Abstract
Complement activation plays a critical role in the pathogenesis of Guillain-Barré syndrome (GBS), a debilitating immune-mediated neuropathy. Mannose-binding lectin (MBL) is a complement activation factor of lectin pathway which as genetic host factor may influence the susceptibility or severity of GBS. We investigated the frequency of MBL2 promoter (− 550H/L and − 221X/Y) and functional region (exon 1 A/O) polymorphisms and their association with disease susceptibility, clinical features and serum MBL among GBS patients (n = 300) and healthy controls (n = 300) in Bangladesh. The median patient age was 30 years (IQR: 18–42; males, 68%). MBL2 polymorphisms were not significantly associated with GBS susceptibility compared to healthy controls. HL heterozygosity in GBS patients was significantly associated with mild functional disability at enrolment (P = 0.0145, OR, 95% CI 2.1, 1.17–3.82). The HY, YA, HA and HYA heterozygous haplotypes were more common among mildly affected (P = 0.0067, P = 0.0086, P = 0.0075, P = 0.0032, respectively) than severely affected patients with GBS. Reduced serum MBL was significantly associated with the LL, OO and no HYA variants and GBS disease severity. No significant association was observed between MBL2 polymorphisms and electrophysiological variants, recent Campylobacter jejuni infection or anti-ganglioside (GM1) antibody responses in GBS. In conclusion, MBL2 gene polymorphisms are related to reduced serum MBL and associated with the severity of GBS.
Similar content being viewed by others
Introduction
Guillain-Barré syndrome (GBS) is a rapidly progressing peripheral neuropathy and the major cause of flaccid paralysis after an acute infection1. The global incidence of GBS is 1–2 per 100,000 person/year, though this rate varies regionally with significant heterogeneity in terms of clinical presentation and severity2,3. Two-third of patients have symptoms of a precedent infection4 and the most identified cause is Campylobacter jejuni5. The recent public health outbreak of Zika virus caused many patients to develop neurological symptoms like GBS6. However, the mechanisms underlying GBS have not yet been elucidated. There are two major variants of GBS: axonal (AMAN; acute motor axonal neuropathy and AMSAN; acute motor-sensory axonal neuropathy) and demyelinating (AIDP; acute inflammatory demyelinating polyradiculoneuropathy)1. Conclusive evidence indicates GBS after C. jejuni infection is an auto-antibody mediated disease triggered by molecular mimicry between the surface epitopes of an infection agent and peripheral nerve gangliosides, which accounts for the pathogenesis of axonal GBS7. Very few patients with a microbial infection develop GBS8, which indicates involvement of host factors triggering this autoimmunity. Genetic susceptibility could be a predisposing factor for GBS, though the genetic factors that affect the interactions between microbial and host are poorly studied.
The host complement system plays a crucial role in the pathogenetic mechanism of GBS; by mediating complement fixations through anti-ganglioside antibody that elevated serum complements and deposited on the surface of Schwann cells and nodes of Ranvier9,10. Over the last decade, mannose-binding lectin (MBL) has been the center of substantial interest as it possesses the ability to turn on the complement pathway through enzymatic regulation and thus may influence disease susceptibility and severity11. MBL is encoded by the MBL2 gene, which contains four exons. Three single nucleotide polymorphisms (SNPs) in the structural region of the MBL2 gene, located in codons 52 (A/D), 54 (A/B), and 57 (A/C) of exon 1, and two promoter polymorphisms, located at − 550H/L and − 221X/Y, are the major determinants of serum MBL concentration and activity12,13,14. The variant B, C and D alleles, collectively referred to as the O allele, are associated with serum MBL deficiency compared to the wild-type A allele12,13. In addition, polymorphisms within the promoter region (− 550H/L and − 221X/Y) determine the serum MBL concentration to an extent by influencing gene expression14. Host MBL2 genetic variations are associated with susceptibility to a wide variety of infectious and autoimmune diseases15, including tuberculosis (TB)16, rheumatoid arthritis17, systemic lupus erythematosus (SLE)18 and GBS19. However, other reported MBL polymorphisms protect against infectious disease like TB20. MBL can act as double-edged sword in post-infectious diseases as GBS depending on its concentration. Serum MBL deficiency has been reported as a predisposing factor for the development of SLE21, atherosclerosis22, TB16 and coronary artery disease23. However, some studies reported elevated serum MBL was associated with increased disease susceptibility24, thus, the data remain inconclusive. These inconsistent results may be due to the relatively small study populations, different ethnic groups, and environmental influences between studies.
Host–pathogen interactions and environmental factors have attracted attention as concepts that may contribute to GBS. Identifying GBS susceptibility genes would represent an advancement in our understanding of the pathogenesis of the disease. Previously, we reported immune-mediated genes, including tumor necrosis factor-alpha25, apoptotic gene FAS26 and toll-like receptor-427 were associated with GBS disease susceptibility, whereas human leukocyte antigen- DQB128, antigen presenting glycoprotein CD1A and CD1E29 genes have no effect on development of GBS. However, very limited data are available on MBL2 gene polymorphisms and their influence on the serum MBL levels in patients with GBS. Therefore, we determined the potential association between MBL2 gene polymorphisms and disease susceptibility, clinical subtypes, severity and level of serum MBL protein in patients with GBS.
Results
Clinical and sociodemographic characteristics
The basic demographic and clinical characteristics of all patients with GBS included in this study are summarized in Table 1. The median age was 30 years (interquartile range [IQR], 18–42 years) for GBS patients (n = 300) and 34 years (IQR, 28–46 years) for healthy controls (n = 300). Almost 225/300 (75%) patients with GBS had a history of preceding events, mostly diarrhea (129/300; 43%) or respiratory tract infections (54/300; 18%). Electrophysiological studies were performed on 240/300 patients (80%), resulting in classifications of the axonal variant for 143/240 (60%) cases (including acute motor axonal neuropathy [AMAN] for 131/240 [55%] cases and acute motor and sensory axonal neuropathy [AMSAN] for 12/240 [5%] cases); acute inflammatory demyelinating polyradiculoneuropathy (AIDP) for 66/240 (28%) cases; unclassified GBS with unexcitable nerves or equivocal findings for 23 (9%) cases and normal for 8/240 (3%). The majority of patients 230/300 (79%) were severely affected at the time of enrolment on the basis of their Medical Research Council (MRC) sum score; 56/300 (19%) patients had dysautonomia and 44/300 (15%) required mechanical ventilation (MV), 248/300 (83%) patients with GBS regained locomotion and 18/300 (6%) died within six months of follow up.
No association between MBL2 gene polymorphisms and GBS susceptibility
The distributions of MBL2 genotypes were compared between patients with GBS and healthy controls to assess the association with GBS susceptibility. All genotype distributions in healthy controls fitted Hardy–Weinberg equilibrium. There was no significant difference in the genotype and allele frequencies between patients with GBS and controls. In particular, genotypes and alleles of − 550 (H/L), − 221 (X/Y) and exon 1 (A/O) SNPs occurred at similar frequencies in patients with GBS and healthy controls (Table 2). In addition to single-site SNP analysis, we compared the haplotypes of the patients with GBS and controls. The haplotypes were determined by combining the following alleles: − 550H/L and − 221X/Y (HY haplotypes); − 550H/L and exon 1A/O (HA haplotype); − 221X/Y and exon 1 A/O (YA haplotypes) and − 550H/L, − 221X/Y and exon 1A/O (HYA haplotypes). The HY haplotype contains heterozygous combination of HY (HY/LX & HY/LY) and homozygous combination of HY (HY/HY) and no combination of HY (LX/LX, LX/LY & LY/LY) genotypes. The distribution of the HY, HA, YA and HYA haplotypes were almost similar between cases and controls (Table 2).
The − 550H/L SNP and MBL2 haplotypes are associated with a less severe form of GBS
The patients enrolled in this study were classified based on their disease severity, as severely affected (MRC sum score of < 40) or mildly affected (MRC sum score between 40 and 60). The frequency of the MBL2 genotypes were compared among these groups to determine the effect of SNPs on disease severity. The heterozygous HL genotype (63% vs. 43%; P = 0.0145, OR = 2.1, 95% CI 1.17–3.82, corrected; Pc = 0.0048, Table 3) was significantly more frequent in mildly affected patients than severely affected patients with GBS. The heterozygous XY genotype (39% vs. 31%) and heterozygous AO genotype (41% vs. 33%) were also more frequent among mildly affected patients than patients with the severe form of GBS, but these trends were not statistically significant (Table 3).
Haplotype analysis of the MBL2 gene polymorphisms revealed a significant association with developing the mild form of GBS. The HYA heterozygous haplotype (HYA/LYO, HYA/LYA, HYA/LXA, HYA/HYO and HYA/LXO) was significantly more prevalent among mildly affected patients (67%) than severely affected patients (44%) (P = 0.0032, OR = 2.39, 95% CI 1.34–4.28, Pc = 0.0011; Table 3). In addition, the heterozygous HY (P = 0.0067, OR = 2.25, 95% CI 1.25–4.06; Pc = 0.0022), heterozygous YA (P = 0.0086, OR = 5.03, 95% CI 1.48–17.1; Pc = 0.0029) and heterozygous HA (P = 0.0075, OR = 2.26, 95% CI 1.26–4.04; Pc = 0.0025) haplotypes were significantly more prevalent in patients with the mild form of GBS (Table 3).
We also compared the MBL2 polymorphisms with other severe clinical features like dysautonomia and requirement of mechanical ventilation in patients with GBS. Higher HL and lower LL genotypes were significantly present in patients with dysautonomia than patients with normal autonomic function (Pa = 0.017, OR = 2.29, 95% CI 1.18–4.44, Table 4). Moreover, AO genotype was also significantly associated with autonomic dysfunction (Pa = 0.032, OR = 1.92, 95% CI 1.06–3.47, Table 4). No significant association was found between MBL2 gene polymorphism and requirement of mechanical ventilation (Table 4). HL and OO genotype were higher among patients who died during the course of the disease.
Association of MBL2 polymorphisms with the clinical and serological features of GBS
We categorized the patients with GBS based on electrophysiological subtypes (axonal and demyelinating) and serological data to assess the associations between these features and MBL2 genotypes. No significant association was found with the MBL2 genotypes and electrophysiological variants (axonal; AMAN & AMSAN and demyelinating; AIDP) compared to the control group (Supplementary Table S1). The comparison of MBL2 genotypes and haplotypes between anti-GM1 antibody-positive and antibody-negative group did not reveal a significant association between the MBL2 polymorphisms and anti-GM1 antibody productivity (Supplementary Table S2). Similarly, there was no significant association found between the MBL2 genotypes and recent C. jejuni infection (data not shown).
Elevated serum MBL level is associated with disease severity
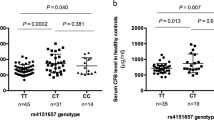
We measured the serum concentration of MBL for a subgroup of patients with GBS (n = 166) and healthy controls (n = 102). The serum MBL levels were slightly higher in patients with GBS than healthy controls (median, [IQR]; 2726 [715–4013] ng/mL vs. 2440 [738–3524] ng/mL, P = 0.3069; Fig. 1a), though serum MBL was not significantly associated with disease susceptibility. The serum concentration of MBL was also compared between the clinical and serological subgroups of GBS. Serum MBL was significantly elevated among severely affected patients compared to mildly affected patients with GBS (median [IQR]; 2957 [903–4093] ng/mL vs. 921 [597–3318] ng/mL, P = 0.0469; Fig. 1b). However, we found no significant difference in the serum MBL between the C. jejuni or anti-GM1 antibody seropositive and negative groups (Fig. 1c,d). In addition, we excluded the patients with OO genotype and compared the MBL level among the groups. We found high serum MBL level was associated with the severely affected patients with GBS (P = 0.0126) and no significant difference was revealed between GBS and healthy control. We compared MBL serum levels with the requirement of mechanical ventilation and development of autonomic, dysfunction in patients with GBS. However, no association was found with the elevated serum MBL and mechanical ventilation or autonomic dysfunction. The median of serum MBL (4417 ng/mL) increased among the patients who died during the course of the disease.
Serum levels of MBL among different subgroups of patients with GBS and healthy controls. Comparisons between (a) GBS patients and controls, (b) severely affected and mildly affected patients with GBS, (c) C. jejuni-positive and -negative patients with GBS, and (d) anti-GM1 antibody-positive and -negative patients. The thick horizontal black lines indicate the median serum MBL value and the vertical lines indicate the IQR; *P < 0.05, Mann–Whitney U-test.
The − 550H/L and Exon 1A/O SNPs are associated with low levels of serum MBL
We compared the serum MBL concentration across the MBL2 genotypes among the patients with GBS and healthy controls to investigate the influence of MBL2 polymorphisms on MBL protein levels. In patients with GBS, the homozygous LL promoter genotype was associated with lower serum MBL compared to the HH homozygous genotype (median [IQR]; 1829 [319–3494] ng/mL, vs. 3550 [2295–4881] ng/mL, P = 0.0082; Fig. 2a). In healthy controls, serum MBL was significantly lower among individuals with the homozygous LL genotype than individuals with the HH homozygous (median [IQR]; 705, [115–2700] ng/mL vs. 3717 [2577–4295] ng/mL, P < 0.05; Fig. 2b) or HL heterozygous genotype (median [IQR]; 705, [115–2700] ng/mL vs. 3193 [2580–3885] ng/mL, P < 0.0001; Fig. 2b). The serum MBL concentration was also lower in individuals with the AO heterozygous or OO homozygous genotypes compared to the AA homozygous genotype in both patients with GBS (median [IQR]; 621 [213–1130] ng/mL vs. 3486 [2377–4174] ng/mL, P < 0.0001 and 83 [56–729] ng/mL vs. 3486 [2377–4174] ng/mL, P < 0.0001; Fig. 2e) and healthy controls (median [IQR]; 595 [108–2426] ng/mL vs. 3520 [2722–4086] ng/mL, P < 0.0001 and 479 [432–526] ng/mL vs. 3520 [2722–4086] ng/mL, P < 0.0001; Fig. 2f). We found no significant difference in the serum MBL levels among individuals with the − 221X/Y polymorphism (Fig. 2c,d). Comparison between MBL haplotypes and serum MBL levels revealed, reduced serum MBL levels were associated with no HYA allele and HYA heterozygous than HYA homozygous in both patients with GBS (P = 0.0014 and P < 0.05; Fig. 2g) and healthy controls haplotypes (P < 0.05 and P < 0.0001; Fig. 2h).
Association of serum MBL levels with MBL2 genotypes in patients with GBS and healthy controls. (a–d) Associations between the promoter − 550H/L and − 221X/Y SNPs and serum MBL levels, (e,f) association between functional region exon 1 SNPs and serum MBL levels, and (g,h) association between HYA haplotypes and serum MBL levels. The violin plots indicate interquartile (Q3-Q1) ranges with the minimum and maximum values; P < 0.05 were determined with Kruskal–Wallis test and the kernel density estimate depicts the multimodal probability distribution.
Discussion
Preliminary studies of genetic variation in the MBL2 gene and its functions over the last decades have focused on disease susceptibility of GBS; however, the roles of MBL2 in immunopathogenesis and regulation of disease severity are poorly understood. The present study focused on the contribution of polymorphisms in the promoter region (− 550H/L and − 221X/Y) and structural exon 1(A/O) region of the MBL2 gene and their association with GBS susceptibility, subtype and severity. The analysis of genetic associations in this study revealed MBL2 gene polymorphisms do not significantly contribute to GBS susceptibility. However, the serum levels of MBL were significantly associated with the HL genotype and heterozygote haplotypes (HY, YA, HA and HYA) of the MBL2 gene and the disease severity of GBS in our Bangladeshi cohort.
Complement activation has recently been reported to be involved in the induction of post-infectious immune-mediated peripheral nerve damage in patients with GBS30. MBL is a key regulatory element that activates the complement system, as part of first-line defense in the pre-immune host in several autoimmune neurological disorders31. Previously, Geleijns et al. reported MBL polymorphisms contributed to the susceptibility in patients with GBS and were associated with disease severity19. The B allele of exon 1 was found to protect against developing severe form of GBS in a Dutch population19. However, this study from Bangladesh revealed no significant association between the B allele and disease severity. The probable explanation could be the inclusion of high number of severe cases (77%) in Bangladeshi cohort due to high severity32. In contrast, the heterozygous HL, HY, YA, HA and HYA haplotypes were significantly associated with developing the mild form of GBS. In addition, HL and AO genotypes were significantly associated with autonomic dysfunction that includes hypertension, hypotension, tachycardia, pupillary abnormality. However, the previous study did not examine any association between the clinical and serological subgroups, including the subtype of GBS, recent infection with C. jejuni and auto-antibodies, with MBL2 polymorphisms or serum MBL levels19. In our population, MBL2 polymorphisms had no influence on the neural damage that occurs in the axonal and demyelinating variants of GBS. More evidence for relation between MBL and disease susceptibility and severity need to be studied to confirm this finding.
MBL contains pattern recognition features that allow the widest range of potential microbial structures to be recognized11. Many infections lead to higher serum MBL levels and increase the disease susceptibility33,34. Several reports suggest that the MBL protein can also modulate disease severity including GBS, leptospirosis and dengue19,34,35. We observed significant elevation of serum MBL in severely affected patients with GBS, in accordance with the Dutch study19. Geleijns et al. reported the only study on GBS and MBL2 polymorphisms thus far, though, they did not measure serum MBL in their control population which is a limitation of their study19. MBL2 gene polymorphisms appear to influence the serum concentration of MBL as we could confirm both for HC and GBS patients. Three single point mutations in structural codons 52 (A/D), 54 (A/B) and 57 (A/C) of exon 1 of the MBL2 gene are the major determinants of MBL deficiency36. The frequencies of these variant alleles vary in different populations37,38. In line with previous observations19, we found polymorphism in structural region of exon 1 is associated with lower serum MBL. Individuals with AO and OO genotype had lower serum MBL than the wild-type variant AA homozygous individuals in GBS. The effect of AO and OO genotype on serum MBL in patients with GBS is consistent with the findings in healthy controls, further indicating this polymorphism affects the serum MBL level. Several reports have suggested the promoter region (− 550) variant allele (L allele) downregulates serum MBL by functionally regulating the immune system in several diseases39. This study also revealed the L allele was associated with lower serum MBL levels in patients with GBS and the healthy control group.
Several studies have presented evidence that MBL deficiency increases the generalized susceptibility of an individual to infectious diseases40,41, including human immunodeficiency virus42,43, Plasmodium falciparum44, cryptosporidium45 and N. meningitidis46. In addition to these associations with infectious disease, there is also evidence of associations between reduced serum MBL and susceptibility to autoimmune diseases. Several reports suggest strong association in the case of systemic lupus erythematosus (SLE). British47, Hong Kong Chinese48, African American49 and Spanish50 patients with SLE all exhibited increased frequencies of mutant MBL alleles or reduced serum MBL levels. In our Bangladeshi population, we did not find any significant association between GBS disease susceptibility and lower levels of MBL protein. As earlier reported, elevated levels of MBL protein have been associated with increased susceptibility towards chronic rheumatic heart disease in patients previously diagnosed with rheumatic fever51. The pathogenesis of rheumatic fever and GBS are similar in terms of antecedent infections, molecular mimicry, and cross-reactive antibodies. Therefore, the higher levels of MBL in severe GBS cases might be explained by binding of MBL to damaged nerve tissue, followed by complement activation, attraction of inflammatory cells, and aggravation of tissue injury. One of the limitations of this study was the lack of determination of complement product activation (C3a and C5a) in serum of GBS patients. Further functional activity of MBL2 polymorphism and its serum levels can be confirmed by measuring the products of complement pathway.
In conclusion, MBL2 haplotypes and serum MBL levels may be one key determinant of the severity of weakness in patients with GBS in Bangladesh. Furthermore, both promoter and functional region polymorphisms in the MBL2 gene are associated with downregulation of serum MBL protein in both patients with GBS and healthy controls. The precise mechanistic role of MBL in GBS is not clear. Further in-depth research is required to elucidate complement activation mediated by MBL and its involvement in the lectin pathway during the pathogenesis of GBS; analysis of samples from the multi-center International GBS Outcome Study (IGOS)52 of a large population of various ethnic groups around the world may to explain the underlying mechanisms.
Materials and methods
Study participants
The study was conducted in the Laboratory of Gut-Brain Signaling, icddr,b, Dhaka, Bangladesh. The patients with GBS and healthy controls were recruited from Dhaka Medical College and Hospital (DMCH) and the National Institute of Neurosciences and Hospital (NINS), Dhaka, Bangladesh. All patients with GBS fulfilled with the National Institute of Neurological Disorders and Stroke (NINDS) criteria and were enrolled within the two weeks of onset of weakness53. The study was reviewed and approved by the Ethical Committee of icddr,b, Dhaka, Bangladesh and all participants gave written informed consent prior enrolment.
Clinical and blood samples were obtained from 300 consecutive patients with GBS and 300 healthy controls for this study. The patients were followed up and clinically assessed at standard time-points (2 weeks, 4 weeks, 6 months and 1 year after entry) to evaluate disease outcome. Disease severity was defined using the Medical Research Council (MRC) sum score for six muscles in the upper and lower limbs on both sides; the sum score ranges from 60 (normal) to 0 (quadriplegic)54. GBS patients with a MRC sum score < 40 were defined as severely affected and 40–60, mildly affected55. Disease outcome was assessed using the GBS disability scale (GBS-DS)56 at six months follow-up; patients able to walk independently (GBS-DS of ≤ 2) were defined as having a good outcome and patients unable to walk independently (GBS-DS of ≥ 3), poor outcome57. Ethnicity-based healthy controls (HC) were recruited from people accompanying the patients to physical examinations at the hospitals mentioned above and pair matched with the GBS cases by age and gender. All control subjects were genetically unrelated inhabitants of Bangladesh without a prior history of other neurological complications or non-communicable diseases, and no recent history of pregnancy or surgery.
Genomic DNA isolation
Genomic DNA was isolated from whole blood for 600 subjects using QIAamp® DNA Blood Midi Kits (100) (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The extracted DNA was dissolved in 1 × TE-buffer (10 mM Tris–Cl, pH 8.0, 1 mM EDTA) and stored at − 80 °C. The stock DNA was diluted to a final concentration of 10 ng/μL in Milli-Q-water and stored at − 20 °C until SNP detection.
Serological assays
Serological tests were performed on the 300 sera of patients with GBS to assess baseline anti-ganglioside antibodies and recent C. jejuni infection at the time of enrolment. Enzyme-linked immunosorbent assay (ELISA) was used to detect IgG, IgM and IgA antibodies against C. jejuni and IgG against the nerve GM1-ganglioside, as described previously58,59. Serum MBL was measured in duplicate from 166 randomly selected serum samples from patients with GBS at the time of enrolment and 102 serum samples from healthy controls by ELISA method using a human MBL oligomer ELISA kit (BioPotro Diagnostics A/S, Gentofte, Denmark), which specifically detects oligomerized forms of MBL protein. Serum samples were stored at − 80 °C and diluted 1:100 in the sample diluent provided in the ELISA kit during the experiment. The ELISA was performed according to the manufacturer’s instructions; absorbance values were measured at 450 nm and serum concentrations were expressed as nanograms of MBL per milliliter (ng/mL).
Genotyping of MBL2 polymorphisms
Single nucleotide polymorphisms (SNPs) in the promoter regions at − 550 (H/L, rs11003125), − 221 (X/Y, rs7096206) and the structural region exon 1 at codon 52 (A/D, rs5030737), codon 54 (A/B, rs1800450) and codon 57 (A/C, rs1800451) of the MBL2 gene were determined in 600 DNA samples extracted from patients with GBS and healthy controls. The real-time reverse transcription polymerase chain reaction (RT-qPCR) and melting curve analysis were performed in LightCycler capillaries (Roche Diagnostics). The primers, probes and the PCR amplification program were based on Geleijns et al.19. The PCR amplification program was designed to determine both promoter regions (− 550H/L and − 221X/Y) and exon 1 (A/O) SNPs using 1 × LightCycler DNA Master Hybridization Probes (Roche Molecular Biochemicals). The melting curve profile was determined after PCR by constant detection of emitted light 19. Approximately 20% of the samples were sequenced to confirm the MBL2 polymorphisms.
Statistical analysis
The chi-squared test was conducted to examine the differences in demographic characteristics and potential confounders between the GBS cases and healthy controls. The MBL2 genotype and haplotype frequencies were determined by a simple counting method and managed using Microsoft Excel 2010 (Microsoft, Redmond, WA) and SPSS (16.0 version, Chicago, IL). Fisher's exact test was used to determine the association of MBL2 polymorphisms and GBS susceptibility; odd ratios (ORs) and 95% confidence intervals (95% CI) were reported. The chi-square test was conducted to confirm the Hardy–Weinberg equilibrium among healthy controls. Serum MBL levels were presented as medians and interquartile ranges (IQR). The non-parametric Mann–Whitney U-test was used to compare the differences in the serum MBL concentrations (ng/mL) between subgroups of patients with GBS and healthy controls. The associations between serum MBL levels and MBL2 genotypes for the studied SNPs in patients with GBS and controls were analyzed using the Kruskal–Wallis test. The associations between the clinical features of GBS and genotypes were analyzed using Fisher's exact test. A probability level (P) of less than or equal 0.05 was considered as the significance criterion. To avoid type I errors in multiple comparisons, the Bonferroni adjustment was performed to correct the P-values obtained by dividing the number of comparisons; the corrected P-values are denoted as Pc60. The significance threshold for Fisher's exact test was adjusted to 0.01667 and 0.025 to obtain Pc for genotypic and allelic differences. All statistical analyses (except for haplotype analyses) were performed on SPSS software (IBM, Chicago, IL, USA, version 20) and GraphPad Prism (version 5 and 9).
References
Willison, H. J., Jacobs, B. C. & van Doorn, P. A. Guillain-barre syndrome. The Lancet 388, 717–727 (2016).
Doets, A. Y. et al. Regional variation of Guillain-Barré syndrome. Brain 141, 2866–2877 (2018).
Wakerley, B. R. et al. Guillain-Barré and Miller Fisher syndromes—new diagnostic classification. Nat. Rev. Neurol. 10, 537 (2014).
Hao, Y. et al. Antecedent infections in Guillain-Barré syndrome: a single-center, prospective study. Ann. Clin. Transl. Neurol. 6, 2510–2517 (2019).
Van Den Berg, B. et al. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 10, 469 (2014).
Cao-Lormeau, V.-M. et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387, 1531–1539 (2016).
Ang, C. W., Jacobs, B. C. & Laman, J. D. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 25, 61–66 (2004).
Tam, C., Rodrigues, L. & Petersen, I Islam A, Hayward A, O’Brien SJ. Incidence of Guillain-Barré syndrome among patients with Campylobacter infection: a general practice research database study. J. Infect. Dis. 194, 95–97 (2006).
Putzu, G. et al. Immunohistochemical localization of cytokines, C5b–9 and ICAM-1 in peripheral nerve of Guillain-Barre syndrome. J. Peripher. Nerv. Syst. 5, 238–239 (2000).
McGonigal, R. et al. C1q-targeted inhibition of the classical complement pathway prevents injury in a novel mouse model of acute motor axonal neuropathy. Acta Neuropathol. Commun. 4, 23 (2016).
Jack, D. L., Klein, N. J. & Turner, M. W. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol. Rev. 180, 86–99 (2001).
Beltrame, M. H., Catarino, S. J., Goeldner, I., Boldt, A. B. W. & de Messias-Reason, I. J. The lectin pathway of complement and rheumatic heart disease. Front. Pediatr. 2, 148 (2015).
Takahashi, K., Ip, W. E., Michelow, I. C. & Ezekowitz, R. A. B. The mannose-binding lectin: A prototypic pattern recognition molecule. Curr. Opin. Immunol. 18, 16–23 (2006).
Madsen, H. O. et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J. Immunol. 155, 3013–3020 (1995).
Koch, A. et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA 285, 1316–1321 (2001).
Liu, C. et al. Association of mannose-binding lectin polymorphisms with tuberculosis susceptibility among Chinese. Sci. Rep. 6, 1–6 (2016).
Dolman, K. M. et al. Mannose-binding lectin deficiency is associated with early onset of polyarticular juvenile rheumatoid arthritis: a cohort study. Arthritis Res. Ther. 10, R32 (2008).
Kieninger-Gräfitsch, A. et al. No association of complement mannose-binding lectin deficiency with cardiovascular disease in patients with Systemic Lupus Erythematosus. Sci. Rep. 10, 1–11 (2020).
Geleijns, K. et al. Mannose-binding lectin contributes to the severity of Guillain-Barre syndrome. J. Immunol. 177, 4211–4217 (2006).
Azad, A. K., Sadee, W. & Schlesinger, L. S. Innate immune gene polymorphisms in tuberculosis. Infect. Immun. 80, 3343–3359 (2012).
Panda, A. K. et al. Low producer MBL genotypes are associated with susceptibility to systemic lupus erythematosus in Odisha, India. Human Immunol. 74, 114–119 (2013).
Madsen, H. O. & Videm, V. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet 352, 959–960 (1998).
Best, L. G. et al. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: The Strong Heart Study. Circulation 109, 471–475 (2004).
Hornum, M. et al. High levels of mannose-binding lectin are associated with lower pulse wave velocity in uraemic patients. BMC Nephrol. 15, 162 (2014).
Jahan, I. et al. Tumor necrosis factor-alpha-863C/A polymorphism is associated with Guillain-Barré syndrome in Bangladesh. J. Neuroimmunol. 310, 46–50 (2017).
Islam, Z. et al. FAS promoter polymorphisms and serum sFas level are associated with increased risk of nerve damage in Bangladeshi patients with Guillain-Barré syndrome. PloS One 13 (2018).
Jahan, I. et al. Toll-like receptor-4 299Gly allele is associated with Guillain-Barré syndrome in Bangladesh. Ann. Clin. Transl. Neurol. 6, 708–715 (2019).
Hayat, S. et al. Human leukocyte antigen-DQB1 polymorphisms and haplotype patterns in Guillain-Barré syndrome. Ann. Clin. Transl. Neurol. 6, 1849–1857 (2019).
Rahman, M. I. et al. CD1A and CD1E gene polymorphisms are not associated with susceptibility to Guillain-Barré syndrome in the Bangladeshi population. J. Neuroimmunol. 314, 8–12 (2018).
Kuwahara, M. & Kusunoki, S. Complement-mediated mechanism and complement inhibitors in Guillain-Barré syndrome. Brain Nerve 71, 581–587 (2019).
Farrokhi, M. et al. Mannose-binding lectin mediated complement pathway in autoimmune neurological disorders. Iran. J. Allergy Asthma Immunol. 15, 251 (2016).
Papri, N. et al. Guillain-Barré syndrome in low-income and middle-income countries: Challenges and prospects. Nat. Rev. Neurol. 17, 285–296 (2021).
Garred, P. et al. Mannan-binding lectin in the sub-Saharan HIV and tuberculosis epidemics. Scand. J. Immunol. 46, 204–208 (1997).
Miranda, K. et al. High levels of serum mannose-binding lectin are associated with the severity of clinical signs of leptospirosis. 42, 353–357 (2009).
Figueiredo, G. G. et al. Mannose-binding lectin gene (MBL2) polymorphisms related to the mannose-binding lectin low levels are associated to dengue disease severity. 77, 571–575 (2016).
Turner, M. W. Mannose-binding lectin: The pluripotent molecule of the innate immune system. Immunol. Today 17, 532–540 (1996).
Figueiredo, G. G. et al. Mannose-binding lectin gene (MBL2) polymorphisms related to the mannose-binding lectin low levels are associated to dengue disease severity. Hum. Immunol. 77, 571–575 (2016).
Amiri, A., Sabooteh, T., Shahsavar, F., Anbari, K. & Pouremadi, F. Mannose-Binding Lectin (MBL) gene polymorphisms in susceptibility to pulmonary tuberculosis among the Lur population of Lorestan Province of Iran. Genom. Data 12, 146–150 (2017).
Navratilova, Z., Gallo, J., Mrazek, F., Lostak, J. & Petrek, M. MBL2 gene variation affecting serum MBL is associated with prosthetic joint infection in Czech patients after total joint arthroplasty. Tissue Antigens 80, 444–451 (2012).
Summerfield, J. et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet 345, 886–889 (1995).
Garred, P., Madsen, H. O., Hofmann, B. & Svejgaard, A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet (London, England) 346, 941–943 (1995).
Garred, P. et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. The Lancet 349, 236–240 (1997).
Nielsen, S., Andersen, P., Koch, C., Jensenius, J. & Thiel, S. The level of the serum opsonin, mannan-binding protein in HIV-1 antibody-positive patients. Clin. Exp. Immunol. 100, 219–222 (1995).
Luty, A. J., Kun, J. F. & Kremsner, P. G. Mannose-binding lectin plasma levels and gene polymorphisms in Plasmodium falciparum malaria. J. Infect. Dis. 178, 1221–1224 (1998).
Kelly, P. et al. Mannose-binding lectin is a component of innate mucosal defense against Cryptosporidium parvum in AIDS. Gastroenterology 119, 1236–1242 (2000).
Hibberd, M. L. et al. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet 353, 1049–1053 (1999).
Davies, E. J. et al. Mannose-binding protein gene polymorphism in systemic lupus erythematosus. Arthritis Rheum. 38, 110–114 (1995).
Lau, Y., Lau, C., Chan, S., Karlberg, J. & Turner, M. Mannose-binding protein in Chinese patients with systemic lupus erythematosus. Arthritis Rheum. 39, 706–708 (1996).
Sullivan, K. E., Wooten, C., Goldman, D. & Petri, M. Mannose-binding protein genetic polymorphisms in black patients with systemic lupus erythematosus. Arthritis Rheum. 39, 2046–2051 (1996).
Davies, E. et al. A dysfunctional allele of the mannose binding protein gene associates with systemic lupus erythematosus in a Spanish population. J. Rheumatol. 24, 485–488 (1997).
Schafranski, M., Stier, A., Nisihara, R. & Messias-Reason, I. Significantly increased levels of mannose-binding lectin (MBL) in rheumatic heart disease: a beneficial role for MBL deficiency. Clin. Exp. Immunol. 138, 521–525 (2004).
Jacobs, B. C. et al. International Guillain-Barré Syndrome Outcome Study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré syndrome. J. Peripher. Nerv. Syst. 22, 68–76 (2017).
Asbury, A. K. & Cornblath, D. R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol 27, S21–S24 (1990).
Kleyweg, R. P., Van Der Meché, F. G. & Schmitz, P. I. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve 14, 1103–1109 (1991).
Geleijns, K. et al. Functional polymorphisms in LPS receptors CD14 and TLR4 are not associated with disease susceptibility or Campylobacter jejuni infection in Guillain-Barré patients. J. Neuroimmunol. 150, 132–138 (2004).
Hughes, R., Newsom-Davis, J., Perkin, G. & Pierce, J. Controlled trial of prednisolone in acute polyneuropathy. The Lancet 312, 750–753 (1978).
Geleijns, K. et al. Genetic polymorphisms of macrophage-mediators in Guillain-Barré syndrome. J. Neuroimmunol. 190, 127–130 (2007).
Ang, C. et al. Validation of an ELISA for the diagnosis of recent Campylobacter infections in Guillain-Barré and reactive arthritis patients. Clin. Microbiol. Infect. 13, 915–922 (2007).
Kuijf, M. L. et al. Diagnostic value of anti-GM1 ganglioside serology and validation of the INCAT-ELISA. J. Neurol. Sci. 239, 37–44 (2005).
Talluri, R., Wang, J. & Shete, S. Calculation of exact p-values when SNPs are tested using multiple genetic models. BMC Genet. 15, 75 (2014).
Acknowledgements
This research activity was funded by the icddr,b, Dhaka, Bangladesh. ZI has received grant support from the Fogarty International Center, National Institute of Neurological Disorders and Stroke of the National Institutes of Health, USA, under Award Number K43TW011447. SH has received grant support from “Global Health Equity Scholars NIH FIC TW010540”, USA. icddr,b acknowledges with gratitude the commitment of the Government of Bangladesh to its research efforts, and also gratefully acknowledges all donors who provide unrestricted support, including the icddr,b is grateful to the governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support. The authors are indebted to the neurologists who encouraged their patients to take part in this study.
Author information
Authors and Affiliations
Contributions
Z.I. and B.C.J. contributed to this conception, design and implementation of this research with his expertise. Preparation of materials, technique optimization and data acquisition were performed by R.U.A., M.M.K., I.J. and S.H. All data were interpreted and analyzed by I.J., S.H., M.M.K. and A.A. Z.I. and I.J. contributed to writing and proofreading the manuscript, which was critically reviewed by all other authors. All authors read and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jahan, I., Hayat, S., Khalid, M.M. et al. Association of mannose-binding lectin 2 gene polymorphisms with Guillain-Barré syndrome. Sci Rep 12, 5791 (2022). https://doi.org/10.1038/s41598-022-09621-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09621-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.