Abstract
There is increasing evidence that autonomic dysfunction is an important factor in the progression of glaucoma. Mechanism of the association between autonomic dysfunction and progression of glaucoma is poorly understood. Since blood circulation is basically regulated by the autonomic nervous system, autonomic dysfunction may contribute to unstable or fluctuating blood pressure. Therefore, It is hypothesized that autonomic dysfunction may contribute to impaired ocular blood flow and lead to glaucoma progression. However, no clinical study yet has evaluated the relationship between ocular blood flow and autonomic nervous function. We enrolled 152 open angle glaucoma patient. Ocular blood flow was assessed by measuring vessel density (VD) using optical coherence tomography angiography, and autonomic nervous function was evaluated with heart-rate variability (HRV) parameters. The low frequency/high frequency (LF/HF) ratio, which is one of the HRV parameters, quantified the degree of sympathovagal balance. This indicator could represent autonomic dysfunction. Higher LF/HF ratio was associated with reduction of the deep parapapillary VD (R = − 0.243, P = 0.003). Linear regression analysis showed a significant negative association between parapapillary choroidal VD and LF/HF ratio (β = − 0.249; 95% confidential interval = − 1.193 to − 0.249; P = 0.002) in multivariate analysis. We demonstarted the association between impaired ocular blood flow (parapapillary choroidal vessel density) and autonomic dysfunction (LF/HF ratio). This study could help understand the role of the autonomic dysfunction in pathophysiology of glaucoma progression.
Similar content being viewed by others
Introduction
Although elevated intraocular pressure (IOP) is considered a major risk factor for the development and progression of glaucoma, vascular dysregulation and perfusion abnormalities have also been considered as important factors in the progression of glaucoma1,2. The potential roles of the blood flow in the pathophysiology of glaucoma have been extensively investigated. Previous studies have reported that ocular blood flow was reduced in the optic nerve head (ONH), retina, choroid, and retrobulbar region in glaucoma3,4,5. Of systemic conditions, vascular diseases, such as low arterial blood pressure (BP) with nocturnal hypotension, migraines, Raynaud’s syndrome, and obstructive sleep apnea have been reported to be associated with glaucoma6,7,8.
Dysfunction of the autonomic nervous system (ANS) is one possible cause leading to insufficient and unstable blood flow. Blood flow abnormalities in vessels of the ONH lead to exacerbation of glaucoma. Many reports have investigate the association between autonomic dysfunction and glaucoma. Several studies have reported that abnormal autonomic nervous system (ANS) function was associated with increased risk for progression of glaucoma. Furthermore, autonomic neuropathies (sympathetic and parasympathetic) have been reported in patients with primary open-angle glaucoma and normal-tension glaucoma (NTG)9,10,11.
Measurement of heart rate variability (HRV) is a well-known tool, that is widely used to evaluate the ANS12,13. HRV permits study of the autonomic modulation of the heart’s sympathovagal balance. In previous studies, HRV has been used to evaluate autonomic dysfunction in glaucoma patients14,15,16,17,18. These studies reported that abnormal HRV parameters were associated with high prevalence of NTG and faster rates of central visual field (VF) loss in glaucoma14,18,19,20.
With the advent of optical coherence tomography angiography (OCT-A), we can visualize the vessel density (VD) in various retinal layers, which could be a surrogate for measuring blood flow21. In particular, lower parapapillary choroidal VD has been reported to be associated with the progression of glaucoma22,23,24. Investigations are ongoing to discover systemic risk factors that may influence impairment or loss of VD in glaucoma25,26. Dips in nocturnal BP have been reported to be associated with localized choroidal VD loss in glaucoma patients27. Furthermore, glaucoma patients with higher systemic BP showed reducted macular VD28. These studies could help us determine the relationship between systemic conditions and blood flow to the ONH or to the retina and help us understand the pathophysiology of progression in glaucoma.
In this study, we investigate the association between autonomic dysfunction and impaired ocular blood flow in glaucoma. Ocular blood flow was assessed by measuring VD using OCT-A, and autonomic dysfunction was evaluated with HRV parameters. We aimed to determine whether HRV parameters and autonomic imbalance are associated with VD status in patients with glaucoma.
Results
A total of 152 glaucoma patients (152 eyes) were included in this study. The demographics features of the subjects are presented in Table 1. We measured the correlations between the mean deviation (MD) or pattern standard deviation (PSD) of VFs, and the HRV parameters. Worse MDs or PSDs of the VFs were correlated with larger IOP fluctuations (R = −0.208, P = 0.011, and R = 0.162, P = 0.049, respectively, Table 2). Lower MD values were correlated with thinner superficial VD in the macular (R = 0.209, P = 0.015) and parapapillary (R = 0.421, P = 0.001) regions (Table 2).
We also measured the correlations between VD and the ocular parameters (Tables 3, 4). Increased age was correlated with superficial VD reduction in the macular (R = −0.171, P = 0.044) and parapapillary (R = −0.217, P = 0.009) regions. Reduced superficial parapapillary VD was associated with longer axial length (R = −0.326, P < 0.001) and greater IOP fluctuations (R = −0.191, P = 0.021). The reduction of deep parapapillary VD was associated with higher low frequency (LF) / high frequency (HF) ratio (R = −0.243, P = 0.003). Deep macular VD did not show significant correlations with the other parameters.
To determine factors related to parapapillary choroidal VD, regression analyses were performed. Lower parapapillary choroidal VD was associated with higher LF/HF ratio (β = −0.249, 95% confidential interval = −1.193 to −0.249; P = 0.002) in multivariate analysis (Table 5). Figure 1 shows a scatter plot between parapapillary choroidal VD and LF/HF ratio, which had a negative relationship.
Discussion
We have demonstrated the association between impaired deep parapapillary vessel density and autonomic dysfunction in NTG. This study showed that autonomic dysfunction has a relationship with ocular blood flow, other than that with systemic blood circulation, which has been reported in other studies. Lower parapapillary choroidal VD is a risk factor for progression of glaucoma22,23,24. Our findings agree with reports proposing that autonomic dysfunction contributes to the pathophysiology of glaucoma in terms of BP or blood flow instability, and this study is, to our knowledge, the first study to directly view VD changes using OCT-A.
In our study, the poor MD of VF correlated with IOP and parapapillary vessel density, respectively. Howerver, impaired parapapillary choroidal VD was associated with autonomic dysfunction, independent of IOP. This result may support non-mechanical theory (IOP-independent mechanism) of glaucoma pathogenesis.
Impaired blood flow is considered to be an important risk factor for glaucoma1,2. According to the Collaborative Normal-Tension Glaucoma Study (CNTGS) and the Early Manifest Glaucoma Trial (EMGT), migraine and disc hemorrhage contribute to the progression of VF loss29,30. Migraine was characterized as a vasospastic disorder and represented systemic blood flow disorders7. Disc hemorrhage is a local indicator of ocular blood flow disturbance6. With the advent of OCT-A, this concept could be directly visualized in terms of VD. Glaucomatous eyes with disc hemorrhage tended to show microvascular dropout or VD reduction on OCT-A22,31. According to other OCT-A studies, systemic conditions such as nocturnal BP dip, higher systemic BP or low arterial BP were associated with reduced ocular VD27,28. These all indicate disturbances or instability of systemic or local blood flow, as capsured by OCT-A, and this tool could be promising in terms of evaluating the vascular status of the retina or ONH in glaucoma.
Blood circulation is basically regulated by the ANS32. Autonomic dysfunction may contribute to unstable or fluctuating blood pressure32. Ocular blood flow is controlled by direct autonomic and indirect autoregulatory system. Vasculature of the optic nerve, choroid, ciliary body, and iris were controlled by direct autonomic system. Retinal blood flow was controlled by indirect autoregulatory system33. Abnormality of the ANS cause disturbance of the blood supply to the ONH and choroid34. Given that the choroid contributes to the prelaminar blood supply of the ONH, decreased choroidal blood flow may promote progression of glaucoma35,36. Choroidal blood flow is innervated by the parasympathetic and sympathetic nervous systems36. These vessel are innervated by postganglionic nerve fibers from the pterygopalatine (parasympathetic) and superior cervical (sympathetic) ganglia33,36.Unlike the choroidal circulation, there is no neuronal innervation in the retinal vessel37. The physiology and anatomy of the retinal circulation are similar to the brain circulation37,38.The presence of endothelial tight junctions results in the blood-retinal barrier, resembling the blood–brain barrier37,38.Histological studies have demonstrated a abundant autonomic innervation for the choroid, but these nerves do not go further into the retina39. Hence, retinal blood flow is mainly controlled through autoregulation by myogenic and local metabolic mechanisms38,39. The mediators of these mechanisms include oxygen, adenosine, nitric oxide, angiotensin-II, carbon dioxide, and endothelin-137. Both superficial and deep macular vessels are supplied by retinal vessels. Radial peripapillary capillaries (RPCs) are mainly supplied by retinal blood flow. Only the deep parapapillary blood flow is controlled by choroidal vessels, which are innervated by the ANS. Hence, autonomic function should show an association with the status of the choroidal vessels and our results show that HRV, an indicator of ANS function, was only correlated with parapapillary choroidal VD.
The vascular plexus of the superficial macular layer is located mainly within retinal nerve fiber layer (RNFL), retinal ganglion cell (RGC) layer and inner plexiform layer (IPL). Measuring macular perfusion has the potential for detecting reduced metabolic rate and dysfunction of the RGCs. The superficial parapapillary layer contains RPCs, which compose a unique capillary plexus within the RNFL, and constitute a critical blood source to satisfy the nutritional demands of RGC axons40. Because glaucoma damages RGCs and the RNFL, superficial macular and parapapillary perfusion could theoretically be used to assess disease severity41,42. Previous studies reported that superficial VD was correlated with MD of VF and the macular RGC layer and IPL41,42. Our results also showed that the MD of VFs is correlated with macular and parapapillary superficial VD. Changes of superficial VD could be the result of RNFL loss and, thus, ANS did not show correlation with superficial VD parameters.
ANS function can be measured by a variety of methods. Among the many relevant procedures, HRV analysis has rapidly expanded in use in the investigation of both normal physiology and pathologic conditions. HRV has also been used to evaluate ANS function in glaucoma patients. A 24-h HRV analysis showed that there was increased sympathetic activity of the ANS in patients with NTG, and that the extent of the autonomic disorder correlated with the severity of glaucoma43,44. Short term HRV analysis showed that patients with NTG had an autonomic imbalance that was related to serum levels of endothelin-115,19,45,46. Because the imbalance between the sympathetic and parasympathetic autonomic systems (i.e., the LF/HF ratio in our study) had a significant association with deep parapapillary VD, we can state that autonomic dysfunction influences choroidal blood flow around the ONH, which may contribute to the progression of glaucoma in patients with autonomic imbalance.
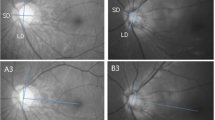
Representative cases are shown in Fig. 2. The picture on the left is the eye of a patient with a high LF/HF ratio (5.41). The VD was measured in the area except for the optic disc region. The optic disc area can be seen in the figure below. The picture on the right is the eye of a patient with a low LF/HF ratio (2.18). The calculated choroidal VD was lower in the left one than in the right one. (47.92% vs 56.61%, respectively).
Two representative cases with contrasting features. Left image is low parapapillary choroidal vessel density with high LF/HF ratio (5.41). Right image is high parapapillary choroidal vessel density with low LF/HF ratio (2.18). The vessel density was measured in whole area except for the optic disc region.
In our study, the poorer MD or PSD of VF was correlated with the lower parapapillary vessel density, which is consistent with previous reports. Howerer, multivariate analysis did not show statistical significance. This is because this study is a cross-sectional study, not a longitudinal study. The impaired blood flow increases the risk of glaucoma progression, but does not necessarily mean that the current glaucoma damange is severe. Another possibility is that the glaucoma damage in this study was an early stage.
There are limitations in this study. First, this study has the intrinsic limitation of a cross-sectional design described above. Second, the VD obtained by OCTA may not perfectly match to blood flow due to their floor effect. Third, we could not exclude the patients with DM that could affect the HRV. However, only two diabetic patients were included in this study. Forth, we could not totally exclude the patients with cataract or multifocal intraocular lens insertion that could affect the quality of OCTA image.
In summary, impaired parapapillary choroidal blood flow was associated with autonomic dysfunction. This study supports the association between glaucomatous damage and autonomic dysfunction, and its possible role in glaucoma progression.
Materials and methods
Study subjects
This study included 152 normal tension glaucoma patients (152 eyes) who visited the Seoul St. Mary's Hospital between January 1, 2020 and December 31, 2020. Institutional Review Board (IRB)/Ethics Committee approval was obtained from the Seoul St. Mary’s hospital’s IRB, which waived the requirement for written informed consent because of the retrospective nature of the study. All relevant principles of the Declaration of Helsinki were followed. All patients had a detailed ophthalmic examination, including slit lamp examination, measurements of IOP (Goldmann applanation tonometry), central corneal thickness (UD-800; Tomey Corporation, Japan), axial length (IOL Master; Carl Zeiss Meditec, Dublin, CA), red-free fundus photography (Canon; Tokyo, Japan), mean RNFL thickness (Cirrus OCT; Carl Zeiss Meditec) and a VF test (Humphrey field analyzer using the Swedish Interactive Threshold Standard 24–2 algorithm; Carl Zeiss Meditec). Histories of hypertension, migraine, diabetes and optic disc hemorrhage were investigated through medical records. Patients taking drugs known to affect the ANS, such as beta-blockers and digoxin, were excluded. Glaucomatous VF defects were defined by the following findings: 1) a cluster of ≥ 3 adjacent points in pattern deviation plots with a probability of < 5% of the normal population, with one of these points having a probability of < 1%, or a cluster of ≥ 2 adjacent points in pattern deviation plots with a probability of < 1%; 2) glaucoma hemifield test results outside the normal limits and PSD < 5%.
The mean IOP was the average of all measurements taken for 3 years preceding th last visit. IOP fluctuation was calculated as the difference between the maximum and minimum IOP values obtained during the follow-up period.
HRV assessment
The participants were asked to avoid activities such as running, jumping, ect at least 2 h before the test. The testing was done over 5 min in very-controlled conditions. The echocardiography was monitored by an experienced technician for 5 min, and the echocardiography signals were transferred to a Medicore Heart Rate Analyzer, Model SA-3000P (Medicore, Seoul, Korea).
The short-term HRV allow to obtain and accurate and meaningful data under more controlled conditions reducing confounder factors, compared to the long-term HRV47,48,49. The reproducibility of short-term HRV parameters has been demonstrated in previous human cohorts studies48.
The standard deviation of the NN-interval (SDNN) index is obtained by measuring the normal R-R interval of QRS complex in the electrocardiogram. Both the sympathetic and parasympathetic nervous systems contribute to the SDNN13. A reduction in SDNN is correlated with left ventricular dysfunction, indicating a high tone of heart sympathetic activity and has been found to be predictive of increased risk of sudden cardiac death13.
The power in the HF is the parameter of the spectral power of heart rate respiratory undulations reflecting the activity level of the respiratory center50,51. The HF band reflects fast changes in beat-to-beat variability, which are due to the parasympathetic activity50,51 The power in the LF modulated by both the sympathetic and parasympathetic nervous system, but LF band is considered to be a sympathetic activity50,51. It reflects the activity of the baroreflex mechanism that ensures BP regulation50,51. “LF/HF” is the ratio of LF power to HF power and reflects the sympathetic-vagal balance. A higher ratio indicates increased sympathetic activity or reduced parasympathetic activity52. Both the SDNN and LF/HF ratio are affected by the sympathetic and parasympathetic nervous systems, but the LF/HF ratio more accurately represents the magnitude and direction of autonomic imbalance52.
OCT angiography
The macula and ONH were scanned using a swept-source OCT-A device. Scans were taken with 4.5-cm × 4.5-cm cubes. Identification of the microvasculature was based on automated layer segmentation, performed by the built-in OCT software. Each cube consisted of 320 clusters of four repeated B-scans centered on the optic disc or macular region.
The superficial macular microvasculature reaches from 2.6 μm below the internal limiting membrane (ILM) to 15.6 μm below the junction of the IPL and inner nuclear layer (INL) (IPL/INL). The deep macular microvasculature reaches from 15.6 μm below IPL/INL to 70.2 μm below IPL/INL. The vascular plexus of the superficial macular layer is located mainly within the RNFL, RGC layer and IPL. The vascular plexus of the deep macular layer is located within the INL53.
The superficial parapapillary microvasculature reaches from the ILM to the RNFL. The deep parapapillary microvasculature reaches from 130 μm below the ILM to 390 μm below the basement membrane.
Measurement of VD from OCT-A images was performed as described in previous studies54,55,56 The binarized image was converted to a red–green–blue color model and then split into three channels (red, green and blue). The red channel was chosen as the reference. After using the “adjust threshold” tool, which automatically sets the lower- and upper-threshold values, images were segmented into the area of interest and the background. The white pixels represented “vessel” and black pixels represented “background”. The VD was calculated as the percentage of the white-pixels area, divided by the total image area.
Statistical analysis
We Sample size calculations were performed using a statistical power analysis program (G*Power 3.1 software). At the significance level of 5% and minimum power of 95% and considering a minimum value of 0.3 for correlation, 138 individuals are required in the sample. Data are presented as means and SDs. Pearson correlation analysis was used to evaluate the relationships between VD and the autonomic dysfunction parameters, such as SDNN, HF, LF and the HF/LF ratio. The linear and logarithmic R2 values were calculated from scatter plots of VD and the LF/HF ratio of HRV. Univariate and multivariate logistic regression analyses were used to identify factors associated with parapapillary choroidal VD. Values of P < 0.05 in the univariate model were included in the multivariate model. Values of P < 0.1 indicated statistical significance. All statistical analyses were performed using SPSS for Windows (v. 24.0; IBM Corporation, Armonk, NY).
References
Flammer, J. et al. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 21, 359–393. https://doi.org/10.1016/s1350-9462(02)00008-3 (2002).
Kaiser, H. J., Schoetzau, A., Stümpfig, D. & Flammer, J. Blood-flow velocities of the extraocular vessels in patients with high-tension and normal-tension primary open-angle glaucoma. Am. J. Ophthalmol. 123, 320–327. https://doi.org/10.1016/s0002-9394(14)70127-8 (1997).
Piltz-Seymour, J. R. Laser Doppler flowmetry of the optic nerve head in glaucoma. Surv. Ophthalmol. 43(Suppl 1), S191-198. https://doi.org/10.1016/s0039-6257(99)00053-3 (1999).
Tobe, L. A. et al. The role of retrobulbar and retinal circulation on optic nerve head and retinal nerve fibre layer structure in patients with open-angle glaucoma over an 18-month period. Br. J. Ophthalmol. 99, 609–612. https://doi.org/10.1136/bjophthalmol-2014-305780 (2015).
Duijm, H. F., van den Berg, T. J. & Greve, E. L. A comparison of retinal and choroidal hemodynamics in patients with primary open-angle glaucoma and normal-pressure glaucoma. Am. J. Ophthalmol. 123, 644–656. https://doi.org/10.1016/s0002-9394(14)71077-3 (1997).
Kwon, J., Lee, J., Choi, J., Jeong, D. & Kook, M. S. Association between nocturnal blood pressure dips and optic disc hemorrhage in patients with normal-tension glaucoma. Am. J. Ophthalmol. 176, 87–101. https://doi.org/10.1016/j.ajo.2017.01.002 (2017).
Gramer, G., Weber, B. H. & Gramer, E. Migraine and vasospasm in glaucoma: Age-related evaluation of 2027 patients with glaucoma or ocular hypertension. Invest. Ophthalmol. Vis. Sci. 56, 7999–8007. https://doi.org/10.1167/iovs.15-17274 (2015).
Lee, S. S. Y. et al. Associations between optic disc measures and obstructive sleep apnea in young adults. Ophthalmology 126, 1372–1384. https://doi.org/10.1016/j.ophtha.2019.04.041 (2019).
Clark, C. V. & Mapstone, R. Systemic autonomic neuropathy in open-angle glaucoma Documenta ophthalmologica. Adv. Ophthalmol. 64, 179–185. https://doi.org/10.1007/bf00159992 (1986).
Kumar, R. & Ahuja, V. M. A study of changes in the status of autonomic nervous system in primary open angle glaucoma cases. Indian J. Med. Sci. 53, 529–534 (1999).
Brown, C. M., Dütsch, M., Michelson, G., Neundörfer, B. & Hilz, M. J. Impaired cardiovascular responses to baroreflex stimulation in open-angle and normal-pressure glaucoma. Clin. Sci. (London, England: 1979) 102, 623–630 (2002).
Ewing, D. J., Neilson, J. M., Shapiro, C. M., Stewart, J. A. & Reid, W. Twenty four hour heart rate variability: Effects of posture, sleep, and time of day in healthy controls and comparison with bedside tests of autonomic function in diabetic patients. Br. Heart J. 65, 239–244. https://doi.org/10.1136/hrt.65.5.239 (1991).
Lucini, D. & Pagani, M. Heart rate variability, autonomic regulation and myocardial ischemia. Int. J. Cardiol. 312, 22–23. https://doi.org/10.1016/j.ijcard.2020.03.012 (2020).
Asefa, N. G., Neustaeter, A., Jansonius, N. M. & Snieder, H. Autonomic dysfunction and blood pressure in glaucoma patients: The lifelines cohort study. Invest. Ophthalmol. Vis. Sci. 61, 25. https://doi.org/10.1167/iovs.61.11.25 (2020).
Na, K. S., Lee, N. Y., Park, S. H. & Park, C. K. Autonomic dysfunction in normal tension glaucoma: the short-term heart rate variability analysis. J. Glaucoma 19, 377–381. https://doi.org/10.1097/IJG.0b013e3181c4ae58 (2010).
Park, H. L., Jung, S. H., Park, S. H. & Park, C. K. Detecting autonomic dysfunction in patients with glaucoma using dynamic pupillometry. Medicine 98, e14658. https://doi.org/10.1097/md.0000000000014658 (2019).
Shin, D. Y., Jeon, S. J., Park, H. Y. L. & Park, C. K. Posterior scleral deformation and autonomic dysfunction in normal tension glaucoma. Sci. Rep. 10, 8203. https://doi.org/10.1038/s41598-020-65037-6 (2020).
Kurysheva, N. I., Shlapak, V. N. & Ryabova, T. Y. Heart rate variability in normal tension glaucoma: A case-control study. Medicine 97, e9744. https://doi.org/10.1097/md.0000000000009744 (2018).
Park, H. Y., Jung, K. I., Na, K. S., Park, S. H. & Park, C. K. Visual field characteristics in normal-tension glaucoma patients with autonomic dysfunction and abnormal peripheral microcirculation. Am. J. Ophthalmol. 154, 466-475.e461. https://doi.org/10.1016/j.ajo.2012.03.028 (2012).
Park, H. Y., Park, S. H. & Park, C. K. Central visual field progression in normal-tension glaucoma patients with autonomic dysfunction. Invest. Ophthalmol. Vis. Sci. 55, 2557–2563. https://doi.org/10.1167/iovs.13-13742 (2014).
Rao, H. L. et al. Optical coherence tomography angiography in glaucoma. J. Glaucoma 29, 312–321. https://doi.org/10.1097/ijg.0000000000001463 (2020).
Park, H. Y., Shin, D. Y., Jeon, S. J. & Park, C. K. Association between parapapillary choroidal vessel density measured with optical coherence tomography angiography and future visual field progression in patients with glaucoma. JAMA Ophthalmol. 137, 681–688. https://doi.org/10.1001/jamaophthalmol.2019.0422 (2019).
Kim, J. A., Lee, E. J. & Kim, T. W. Evaluation of parapapillary choroidal microvasculature dropout and progressive retinal nerve fiber layer thinning in patients with glaucoma. JAMA Ophthalmol. 137, 810–816. https://doi.org/10.1001/jamaophthalmol.2019.1212 (2019).
Lin, S. et al. Parapapillary choroidal microvasculature dropout is associated with the decrease in retinal nerve fiber layer thickness: A prospective study. Invest. Ophthalmol. Vis. Sci. 60, 838–842. https://doi.org/10.1167/iovs.18-26115 (2019).
Yarmohammadi, A. et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 123, 2498–2508. https://doi.org/10.1016/j.ophtha.2016.08.041 (2016).
Suh, M. H., Na, J. H., Zangwill, L. M. & Weinreb, R. N. Deep-layer microvasculature dropout in preperimetric glaucoma patients. J. Glaucoma 29, 423–428. https://doi.org/10.1097/ijg.0000000000001489 (2020).
Shin, J. W., Jo, Y. H., Song, M. K., Won, H. J. & Kook, M. S. Nocturnal blood pressure dip and parapapillary choroidal microvasculature dropout in normal-tension glaucoma. Sci. Rep. 11, 206. https://doi.org/10.1038/s41598-020-80705-3 (2021).
Park, C. K. et al. Effect of systemic blood pressure on optical coherence tomography angiography in glaucoma patients. Eye (Lond.) 35, 1967–1976. https://doi.org/10.1038/s41433-020-01199-x (2021).
Drance, S., Anderson, D. R. & Schulzer, M. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am. J. Ophthalmol. 131, 699–708. https://doi.org/10.1016/s0002-9394(01)00964-3 (2001).
Founti, P. et al. Risk factors for visual field deterioration in the United Kingdom glaucoma treatment study. Ophthalmology 127, 1642–1651. https://doi.org/10.1016/j.ophtha.2020.06.009 (2020).
Park, H. L., Kim, J. W. & Park, C. K. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology 125, 1003–1013. https://doi.org/10.1016/j.ophtha.2018.01.016 (2018).
Sheng, Y. & Zhu, L. The crosstalk between autonomic nervous system and blood vessels. Int. J. Physiol. Pathophysiol. Pharmacol. 10, 17–28 (2018).
McDougal, D. H. & Gamlin, P. D. Autonomic control of the eye. Compr. Physiol. 5, 439–473. https://doi.org/10.1002/cphy.c140014 (2015).
Flammer, J., Konieczka, K. & Flammer, A. J. The primary vascular dysregulation syndrome: Implications for eye diseases. EPMA J. 4, 14. https://doi.org/10.1186/1878-5085-4-14 (2013).
Grunwald, J. E., Piltz, J., Hariprasad, S. M. & DuPont, J. Optic nerve and choroidal circulation in glaucoma. Invest. Ophthalmol. Vis. Sci. 39, 2329–2336 (1998).
Reiner, A., Fitzgerald, M. E. C., Del Mar, N. & Li, C. Neural control of choroidal blood flow. Prog. Retin. Eye Res. 64, 96–130. https://doi.org/10.1016/j.preteyeres.2017.12.001 (2018).
Luo, X., Shen, Y. M., Jiang, M. N., Lou, X. F. & Shen, Y. Ocular Blood Flow Autoregulation Mechanisms and Methods. J. Ophthalmol. 2015, 864871. https://doi.org/10.1155/2015/864871 (2015).
Iester, M., Torre, P. G., Bricola, G., Bagnis, A. & Calabria, G. Retinal blood flow autoregulation after dynamic exercise in healthy young subjects. Ophthalmologica. Int. J. Ophthalmol. 221, 180–185, https://doi.org/10.1159/000099298 (2007).
Laties, A. M. Central retinal artery innervation: Absence of adrenergic innervation to the intraocular branches. Arch. Ophthalmol. (Chicago, Ill.: 1960) 77, 405–409. https://doi.org/10.1001/archopht.1967.00980020407021 (1967).
Yu, P. K. et al. Label-free density measurements of radial peripapillary capillaries in the human retina. PLoS ONE 10, e0135151. https://doi.org/10.1371/journal.pone.0135151 (2015).
Takusagawa, H. L. et al. Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology 124, 1589–1599. https://doi.org/10.1016/j.ophtha.2017.06.002 (2017).
Akil, H. et al. Swept-source OCT angiography imaging of the macular capillary network in glaucoma. Br. J. Ophthalmol. https://doi.org/10.1136/bjophthalmol-2016-309816 (2017).
Wierzbowska, J., Wierzbowski, R., Stankiewicz, A., Siesky, B. & Harris, A. Cardiac autonomic dysfunction in patients with normal tension glaucoma: 24-h heart rate and blood pressure variability analysis. Br. J. Ophthalmol. 96, 624–628. https://doi.org/10.1136/bjophthalmol-2011-300945 (2012).
Riccadonna, M. et al. Autonomic system activity and 24-hour blood pressure variations in subjects with normal- and high-tension glaucoma. J. Glaucoma 12, 156–163. https://doi.org/10.1097/00061198-200304000-00011 (2003).
Lee, N. Y., Park, H. Y., Na, K. S., Park, S. H. & Park, C. K. Association between heart rate variability and systemic endothelin-1 concentration in normal-tension glaucoma. Curr. Eye Res. 38, 516–519. https://doi.org/10.3109/02713683.2012.745881 (2013).
Lee, N. Y., Park, H. Y., Park, C. K. & Ahn, M. D. Analysis of systemic endothelin-1, matrix metalloproteinase-9, macrophage chemoattractant protein-1, and high-sensitivity C-reactive protein in normal-tension glaucoma. Curr. Eye Res. 37, 1121–1126. https://doi.org/10.3109/02713683.2012.725798 (2012).
Shaffer, F. & Ginsberg, J. P. An overview of heart rate variability metrics and norms. Front. Public Health 5, 258. https://doi.org/10.3389/fpubh.2017.00258 (2017).
Plaza-Florido, A. et al. Inter- and intra-researcher reproducibility of heart rate variability parameters in three human cohorts. Sci. Rep. 10, 11399. https://doi.org/10.1038/s41598-020-68197-7 (2020).
Plaza-Florido, A., Sacha, J. & Alcantara, J. M. A. Short-term heart rate variability in resting conditions: methodological considerations. Kardiologia polska 79, 745–755, https://doi.org/10.33963/KP.a2021.0054 (2021).
Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European heart journal 17, 354–381 (1996).
Kurysheva, N. I., Ryabova, T. Y. & Shlapak, V. N. Heart rate variability: The comparison between high tension and normal tension glaucoma. EPMA J. 9, 35–45. https://doi.org/10.1007/s13167-017-0124-4 (2018).
Sollers, J. J. 3rd., Buchanan, T. W., Mowrer, S. M., Hill, L. K. & Thayer, J. F. Comparison of the ratio of the standard deviation of the R-R interval and the root mean squared successive differences (SD/rMSSD) to the low frequency-to-high frequency (LF/HF) ratio in a patient population and normal healthy controls. Biomed. Sci. Instrum. 43, 158–163 (2007).
Nemiroff, J., Phasukkijwatana, N. & Sarraf, D. Optical coherence tomography angiography of deep capillary ischemia. Dev. Ophthalmol. 56, 139–145. https://doi.org/10.1159/000442806 (2016).
Jeon, S. J., Park, H. L. & Park, C. K. Effect of macular vascular density on central visual function and macular structure in glaucoma patients. Sci. Rep. 8, 16009. https://doi.org/10.1038/s41598-018-34417-4 (2018).
Jeon, S. J., Shin, D. Y., Park, H. L. & Park, C. K. Association of retinal blood flow with progression of visual field in glaucoma. Sci. Rep. 9, 16813. https://doi.org/10.1038/s41598-019-53354-4 (2019).
Jeon, S. J., Park, H. L. & Park, C. K. Vessel Density Loss of the Deep Peripapillary Area in Glaucoma Suspects and Its Association with Features of the Lamina Cribrosa. J. Clin. Med. 10, 1. https://doi.org/10.3390/jcm10112373 (2021).
Acknowledgements
We thank Claire Barnes, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
D.Y.S. wrote the main manuscript text. D.Y.S., K.E., N.Y.L. and C.K.P. performed the data review and analysis. The critical revision of the manuscript was done by H.-Y.L.P. All authors discussed the results, and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, D.Y., Hong, K.E., Lee, N.Y. et al. Association of choroidal blood flow with autonomic dysfunction in patients with normal tension glaucoma. Sci Rep 12, 5136 (2022). https://doi.org/10.1038/s41598-022-09162-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09162-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.