part of the HipSci project (REC Reference 09/H0304/77, covered under HMDMC 14/013 and REC reference 14/LO/0345), or the EU IMI project, StemBANCC (REC Reference 10/H0505/71)19.
iPSC lines
The three lines (all from healthy donors) used in this study form part of the HipSci or StemBANCC initiatives and are listed in (Supplementary Table S1).
KOLF2.1S was generated from the parental KOLF2-C1 line (https://hpscreg.eu/cell-line/WTSIi018-B-1) itself a clonal isolate of KOLF2 (https://hpscreg.eu/cell-line/WTSIi018-B) by correction of a heterozygous loss of function mutation in ARID2 using CRISPR based homology directed repair. Cells were nucleofected (Lonza) with recombinant enhanced specificity Cas9 protein (eSpCas9_1.1), a synthetic sgRNA (target site AAAAGATCACTTGCTAATGC CGG, Synthego) and a single-stranded DNA oligonucleotide homology directed repair template (5′-CACTCTCCTATCAAATGAAAGCAAGCACGTCATGCAACTTGAAAAAGATCCTAAAATCATCACTTTACTACTTGCTAATGCCGGGGTGTTTGACGACAGTAAGTTTTAAGCTGAATGTA-3′, IDT Ultramer) followed by clonal isolation20. Repaired clones were identified using PCR across the edited region, and sequencing of the amplicons by high throughput sequencing (Illumina MiSeq) and validated by Sanger sequencing. These lines were generated by the Cellular Operations Gene Editing team at the Wellcome Sanger Institute.
SFC841-03-0121 and SFC856-03-0410 were generated at the James and Lillian Martin Centre for Stem Cell Research, University of Oxford, and are available from EBiSC.
iPSC culture
All differentiation reagents are listed in (Supplementary Table S2) and all medium compositions are listed in (Supplementary Table S3). iPSC were cultured in ‘OxE8’ medium22 (based on the published E8 medium formulation23) on Geltrex (Gibco A1413302) coated tissue culture dishes and passaged at 80% confluency with 0.5 mM EDTA. Cells were incubated at 37 °C, 5% CO2 and fed daily with fresh OxE8 medium. HipSci lines were cultured in Essential 8 medium (Gibco A1517001) on vitronectin (Gibco A14700) coated tissue culture wells.
Differentiation to microglia precursors
iPSC were differentiated into primitive macrophage/microglia precursors as described previously24. In brief, an Aggrewell 800 plate (STEMCELL Technologies, no. 34815) was prepared by addition of 0.5 mL of Anti-Adherence Rinsing Solution (STEMCELL Technologies, no 07010) and centrifuged at 3000×g for 3 min to remove bubbles from the microwells. Rinse solution was then removed and the wells washed with 1 mL PBS before the addition of 1 mL of 2 × concentrated EB medium (1 × EB medium: OxE8 medium supplemented with 50 ng/mL BMP4 (PeproTech no PHC9534), 50 ng/mL VEGF (PeproTech no PHC9394), and 20 ng/mL SCF (Miltenyi Biotec no 130-096-695)) supplemented with 10 µM Y-27632 ROCK inhibitor. iPSC were cultured to 70–80% confluency before washing with 1 mL PBS then incubation in TrypLE Express (Gibco, no 12604013) for 3–5 min at 37 °C, 5% CO2. Cells were then lifted and transferred to a falcon tube containing PBS to prepare a single cell solution. Cells were counted and pelleted by centrifugation at 400×g for 5 min. iPSC were then resuspended in OxE8 with 10 µM Y-27632 ROCK inhibitor at a concentration of 4 × 106 cells/mL and 1 mL of cells were added to one well of the Aggrewell 800 containing 2 × EB media. The Aggrewell plate was then spun at 100×g for 3 min with no braking to encourage even distribution of cells. Aggrewells were incubated for 6 days at 37 °C, 5% CO2 with daily feeding of 75% media change with EB media. After 6 days EB were removed from the plate using a 5 mL stripette and transferred to a 40 µm cell strainer, remaining EB were flushed from the well with 2 × 2 mL PBS washes. Dead cells were washed from the EB in the strainer using 4 mL of PBS. The filter was then inverted over a six well tissue culture plate and EB washed off using 4 mL of differentiation media. EB were then divided evenly across two six well plates before being transferred to two T175 flasks containing 18 mL of differentiation media (total medium 20 mL). These “Factories” were incubated at 37 °C, 5% CO2 with weekly feeding of 10 mL differentiation media until macrophage/microglia precursors (PreMac) cells started to appear in the supernatant. After this point, usually 5 weeks into differentiation, 50% of media was harvested from the factories and PreMac cells were collected by centrifugation at 400×g for 5 min. Media removed was replaced with fresh Factory media. Cells were used immediately for experiments or stored for use later in a 1 L bioreactor (Corning) containing Factory media spinning at a constant speed of 30 rpm as described by25.
For 19 HipSci lines, iPSCs were dissociated with Accutase (LifeTech A1110501) for 5 min at 37 °C, 5% CO2. Cells were pelleted and resuspended in Essential 8 (Gibco) with 10 µM Y-27632 (Stemcell Technologies 72304) and strained through 40 µM Pluristrainers (Cambridge Bioscience 43-10040-40) to obtain single cell suspensions. HipSci lines were counted and pooled in equal proportions in Essential 8 with 10 µM Y-27632 at a concentration of 200,000 cells/mL. The pooled cell suspension was added in equal volume to 2 × EB media for a final concentration of 100,000 cells/mL and transferred to a sterile reservoir. 100 µL cell suspension containing 10,000 cells was added to each well in Corning CoStar Ultra Low Cluster round bottom ULA 96-well plates (Corning 7007). The ULA plates were centrifuged at 300×g for 3 min and incubated at 37 °C, 5% CO2 until Day 3. The 96-well plates were fed with 100 µL per well EB media after removing 50 µL per well spent media at day 3. The plates were incubated at 37 °C, 5% CO2 until day 6 when the EBs were transferred using a multichannel pipette with wide orifice tips into a sterile reservoir. The EBs were washed as described above and transferred to gelatin (Sigma G1890) coated T75 flasks (Corning 430641U) in a total of 15 mL Factory media. These “Factories” were maintained at 37 °C, 5% CO2 with total volume incrementally increased over the first 3 feeds from 15 mL to the maximum volume of 50 mL, followed by bi-weekly 60–80% media change. Around 3 weeks after Factory set up, spent media harvested at each feed contained PreMac cells. To start microglial differentiation, the spent media was passed through 40 µm cell strainer (Falcon 352340) and PreMac cells were collected by centrifugation at 400×g for 5 min.
Maturation to microglia
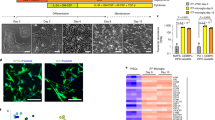
Microglial precursors were plated in 6 or 12-well plates at a density of 100,000 cells/cm2. For Geltrex coating, plates were incubated with Geltrex (Thermofisher, A1413202) for 1 h at 37 °C, 5% CO2, before removal and direct addition of cells. Where fibronectin (Sigma, F0895) was used, it was diluted to 10 µg/mL in PBS and plates were incubated for 3 h at room temperature, removed, and wells washed three times with water before adding cells. Factors used for the experiments and concentrations are outlined in (Supplementary Table S4) with the following acronym when included in a medium: I (IL-34), T (TGF-β1), M (M-CSF), G (GM-CSF), C (CD200/CX3CL1), B (β-mercaptoethanol), N (N-2), F (FBS) (Table 2). 50% media change was performed to minimise cell loss and metabolic shock every 3–4 days and maturation was allowed to continue for a total of 14 days. For media containing CD200/CX3CL1, these were added at day 10 of the maturation, as described in12. An overview of the protocol is provided in (Fig. 1a). The triaging of factors in the test microglial media, including selection criteria, is shown in Fig. 1b, exclusion criteria are listed in Fig. 1c and the final comparison is shown in Fig. 1d.
A flowchart of the differentiation process from iPSC to Microglia as described by Haenseler et al. (A) iPSC are cultured in OxE8 medium for 7 days before differentiation into embryoid bodies in Aggrewell plates for 6 days in embryoid body medium (BMP4/VEGF/SCF). After 6 days embryoid bodies are transferred to T175 flasks and cultured in myeloid differentiation media of IL-3 and M-CSF. At approximately d40 these factories produce microglial precursor cells which are harvested and plated in microglial media and differentiated for 14 days. This final step is the target for optimisation in this manuscript. iPSC induced pluripotent stem cell, EB embryoid body, SCF stem cell factor, BMP4 bone morphogenetic protein 4, VEGF vascular endothelial growth factor, IL-3 Interleukin 3, M-CSF macrophage colony stimulation factor, d day. Created with BioRender.com. (B) A flowchart describing the systematic identification of factors required for iPSC-microglial differentiation. After the first triage of 15 media combinations, the morphology analysis removed three conditions due to poor differentiation. Following qPCR analysis four factors could be removed from further medium development. The second set of differentiations focused on IL-34, TGF-β1, M-CSF, and GM-CSF as these were shown to have an effect in the first round of differentiations. Several conditions were removed due to poor microglial identity following scRNA-seq and morphology analysis. In the final differentiation, we identified that our new medium (ITMG) produced iPSC-microglia with a microglial-like transcriptome, improved survival, and performed similarly to our previous medium in functional assays. Created with BioRender.com. (C) Conditions and media tested and their reason for exclusion. (D) Representative images of d14 microglia cultured in our previous media (IGBN) and the media presented in this manuscript (ITMG). Scale bar 100 µm.
RNA extraction
RNA was extracted from cells using TRIzol LS (Invitrogen, 10296010) and purified using the Zymo DirectZol RNA Miniprep Kit (Zymo Research, R2050) following manufacturer’s instructions. Briefly, media was removed from the microglia and wells washed with PBS, 250 µL TRIzol LS reagent was then added to each well and cells flushed off using a p1000 pipette. Lysates were collected into a 1.5 mL tube and either frozen at − 80 °C or immediately extracted following the Zymo DirectZol protocol. RNA was eluted in 25 µL RNase/DNase free water and RNA concentrations quantified by Nanodrop.
For HipSci pooled differentiations, RNA was extracted using AllPrep DNA/RNA Mini kit (QIAGEN 80204) or RNeasy Mini kit (QIAGEN 74104) with QIAshredder (QIAGEN 79654) and treated with Turbo DNase (Life Tech AM1907) following manufacturer’s instructions.
qPCR
Maximal RNA was converted to cDNA using the High Capacity RNA-cDNA conversion kit (Applied Biosystems, 4387406) following manufacturer’s instructions. Assuming a 100% conversion rate, cDNA was then diluted to 1 ng/µL using RNase/DNase free water for use in qPCR. qPCR reactions were undertaken in triplicate using Power SYBR Green PCR Master Mix (Applied Biosystems, 4367659) following manufacturer’s instructions in 384 well plate format with the QuantStudio 5 system. For each cDNA conversion a qPCR for housekeeping gene panel was undertaken to confirm the most stably expressed housekeeper to use for the marker analysis. Primers are listed in (Supplementary Table S5). GeNorm was used to analyse housekeeper stability and the two most stable housekeepers were taken forward for use in the marker qPCR panel26. We undertook qPCR on a panel of known microglial and perivascular marker genes. Data was analysed in the statistical package R using linear regression, multiple testing of p values were corrected by Bonferroni. Raw Ct values were collected from the software, SDs of the triplicates were calculated, any outliers (determined by ± 2.5 SD about the mean) were excluded and the mean Ct value was recalculated. Differential expression was calculated using the 2−ΔΔCT method using the selected housekeepers as normalising controls27. The baseline condition is defined in each experiment as either RNA extracted from cells in the baseline microglial media (IGBN) or from microglial precursors. Heatmaps were generated in R using the pheatmap package28.
Single cell RNA sequencing
To harvest cells for single-cell RNAseq, the cells were incubated in a dissociation buffer of DPBS with 5 mM EDTA (Invitrogen 15575-038) and 4 mg/mL lidocaine hydrochloride monohydrate (Sigma L5647) at 37 °C for 15 min. An equal volume of 0.04% BSA in DPBS was added to each well to avoid sticking of cells to vessels. Cells were collected into centrifuge tubes pre-coated with 0.04% BSA and centrifuged at 400×g for 5 min. The pellet was resuspended in 0.04% BSA and strained through MACS 30 µm Cell Strainer (Miltenyi Biotec Ltd 130-041-407) twice. The single cell suspension was centrifuged at 400×g for 5 min and resuspended in 0.04% BSA for counting. The cell suspension was placed on ice when possible.
Cell suspensions containing 17,400 viable cells (aiming for recovery of 10,000 cells) were processed by the Chromium Controller (10 × Genomics) and barcoded libraries constructed using the Chromium Next GEM Single Cell 5′ v2 kit (PN-1000263) following manufacturer’s instructions. Samples were sequenced with Illumina HiSeq and reads were mapped and UMI count matrices generated with 10 × Genomics CellRanger v.6.0.1 using default parameters and reference transcriptome v. GRCh38-2020. Singlet donor and doublet identity was inferred with cellSNP-lite v. 1.2.0 followed by Vireo v. 0.2.129,30. Single cell data was analysed with Seurat v. 4.0.0 in R v. 4.0.331. Briefly, cells with over 1000 genes, UMI counts under 40,000, under 10% mitochondrial genes, and that had singlet identity predicted by Vireo were retained (doublets ranged from 7 to 13% of all cells). Cell cycle information for every cell was calculated using Seurat’s CellCycleScoring() with marker genes from Tirosh et al.32. Libraries were normalised with SCTransform(), and clustering and dimensionality reduction was performed using UMAP within Seurat following the standard pipeline on the merged libraries. Pairwise differentially expressed genes per media contrast were identified with FindMarkers() using the Wilcoxon test on log-normalised data, and p values were adjusted using Bonferroni correction. Cellular identity was inferred by automatic cell type annotation per media sample using singleR33, which uses a scoring metric for each cell based on correlation of gene expression between the test and the train dataset, using genes that are differentially expressed between the training data labels. The datasets used for annotation were human iPSC-derived microglia matured in mouse xenografts16 and human foetal macrophage precursors34. Figures were made with tidyverse35 and SCpubr36.
Principal component analysis of scRNA-seq samples and external datasets
We compared our transcriptomic dataset to induced, ex vivo and primary cell types from external published bulk RNA-seq datasets, from9,37,38,39,40. We also included the data from human iPSC-derived microglia matured in mouse xenografts for comparison to other ex vivo microglia. All scRNA-seq datasets were converted to pseudobulk. After merging the datasets to raw counts of shared genes, and removing genes with fewer than a sum of 15 counts in total, 10,345 genes were left for normalisation and mean–variance adjustment (with limma’s voom41) and batch correction of the study of origin (with limma’s removeBatchEffect). We finally performed principal component analysis on the corrected counts.
Identification of perivascular macrophage markers
Publically available data from 1.3 million mouse brain cells was processed as follows16,34: from a subsample of 20 k cells, markers of the microglial/macrophage subpopulation were identified by FindAllMarkers() in Seurat; then, a microglial/macrophage identity classifier was built from the top 10 microglial markers with AddModuleScore(), which was then applied to score the whole population of 1.3 million cells; cells were considered microglia or macrophages if the score was > 1 (~ 5 k cells). After normalisation and clustering, three separate clusters of brain macrophage cells (microglia, perivascular macrophages, and macrophage/monocytes) were identified and classified according to the presence of pre-existing markers in the literature in differentially expressed genes calculated via FindMarkers(), and converted to human orthologs (Supplementary Table S6). A subset of perivascular macrophage markers (F13A1, LYVE1, COLEC12 and CD163) were selected for qPCR according to fold expression changes, fraction of microglial cells that express them vs perivascular macrophages, and expression in primary microglia and other reference datasets (data not shown).
Generation of double fluorescent SH-SY5Y cell line for measuring phagocytic uptake and acidification by microglia
Generation of the SH-SY5Y cell line that reports on its phagocytic uptake and subsequent acidification by microglia (efferocytosis), was as follows. The mCherry-EGFP fragment of pBABE-puro mCherry-EGFP-LC3B (Addgene 22418) was PCR amplified and cloned into a lentivirus backbone (Ef1αF IRES Puro) downstream of EF1α the promotor, using SpeI and BamHI cloning sites (Supplementary Figure S1). This generated the construct EF1α pmChGIP IRES Puro (pmChGIP, Supplementary Figure S1) (plasmid available on request). Dual expression of mCherry-eGFP in pmChGIP was confirmed following transfection into HEK293T cells with FuGENE HD (Promega, E2311) (Supplementary Figure S1). pmChGIP lentiviral particles were generated by transfecting 4 × 106 HEK293T cells in 10 cm dishes with 4 μg EF1α pmChGIP IRES Puro, 2 μg psPAX2 (Addgene 12260), 2 μg pMD2.G (Addgene 12259). Lentiviruses were harvested at 48 h and 72 h post transfection before centrifugation at 3000 rpm to remove any HEK293T cells followed by filtering through a 0.45 μm filter low bind PES, processed lentiviruses were stored in single use aliquots at −80 °C. Lentiviral titre was calculated using flow cytometry. P10 SH-SY5Y (ATCC, CRL-2266) neuroblastoma cells were transduced at a multiplicity of infection of 2 or 0.3, and double positive mCherry-eGFP cells were single cell sorted at 24 h post transduction into 96 well plates to generate monocultures (Supplementary Figure S1). Cells were allowed to clonally expand for several weeks before passaging and banking. Generated clonal lines underwent quality control using flow cytometry to confirm a uniform population of double positive mCherry-eGFP expression (Supplementary Figure S1). Clone F4 2 was used for assays, due to its high fluorochrome expression. Lines are known as pmChGIP SH-SY5Y and are available upon request.
Phagocytosis assays
Microglial precursors were plated at 8.8 × 105 cells per well in 48 well plates in either the newly optimised medium (ITMG, no coating) or the previous medium (IGBN, Geltrex coating (Haenseler et al.)), and matured to microglia for 14d. For imaging, cells were stained for 1 h at 37 °C/5% CO2 with 1 μM CellTracker Deep Red (Invitrogen, C34565) and 1 drop/mL NucBlue Live ReadyProbes Reagent (Invitrogen, R37605). Cells were washed with PBS, and then incubated for 2 h at 37 °C/5% CO2 with phagocytic cargo. The three cargo were prepared as follows: (1) Amyloid β aggregates were generated by spiking fluorescent labelled β-Amyloid (Beta-Amyloid [1-42] HiLyte Fluor 488, Anaspec, AS-60479-01) 1:10 with unlabelled β-Amyloid (Beta-Amyloid [1-42] Aggregation Kit, rPeptide, A-1170-1), with shaking incubation for 2 days at 37 °C. Aggregates were stored at − 80 °C and added at a final concentration of 3 µM; (2) 3 µm carboxylated silica beads (Kisker Biotech, PSI-3.0COOH) were washed in PBS before resuspension in 25 mg/mL cyanamide for 15 min on a rotator. Beads were washed twice with coupling buffer (0.1 M Sodium tetraborate, ddH2O, pH 8.0) before labelling for 1 h with 50 µg/mL AF-488 NHS Ester (ThermoFisher, A20000) in coupling buffer. Beads were washed 3 times with quenching buffer (250 mM glycine, PBS, pH 7.2) before storage in 0.02% NaN3 PBS at 4 °C. Beads were added at a 2:1 ratio beads:microglia; (3) pmChGIP SH-SY5Y neuroblastoma cells were maintained in 10% FBS, DMEM/F-12 media at 37 °C/5% CO2. Cells were harvested with TrypLE Express (Gibco), centrifuged at 400×g for 5 min and resuspended in Hank’s Balanced Salt Solution (HBSS, Lonza). Paraformaldehyde (PFA) (Alfa Aesar) was added to a final concentration of 2% and fixed for 10 min at room temperature. PFA was then diluted in HBSS before centrifugation at 1200×g for 5 min, the cell pellet was washed twice with HBSS. SH-SY5Y cells were added at a 2:1 ratio SH-SY5Y:microglia. Following incubation, iPSC-microglia were washed with PBS to remove non-phagocytosed cargo. iPSC-microglia were live imaged on an EVOS microscope (ThermoFisher), image analysis was undertaken in ImageJ. For flow cytometry, iPSC-microglia were lifted with TrypLE Express (Gibco) and fixed in 2% at room temperature for 10 min. Cells were pelleted at 400×g for 5 min and washed with PBS twice before flow cytometry (Beckman Coulter CytoFLEX Flow Cytometer). Analysis of phagocytic ability was undertaken using FCS Express.
Results
IL-34, TGF-β1, M-CSF, and GM-CSF differentially influence morphology and gene expression in iPSC-microglia in monoculture
The previous medium developed by our lab for culturing iPSC-microglia had been optimised for co-culture with neurons, as a result it contained several components that might not be required or optimal for microglial monoculture, including N-2 supplement, β-mercaptoethanol and Geltrex substrate10. It also did not contain factors that would be expected to be present in co-culture but which might enhance microglial identity if supplemented into monoculture medium—specifically, TGF-β1 (produced by astrocytes and also by microglia42,43) and CX3CL1/CD200 (produced predominantly by neurons44,45). These factors have been used in some other microglial protocols12,16,17. We set out to identify whether removal of neuron-relevant components or addition of these additional factors to the media resulted in differential morphological or transcriptional changes to monocultured iPSC-microglia. In differentiation experimental series 1 (Fig. 1b), we matured microglial precursors to microglia for 14 days using 15 different combinations of factors (Supplementary Table S4), including the original Haenseler et al.10 microglia monoculture medium as the baseline condition (IGBN), the modification of that protocol used by Brownjohn et al.11, (IGF) and also the widely adopted protocol that includes TGF-β1/CX3CL1/CD200 by McQuade et al. (ITMCBN)12.
Visual inspection was used as an initial triage of the 15 conditions (Fig. 2a). IL-34 as the sole cytokine (I and IBN) was not sufficient for microglial survival, despite its use at 100 ng/mL and was therefore not taken forward as sole cytokine. However, co-supplementation with low-dose GM-CSF (IG) or supplementation of IL-34 with M-CSF (MG) supported survival. Removal of either N-2 (IGB) or β-mercaptoethanol (IGN) or both (IG) had no effect on microglial morphology or survival compared with IGBN, consistent with their role in neuronal support rather than for microglia in the iPSC-microglia/neuronal co-culture protocol10. TGF-β1 induced a clear difference in morphology, microglia were less adherent and more rounded, regardless of further supplementation with other factors (ITG/ITGC/ITGBN/ITGCBN/ITMCBN). CX3CL1/CD200 addition for the final 4 days of differentiation, as described by12,16, had no effect on morphology (ITGC/ITGBN/ITGCBN/ITMCBN). FBS (IGF) induced expansion of fibroblasts in the culture, therefore this condition was discarded from future experiments (Fig. 2a).
IL-34, TGF-β1, M-CSF, and GM-CSF affect iPSC-microglia morphology and result in transcriptional changes. (A) Morphology of microglia cultured for 14 days in 15 different media combinations on Geltrex. Each letter corresponds to a growth factor or compound; I IL-34, T TGF-β1, M M-CSF, G GM-CSF, C CX3CL1/CD200, B β-mercaptoethanol, N N-2 supplement, F Foetal Bovine Serum. Phase contrast images taken at ×10 magnification, scale bar 100 µm. * media IGBN is media published by Haenseler et al., and is the baseline for comparison. (B) qPCR results of known microglial marker genes in medium containing different growth factors. Fold changes initially calculated to IGBN before undertaking linear regression, regressing out each individual factor. TGF-β1, M-CSF, GM-CSF all result in transcriptional changes. CD200/CX3CL1, β-mercaptoethanol, and N-2 supplement result in no significant change in microglial gene expression. Fold changes are shown by colour, where green is increased expression and red is decreased and the number within cells. Stars indicate Bonferroni corrected significance, ***p < 0.001, **p < 0.01, *p < 0.05. Heatmap generated in R using the pheatmap package.
qPCR analysis of microglial markers was consistent with the findings of the morphology observations. Fold changes were calculated to condition IGBN, followed by linear regression (see “Methods”) to examine the effects of inclusion of the different factors (Fig. 2b). Removal of β-mercaptoethanol or N-2 resulted in no significant differences in gene expression compared to medium where each were included, confirming that β-mercaptoethanol and N-2 have no effect on microglial identity in monoculture. Equally, supplementation with CX3CL1/CD200 for the final 3 days resulted in no strong transcriptional changes, and were therefore excluded from further experiments. Inclusion or removal of IL-34 resulted in no significant changes to gene expression across all the media conditions versus the baseline condition IGBN, however only two of the fifteen conditions did not include IL-34. M-CSF supplementation resulted in a significant decrease in IL-1β (FC, 0.15) and TGF-β1 (FC, 0.49), with C1QA (FC, 2.01) significantly increased. TGF-β1 supplementation resulted in a significant decrease in CD200R1 (FC, 0.09) expression and significant increases in IL-1β (FC, 6.04) and MERTK (FC, 2.79). Medium in which GM-CSF was included resulted in a significant decrease in expression of TMEM119 (Fold Change (FC), 0.26), MERTK (FC, 0.54), IL-1β (FC, 0.36), CSF1R (FC, 0.53) and an increase in PROS1 (FC, 1.86) and CD200R1 (FC, 2.78).
Combining both the morphology and qPCR data from these initial 15 conditions we concluded that while IL-34, TGF-β1, M-CSF, and GM-CSF result in differential microglial phenotypes, CX3CL1/CD200, β-mercaptoethanol and N-2, have no effect in this experimental setup, and FBS is clearly detrimental.
Supplementation with TGF-β1 promotes microglial-like identity, whereas M-CSF promotes macrophage identity, these changes are conserved regardless of culturing matrix
Next we sought to identify if supplementation with M-CSF, TGF-β1, or a combination of both would support microglial differentiation compared to our original baseline condition, IGBN medium. In parallel, we tested different tissue culture matrices that have been used in published protocols10,12,17.
We trialled five media conditions in differentiation two with combinations of factors identified by our initial triage: the baseline condition IGBN (Haenseler et al.10) (IL-34, low-dose GM-CSF, β-mercaptoethanol, N-2); IMBN (IL-34, M-CSF, β-mercaptoethanol, N-2), where GM-CSF is replaced with M-CSF; ITGBN (IL-34, TGF-β1, GM-CSF, β-mercaptoethanol, N-2) where the baseline IGBN condition is supplemented with TGF-β1; ITM RPMI (IL-34, TGF-β1, M-CSF) a medium previously described by17 and ITM ADMEM (IL-34, TGF-β1, M-CSF, Advanced DMEM basal media). ITM ADMEM ensured the same basal media was used for all factor cocktails. We plated cells at the same density on three different matrices: standard TC treated plastic; Geltrex coated or 10 μg/mL fibronectin coated.
iPSC-microglia cultured on tissue-culture plastic were observed to have greater attachment and confluency than on Geltrex, which was greater than fibronectin (Fig. 3a). There was no notable difference in morphology between IGBN, IMBN, and ITGBN on any of the different matrices. However, ITM resulted in poor attachment, especially in RPMI base, and this was only partly rescued by ADMEM base in combination with TC plastic. The minimal composition of RPMI media versus ADMEM may play a role here.
TGF-β1 promotes microglial identity, and M-CSF promotes macrophage identity, tissue culture coating influences morphology but medium factors are the main driver of identity. (A) Morphology of iPSC-microglia cultured for 14 days in five different media combinations on either tissue culture treated plastic, Geltrex, or 10 µg/mL fibronectin. Each letter corresponds to a growth factor or compound; I IL-34, T TGF-β1, M M-CSF, G GM-CSF, B β-mercaptoethanol, N N-2 supplement. Phase contrast images taken at ×10 magnification, scale bar 100 µm. * media IGBN is media published by Haenseler et al., and is the baseline for comparison. Geltrex resulted in the fewest cells per media condition, followed by fibronectin, with TC treated plastic resulted in the highest cell number. ITM with RPMI base medium results in a large reduction of cell numbers compared to the medium with ADMEM/F12 base. (B) qPCR of the 15 samples in (A) for microglial and perivascular macrophage markers, fold changes represented relative to IGBN Geltrex (the Haenseler et al. protocol). Fold changes are presented as both colour changes (green indicates increased expression, red decrease expression) and the number within each cell. Samples cluster by medium, with medium containing TGF-β1 showing lower expression of perivascular macrophage genes (F13A1/LYVE1/COLEC12) and increased microglial genes (CX3CR1/MERTK/OLFML3). Heatmap generated in R using the pheatmap package.
qPCR analysis identified that medium composition was the main driver of clustering, resulting in much larger transcriptional changes than tissue culture coating (Fig. 3b). For this qPCR panel we included perivascular macrophage markers (F13A1/LYVE1/COLEC12/CD163), as identified by differential expression of brain macrophage subpopulations (see “Methods”), and which have been described previously in the literature46,47. These were highest within the baseline IGBN media and were downregulated when the media was supplemented with TGF-β1. Conversely, TGF-β1 supplementation (ITGBN) resulted in increased expression of microglial identity genes CX3CR1 (29/197/270 fold increase on plastic, Geltrex, and fibronectin respectively), MERTK (0.8/2.6/4.3), OLFM3 (0.3/2.3/1.3) and P2RY12 (0.4/5.5/2.9). Note also that these microglial identity genes all are increased when cells are cultured on a matrix. Replacing GM-CSF with M-CSF (IGBN vs IMBN) reduced expression of perivascular macrophage markers. Microglial markers (CX3CR1/MERTK/OLFML3) were also increased in IMBN, however the fold changes were lower than for ITGBN, indicating that TGF-β1 is the key driver in microglial identity.
Interestingly, co-supplementation of TGF-β1 with M-CSF resulted in the strongest microglial identity signal (ITM), with MERTK, CXCL8, and OLFML3 showing the largest fold change (CX3CR1 was also upregulated but the changes were not as great as ITGBN). Basal media (ADMEM/F12 vs RPMI ATCC) had little effect on the gene expression profile of the microglia.
These gene expression profiles correlated well between independent differentiations at two independent sites, using iPSC with different genetic backgrounds (Supplementary Figure S2), indicating that the media conditions tested result in robust changes in gene expression (IMBN, R2 = 0.236, p value = 0.03. ITGBN, R2 = 0.348, p value = 0.006). Given the close correlation in gene expression between ITGBN and ITM we next assessed whether inclusion of low-dose GM-CSF would improve cell retention while maintaining microglial identity.
Low-dose GM-CSF promotes cellular retention while maintaining microglial identity
Our final differentiation series (differentiation three, Fig. 1b) examined the effect of low-dose GM-CSF (10 ng/mL) on the retention (defined as the cells remaining at the end of the differentiation which depends on adherence, survival, and proliferation) of iPSC-microglia in monoculture We assessed six conditions: the baseline IGBN medium10; modified ITM ADMEM medium (from17); and ITM supplemented with GM-CSF (ITMG), each on tissue culture treated plastic versus fibronectin-coating, then undertook qPCR for microglial and perivascular marker gene expression.
Tissue culture treated plastic versus fibronectin coating had no effect on morphology across all three media (Fig. 4a). ITM ADMEM medium resulted in decreased adherence and a more rounded morphology compared to IGBN/ITMG, in agreement with the previous experimental series. Supplementation with low-dose GM-CSF (ITMG, versus ITM) resulted in stronger retention on the substrate and resultant increased yield of cells at the end of the 14 days of culture, this is supported by previous work in neutrophils and macrophages48,49. A caveat here is that reduced adherence of ITM cells and our use of 50% media changes may have contributed to the diminished retention of ITM cells. Prior studies have similarly observed decreased adherence of cells matured in medium containing ITM and used media addition instead of 50% media replacement to avoid such loss12,50.
Low level supplementation with GM-CSF promotes cellular survival while maintaining microglial identity. (A) Morphology of microglia cultured for 14 days in three different media combinations on either tissue culture treated plastic or 10 µg/mL fibronectin. Each letter corresponds to a growth factor or compound; I IL-34, T TGF-β1, M M-CSF, G GM-CSF, B β-mercaptoethanol, N N-2 supplement. Phase contrast images taken at ×10 magnification, scale bar 100 µm. Media IGBN (Haenseler et al.10) is the baseline for comparison. ITM ADMEM results in low survival and adherence on both TC treated plastic and 10 µg/mL fibronectin, however when supplemented with GM-CSF (ITMG) the survival and adherence is improved. (B) qPCR of the six samples from (A) for microglial and perivascular macrophage markers, fold changes represented as changes to IGBN TC treated plastic. Fold changes are presented as both colour changes (green indicates increased expression, red decrease expression) and the number within each cell. Samples cluster by medium followed by coating. ITMG promotes microglial identity with increased expression of CX3CR1 and OLFM3 and a reduction in perivascular macrophage markers (LYVE1/F13A1). Coating with fibronectin results in an increased expression of microglial identity genes in ITM and ITMG. Heatmap generated in R using the pheatmap package.
qPCR indicated strong clustering by media rather than by coating (Fig. 4b), in accordance with the previous experimental series. As previously, inclusion of TGF-β1 decreased perivascular marker expression (COLEC12/LYVE1/F13A1), and increased standard microglial marker gene expression (CX3CR1, OLFML3, MERTK). Microglia cultured in ITM ADMEM had decreased expression of CD200R1, P2RY12 and C1QA, but increased OLFML3 versus IGBN/ITMG. The strongest microglial identity signal was in iPSC-microglia cultured in ITMG on fibronectin. Again, these results were highly correlated between two independent labs, independent differentiations, and different genetic backgrounds (ITM, R2 = 0.639, p value = 2.36 × 10–5, ITMG, R2 = 0.422, p value = 0.00195) (Supplementary Figures S3, S4).
While the panel of qPCR markers helped to confirm microglial identity, this was limited to a few select marker genes, so we next explored whether the different media result in different subtypes of microglia51,52 by undertaking single cell RNAseq on a subset of conditions.
Single cell RNA-seq confirms that TGF-β1 promotes microglial identity, while medium containing M-CSF only promotes macrophage identity
To get a more detailed picture of the transcriptional landscape of iPSC-microglia generated by the different media, we undertook scRNA-seq of cells cultured in the baseline medium IGBN, and IM, IMBN, ITGBN, ITM ADMEM and ITMG, all on TC plastic. After quality control, filtering of doublets and low-quality cells, normalization and clustering (see “Methods”), we could infer that the transcriptome of our samples (six different media) is similarly placed within the principal component space as iPSC-derived microglia derived from other protocols (Supplementary Figure S5). We also observed higher transcriptomic similarity between ITM ADMEM and ITMG media (Fig. 5a, Supplementary Figure S6). The examination of cell marker distribution indicated some heterogeneity in microglial marker expression among the different media, with reduced density of perivascular marker gene expression in ITM ADMEM and ITMG (Supplementary Figure S6). Differential expression analysis of each medium against the IGBN baseline resulted in a mean of 1196 significant differentially expressed (DE) genes, with the ITM ADMEM and ITMG comparisons resulting in the largest amount of DE genes (Supplementary Figure S6, Supplementary Table S7). These DE genes included many of the previously highlighted microglia and perivascular macrophage markers (Fig. 5b). Analysis of microglial and perivascular markers for these media showed again a significant increase in the expression of the microglial genes in media including TGF-β1, and the highest perivascular macrophage gene expression in IGBN, IM and IMBN media.
Single cell RNA-seq confirms that TGF-β1 promotes microglial identity. (A) Uniform manifold approximation and projection (UMAP) plot of quality-controlled iPSC-derived microglia cells, coloured by differentiation media. (B) Violin plots of SCT-normalised expression (y-axis) per media (x-axis) per perivascular macrophage or microglia marker gene. (C) Label transfer with singleR: cumulative percentage of cells (y-axis) of each label (colour) per media (x-axis). Mac precursor 1–3; macrophage precursors subtypes, CD7loP CD7lo progenitor, ErP erythroid progenitor, YSMP yolk sac-derived myeloid-biased progenitor, MkP megakaryocyte progenitor, Cytokine/Amyloid resp. microglia Cytokine/Amyloid response microglia.
We then expanded our investigation of the transcriptional identity of these cells to the whole genome by performing unbiased cell type annotation (“label transfer”) with singleR using two reference datasets. The first one comprises of foetal macrophage precursors and other hematopoietic cells isolated from human embryos34, including developing microglia, and was chosen due to the perceived similarities between iPSC-derived cells and immature microglia. The second dataset is comprised of iPSC-derived microglia whose last step of maturation has been performed as xenografts in mouse brain16, and represents the in vitro-generated microglia that would be most similar transcriptomically to human primary microglia. Label transfer results confirm the increased microglial identity when using media including TGF-β1 (Fig. 5c, Supplementary Figure S6), particularly ITM ADMEM and ITMG, as denoted by the larger proportion of cells annotated as “microglial precursors” in the foetal macrophage dataset, and the reduced number of perivascular macrophages as classified by the xenografted iPSC-microglia dataset.
While cells matured in ITM ADMEM and ITMG are both highly classified as microglia, the reduced reproducibility and poorer retention of cells cultured in ITM across different differentiations led us to conclude that ITMG is preferable, as it not only has a similar transcriptional profile to ITM ADMEM but also has improved cell retention and reproducibility across differentiations. Therefore ITMG was taken forward for final functional characterisation versus our original IGBN medium.
Microglia differentiated in optimised ITMG medium exhibit phagocytic competence
Finally we examined the ability of microglia matured in the newly optimised ITMG medium to take up phagocytic cargo, compared to the baseline medium, IGBN. We utilised three different cargos: fluorescent Amyloid-β aggregates; 488-labelled silica beads; and a novel double-fluorescent SH-SY5Y cell line to measure dead neuron uptake and acidification in the phagolysosome. The double fluorescent SH-SY5Y cell line expresses a GFP-mCherry fusion protein which becomes single mCherry fluorescent when phagocytosed into the low pH environment of the phagolysosome due to pH sensitive conformational changes resulting in loss of GFP fluorescence. As expected, different cargos were taken up by the cells at different rates (Amyloid-β > silica beads > dead SH-SY5Y) (Fig. 6). Importantly, regardless of the cargo, we observed no significant difference between microglia cultured in IGBN versus ITMG media, demonstrating that the improved medium does not alter this important microglial function.
Functional analysis of phagocytosis results in no difference between the new ITMG medium and our previously published IGBN medium. (A) To examine phagocytosis in differentiated iPSC-microglia in our new medium, we incubated cells with three different cargos; Amyloid-β aggregates, 488 labelled silica beads, and fluorescent labelled dead SH-SH5Y neuroblastoma. Phagocytosis occurred for 2 h before fixing the microglia and quantifying fluorescence using FACS, and representative images taken by microscopy. Created with BioRender.com. (B) There is a difference in the phagocytic ability of the different cargos, however there is no difference between the two medium. Data presented as mean ± SEM, N = 3 independent replicates, with individual data points shown. Each independent replicate contained three technical repeats. (C) Representative images of Amyloid-β phagocytosis at 2 h. (D) Representative images of AF-488 labelled bead phagocytosis at 2 h. (E) Representative images of labelled dead SH-SY5Y neuroblastoma phagocytosis is 2 h, red indicates a SH-SY5Y which has been phagocytosed and is in low pH environment. All images are shown at ×40 magnification, microglia are marked with CellTracker, and nuclei are stained with DAPI.
Discussion
Multiple papers over the last 5 years have described different methods for differentiating iPSC to microglia, resulting in variation between labs and potential reproducibility issues when translated to different institutes or genetic backgrounds. For iPSC-microglia in monoculture the field has not yet fully consolidated which factors are crucial for the development of iPSC-microglia and some media contain redundant “legacy” factors, their role having never been assessed systematically. Moreover, some protocols use poorly defined components, including conditioned media53. We set out to systematically identify the key defined factors required for microglial identity in monoculture, utilising morphology, qPCR for both microglial and non-microglial brain macrophages, and scRNA-seq, across two laboratories and multiple genetic backgrounds, to provide a highly reproducible differentiation and single-cell level characterisation of the resulting microglial population.
We started by identifying the defined components used in various iPSC-microglial protocols and tested in parallel their ability to produce iPSC-microglia in monoculture, compared to our original medium (IGBN). Initial morphological assessment allowed us to exclude medium containing FBS (fibroblast outgrowth) and IL-34-only supplementation (increased cell clumping and death). Media where IL-34 was supplemented with M-CSF or low-dose GM-CSF showed increased survival. M-CSF and IL-34 engage the myeloid receptor CSF1R, and GM-CSF acts on the receptor CSF2R, both of which activate survival signalling pathways54,55. IL-34 is the main ligand for CSF1R in the brain, whereas M-CSF is the main ligand for CSF1R in the periphery, both mediating a similar intracellular response54. Supplementation with M-CSF is known to promote a macrophage phenotype and has been used in multiple iPSC-macrophage protocols, and the scRNA-seq analysis here shows that M-CSF-alone induces a more macrophage-like phenotype22,24,56. This supports a unique role of IL-34 in microglial development in the brain which cannot be replicated by peripheral M-CSF alone57,58.
qPCR and morphological analysis confirmed that β-mercaptoethanol and N-2 supplement have no effect iPSC-microglial differentiation or transcription so can be included if microglia are to be used in co-culture10. Most surprisingly was the lack of effect of CX3CL1/CD200 on the phenotype of iPSC-microglia when others have observed changes16,59. Both CX3CL1/CD200 are immunoregulators expressed by neurons, and ligands for CX3CR1/CD200R on microglia. While we see no effect of CX3CR1/CD200 in our model, this does not mean they might exhibit the same response in models using different methods of early hematopoiesis, such as 2D (i.e. non-Embryoid Body-based) protocols12,16. Nonetheless, the addition of TGF-β1 (another potent immunoregulator) could make the role of CX3CL1/CD200 redundant44,45,60,61. These initial results reduced our factor list from nine to four (IL-34, TGF-β1, M-CSF, GM-CSF) for further exploration.
We identified consistent upregulation of microglial marker genes CX3CR1, MERTK and OLFML3 in medium containing TGF-β1. This is unsurprising, as TGF-β1 has been shown to be critical to microglial maturation in mice62. Furthermore, adult primary microglia cultured in M-CSF and TGF-β1 have been shown to have increased microglial identity versus M-CSF or GM-CSF alone62, which is replicated here in iPSC-microglia. In human iPSC microglia, the importance of TGF-β1 has been noted to change expression of known microglial targets9. In our study, the scRNA-seq data further supports the role of TGF-β1 in microglial maturation versus M-CSF in macrophage maturation.
The low cell retention and poor reproducibility of microglia matured in ITM led us to explore if GM-CSF would promote cellular retention while maintaining microglial identity. GM-CSF is a cytokine expressed by astrocytes and during neurodevelopment63,64. GM-CSF has been identified to improve antigen presentation in murine primary microglia65 and has been known to modify microglial morphology10,66. This is supported in our findings, where ITMG microglia are more adherent and ramified versus ITM microglia, regardless of culturing matrix, and the phenotype more consistent across differentiations than ITM microglia. Examining the transcriptome through qPCR identified several changes between IGBN and ITMG. Notably, the reduction in the perivascular macrophage markers (F13A1/LYVE1) and increase in microglial markers (CX3CR1/OLFML3/P2RY12). The use of ITMG is, therefore, preferable for experiments requiring consistent adherence, whereas ITM may be preferable for experiments where non-adherent microglia are acceptable. Finally, while microglial identity is further improved when cells are cultured on fibronectin, media is the main driver of identity so the use of fibronectin is left to the discretion of the user.
Conclusion
Coupling the results of morphological assessment, qPCR, scRNAseq, and phagocytosis assays, we have demonstrated that the optimised ITMG medium described here improves on our previously published IGBN medium for culturing iPSC-microglia. Differentiation of iPSC to mesoderm and hemogenic endothelium via embryoid bodies, then bulk myeloid differentiation to primitive macrophage/microglia precursors, and final maturation of monoculture iPSC-microglia in ITMG medium, provides a highly scalable pipeline for routine experiments investigating neuroinflammation.
Data availability
All single cell RNAseq data is available from the European Nucleotide Archive (www.ebi.ac.uk/ena/browser/home), project number PRJEB55440. Code for the data analysis can be found on GitHub (https://github.com/TrynkaLab/washer_et_al_microglia_media).
References
Prinz, M., Jung, S. & Priller, J. Microglia biology: One century of evolving concepts. Cell 179(2), 292–311. https://doi.org/10.1016/j.cell.2019.08.053 (2019).
Novikova, G. et al. Integration of Alzheimer’s disease genetics and myeloid genomics identifies disease risk regulatory elements and genes. Nat. Commun. 12, 1. https://doi.org/10.1038/s41467-021-21823-y (2021).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53(9), 1276–1282 (2021).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51(3), 414–430 (2019).
Bryois, J. et al. Genetic identification of cell types underlying brain complex traits yields novel insights into the etiology of Parkinson’s disease. Nat. Genet. 52, 482–493 (2020).
Clarke, B. E. & Patani, R. The microglial component of amyotrophic lateral sclerosis. Brain 143(12), 3526–3539 (2020).
Dachet, F. et al. Selective time-dependent changes in activity and cell-specific gene expression in human postmortem brain. Sci. Rep. 11(1), 1–11 (2021).
Pandya, H. et al. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat. Neurosci. 20(5), 753–759 (2017).
Abud, E. M. et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94(2), 278–293 (2017).
Haenseler, W. et al. A Highly efficient human pluripotent stem cell microglia model displays a neuronal-co-culture-specific expression profile and inflammatory response. Stem Cell Rep. 8(6), 1727–1742 (2017).
Brownjohn, P. W. et al. Functional studies of missense TREM2 mutations in human stem cell-derived microglia. Stem Cell Rep. 10(4), 1294–1307 (2018).
Mcquade, A., Coburn, M., Tu, C. H., Hasselmann, J. & Davtyan, H. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol. Neurodegener. 13(67), 1–13 (2018).
Konttinen, H. et al. PSEN1ΔE9, APPswe, and APOE4 confer disparate phenotypes in human iPSC-derived microglia. Stem Cell Rep. 13(4), 669–683 (2019).
Claes, C. et al. Human stem cell-derived monocytes and microglia-like cells reveal impaired amyloid plaque clearance upon heterozygous or homozygous loss of TREM2. Alzheimer’s Dementia 15(3), 453–464 (2019).
Svoboda, D. S. et al. Human iPSC-derived microglia assume a primary microglia-like state after transplantation into the neonatal mouse brain. PNAS 116(50), 25293–25303 (2019).
Mancuso, R. et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat. Neurosci. 22, 25 (2019).
Reich, M. et al. Alzheimer’s risk gene TREM2 determines functional properties of new type of human iPSC-derived microglia. Front. Immunol. 11(February), 1–15 (2021).
Guttikonda, S. et al. Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer’s disease. Nat. Neurosci. 24(3), 343–354 (2021).
Morrison, M. et al. StemBANCC: Governing access to material and data in a large stem cell research consortium. Stem Cell Rev. Rep. 11(5), 681–687 (2015).
Bruntraeger, M., Byrne, M., Long, K. & Bassett, A. R. Editing the Genome of Human Induced Pluripotent Stem Cells Using CRISPR/Cas9 Ribonucleoprotein Complexes. CRISPR Gene Editing: Methods and Protocols 153–183 (Springer, 2019).
Dafinca, R. et al. C9orf72 hexanucleotide expansions are associated with altered endoplasmic reticulum calcium homeostasis and stress granule formation in induced pluripotent stem cell-derived neurons from patients with amyotrophic lateral sclerosis and frontotemporal dementia. Stem Cells 34(8), 2063–2078 (2016).
Vaughan-Jackson, A. et al. Differentiation of human induced pluripotent stem cells to authentic macrophages using a defined, serum-free, open-source medium. Stem Cell Rep. 16(7), 1735–1748 (2021).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8(5), 424–429 (2011).
van Wilgenburg, B., Browne, C., Vowles, J. & Cowley, S. A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One 8, 8 (2013).
Gutbier, S. et al. Large-scale production of human iPSC-derived macrophages for drug screening. Int. J. Mol. Sci. 21(4808), 1–23 (2020).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3(7), 1–12 (2002).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 28, 402–408 (2001).
Kolde R. pheatmap: Pretty Heatmaps. R Package version 1.02.12 (2019). https://CRAN.R-project.org/package=pheatmap.
Huang, X. & Huang, Y. Cellsnp-lite: An efficient tool for genotyping single cells. Bioinformatics 37(23), 4569–4571 (2021).
Huang, Y., McCarthy, D. J. & Stegle, O. Vireo: Bayesian demultiplexing of pooled single-cell RNA-seq data without genotype reference. Genome Biol. 20(1), 273 (2019).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184(13), 3573–3587 (2021).
Itay, T. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352(6282), 189–196 (2016).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20(2), 163–172 (2019).
Bian, Z. et al. Deciphering human macrophage development at single-cell resolution. Nature 582(7813), 571–576 (2020).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4(43), 1686 (2019).
Blanco-Carmona, E. Generating publication ready visualizations for single cell transcriptomics using SCpubr. bioRxiv 48, 2303 (2022).
Douvaras, P. et al. Directed differentiation of human pluripotent stem cells to microglia. Stem Cell Rep. 8(6), 1516–1524 (2017).
Galatro, T. F. et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 20(8), 1162–1171 (2017).
Muffat, J. et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 22(11), 1358–1367 (2016).
Grubman, A. et al. A CX3CR1 reporter hESC line facilitates integrative analysis of in-vitro-derived microglia and improved microglial identity upon neuron-glia co-culture. Stem Cell Rep. 14, 1018–1032 (2020).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43(7), 1–13 (2015).
Diniz, L. P. et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor β signaling through modulation of d-serine levels in cerebral cortex neurons. J. Biol. Chem. 287(49), 41432–41445 (2012).
Spittau, B., Rilka, J., Steinfath, E., Zöller, T. & Krieglstein, K. TGFβ1 increases microglia-mediated engulfment of apoptotic cells via upregulation of the milk fat globule-EGF factor 8. Glia 63(1), 142–153 (2015).
Harrison, J. K. et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. PNAS 95(18), 10896–10901 (1998).
Manich, G. et al. Role of the CD200-CD200R axis during homeostasis and neuroinflammation. Neuroscience 405, 118–136 (2019).
Masuda, T. et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature 20, 22 (2022).
Prinz, M., Erny, D. & Hagemeyer, N. Ontogeny and homeostasis of CNS myeloid cells. Nat. Immunol. 18(4), 385–392 (2017).
Griffin, J. D. et al. Granulocyte-macrophage colony-stimulating factor and other cytokines regulate surface expression of the leukocyte adhesion molecule-1 on human neutrophils, monocytes, and their precursors. J. Immunol. 145(2), 576–584 (1990).
Lacey, D. C. et al. Defining GM-CSF– and macrophage-CSF–dependent macrophage responses by in vitro models. J. Immunol. 188(11), 5752–5765 (2012).
McQuade, A. & Blurton-Jones, M. Human induced pluripotent stem cell-derived microglia (hiPSC-microglia). In Induced Pluripotent Stem (iPS) Cells. Methods in Molecular Biology vol 2454 (eds Nagy, A. & Turksen, K.) (Humana Press, 2021).
Stratoulias, V., Venero, J. L., Tremblay, M. & Joseph, B. Microglial subtypes: Diversity within the microglial community. EMBO J. 38(17), 1–18 (2019).
Olah, M. et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11, 6129 (2020).
Banerjee, P. et al. Generation of pure monocultures of human microglia-like cells from induced pluripotent stem cells. Stem Cell Res. 49, 102046 (2020).
Muñoz-Garcia, J. et al. The twin cytokines interleukin-34 and CSF-1: Masterful conductors of macrophage homeostasis. Theranostics 11(4), 1568–1593 (2021).
Ushach, I. & Zlotnik, A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J. Leukoc. Biol. 100, 481–489 (2016).
Hall-Roberts, H. et al. TREM2 Alzheimer’s variant R47H causes similar transcriptional dysregulation to knockout, yet only subtle functional phenotypes in human iPSC-derived macrophages. Alzheimer’s Res. Therap. 12(1), 1–27 (2020).
Nandi, S. et al. Receptor-type protein-tyrosine phosphatase ζ Is a functional receptor for interleukin-34. J. Biol. Chem. 288(30), 21972–21986 (2013).
Wang, Y. et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 13, 8 (2012).
Mcquade, A. et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat. Commun. 20, 1–17 (2020).
Rabaneda-Lombarte, N. et al. Altered expression of the immunoregulatory ligand-receptor pair CD200-CD200R1 in the brain of Parkinson’s disease patients. Nat. Partner J. Parkinson’s Dis. 8, 1 (2022).
Sheridan, G. K. & Murphy, K. J. Neuron-glia crosstalk in health and disease: Fractalkine and CX3CR1 take centre stage. Open Biol. 3, 130181 (2013).
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17(1), 131–143 (2014).
Dame, J. B., Christensen, R. D. & Juul, S. E. The distribution of granulocyte-macrophage colony-stimulating factor and its receptor in the developing human fetus. Pediatr. Res. 46, 358 (1999).
Choi, S. S., Lee, H. J., Lim, I., Satoh, J. I. & Kim, S. U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS One 9, 4 (2014).
Fischer, H. G. et al. Differentiation driven by granulocyte-macrophage colony-stimulating factor endows microglia with interferon-γ-independent antigen presentation function. J. Neuroimmunol. 42(1), 87–95 (1993).
Suzumura, A., Marunouchi, T. & Yamamoto, H. Morphological transformation of microglia in vitro. Brain Res. 545(1–2), 301–306 (1991).
Acknowledgements
This work was supported by a research Grant from Open Targets (Project OTAR2065).
Author information
Authors and Affiliations
Contributions
S.J.W., Y.C., J.S. performed all laboratory experiments. S.J.W., Y.C., J.S., M.P.A. analyzed the data. M.P.A. provided statistical and computational insight. W.S.J., S.J.W., S.A.C., conceived and planned experiments. S.J.W., M.P.A., Y.C., J.S., W.S.J., G.T., S.A.C., A.R.B. prepared the manuscript. All authors discussed results and contributed to the final article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Washer, S.J., Perez-Alcantara, M., Chen, Y. et al. Single-cell transcriptomics defines an improved, validated monoculture protocol for differentiation of human iPSC to microglia. Sci Rep 12, 19454 (2022). https://doi.org/10.1038/s41598-022-23477-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-23477-2
This article is cited by
-
An integrated toolkit for human microglia functional genomics
Stem Cell Research & Therapy (2024)
-
Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications
Signal Transduction and Targeted Therapy (2024)
-
An adapted protocol to derive microglia from stem cells and its application in the study of CSF1R-related disorders
Molecular Neurodegeneration (2024)
-
Human iPSC-derived glia models for the study of neuroinflammation
Journal of Neuroinflammation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.