Abstract
Choroidal microvasculature dropout (CMvD) implies compromised optic nerve head perfusion in glaucoma patients. However, there are conflicting findings whether office-hour systemic blood pressure (BP) is related to the presence of CMvD. The present study investigated which systemic BP parameters, derived from 24-h ambulatory BP monitoring (ABPM), are associated with CMvD as assessed by optical coherence tomography angiography (OCT-A) in normal-tension glaucoma (NTG). This study included 88 eyes of 88 NTG patients who underwent 24-h ABPM and OCT-A imaging. Various systemic BP parameters associated with the presence of CMvD were evaluated using logistic regression analyses. CMvD was detected in 38 NTG eyes (43.2%). NTG eyes with CMvD had nighttime diastolic BP (DBP) dip of greater magnitude and longer duration than eyes without CMvD. In multivariate logistic regression, worse VF mean deviation (MD) (odds ratio [OR] 0.786; P = 0.001), greater nighttime DBP dip “%” (OR 1.051; P = 0.034), and higher daytime peak IOP (OR 1.459; P = 0.013) were significantly associated with the presence of CMvD. Based on our findings that the eyes with CMvD are closely associated with having nighttime DBP dip, NTG patients with CMvD should be recommended to undergo 24-h ABPM.
Similar content being viewed by others
Introduction
The role of systemic hypotension in glaucoma has been extensively explored in various population-based as well as clinical studies. Low systemic blood pressure (BP) and reduced ocular perfusion pressure (OPP) were identified as important risk factors for the development and progression of open-angle glaucoma (OAG)1,2,3,4,5,6,7,8,9. Studies including our recent reports have also shown that nocturnal hypotension, particularly its duration and magnitude as determined by 24-h ambulatory BP monitoring (ABPM), significantly impacts on future visual field (VF) progression in patients with normal-tension glaucoma (NTG)5,6,7,8,9,10,11. Moreover, continuous BP monitoring over 24 h may better reveal circulatory insufficiency related to systemic hypotension than snapshot readings of daytime BP in these patients7,8,9. Thus, 24-h ABPM may facilitate understanding of glaucoma pathogenesis related to systemic hypotension.
The recent advent of optical coherence tomography angiography (OCT-A) has allowed objective and reproducible visualization and measurement of the retinal and choroidal microvasculature. OCT-A has shown localized parapapillary choroidal perfusion impairment, such as choroidal microvasculature dropout (CMvD), in glaucomatous eyes12,13. To date, CMvD has been detected more frequently in glaucomatous eyes with central VF defects and advanced VF damage at initial presentation13,14,15,16, progressive retinal nerve fibre layer (RNFL) and/or VF loss17,18, and lower choroid thickness13. The presence of CMvD may imply compromised optic nerve head (ONH) perfusion in glaucoma patients, since choroidal circulation within the parapapillary area provides microvascular networks that supply deep ONH structures, such as the lamina and prelaminar tissue19,20. While a few studies have reported that CMvD is associated with abnormal BP parameters, such as low diastolic BP (DBP), OPP, or mean arterial pressure (MAP)13,15, other studies have not confirmed its association with systemic hypotension in glaucoma patients16,21. These conflicting findings may stem from the use of snapshot daytime BP measurements, which may not accurately reflect the true nature of the BP abnormality or its circadian rhythm as related to glaucoma pathogenesis.
Given that nocturnal hypotension is a known systemic vascular risk factor for glaucoma7,8,9, we hypothesized that a nighttime BP dip may be closely linked to CMvD, since it can give rise to a localized choroidal perfusion defect in the form of microvasculature dropout, and these conditions may share circulatory insufficiency to the ONH in common. Therefore, we here investigated the relationship of various 24-h BP parameters, including nocturnal BP dip parameters as determined utilizing 24-h ABPM, with the presence of CMvD in NTG patients. The clarification of this relationship could provide important insights into the role of CMvD and vascular insufficiency in the pathophysiology of NTG.
Results
Of the 95 eyes of 95 NTG patients who initially met the inclusion criteria, 7 eyes were excluded because of poor quality OCT-A images. Eighty-eight eyes of 88 NTG patients (35 men and 53 women; mean age of 56.0 ± 12.2 years) were included in the final analysis. Of these patients, 19 (21.6%) had a history of systemic hypertension and were taking oral antihypertensive medication. In subgroup analysis using eyes with and without CMvD, matched by VF mean deviation (MD; ≤ 1 dB) and age (≤ 10 years), 30 eyes were assigned to each subgroup, with a mean age of 57.1 ± 12.2 years. There were excellent interobserver agreement in terms of determining the presence of CMvD (k = 0.904; 95% confidence interval [CI] 0.832–0.975, P < 0.001). The interobserver intraclass correlation coefficient (ICC) for β-zone parapapillary atrophy (β-PPA) area measurement was 0.944 (95% CI 0.908–0.979).
CMvD was identified in 38 (43.2%) of 88 eyes of NTG patients. Table 1 summarizes the demographic, ocular, and systemic characteristics of eyes with and without CMvD. Eyes with CMvD had significantly worse VF MD, worse VF pattern standard deviation (PSD), lower average circumpapillary vessel density (cpVD), lower circumpapillary RNFL thickness (cpRNFLT), and larger β-PPA area than eyes without CMvD (all P < 0.05). There were no significant differences between the 2 groups with respect to age, sex, refractive error (RE), axial length (AL), central corneal thickness (CCT), untreated office intraocular pressure (IOP), office systolic BP (SBP), DBP, the presence of optic disc hemorrhage, number of patients with hypertension and antihypertensive medication use, diabetes mellitus, stroke, migraine, and cold extremities. In subgroup analysis using eyes with and without CMvD, matched for glaucoma severity and age, eyes with CMvD showed more myopic RE and AL measurements (P = 0.028 and 0.047) than eyes without CMvD, whereas VF MD, PSD, cpVD, cpRNFLT, and β-PPA area did not differ between the 2 subgroups. Other demographic, ocular, and systemic characteristics did not differ between the 2 subgroups (Table 1).
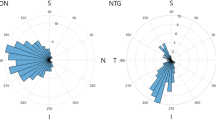
Table 2 shows in-hospital 24-h daytime and nighttime BP and IOP measurements in eyes with and without CMvD. In all subjects, daytime and nighttime SBP parameters did not differ significantly between eyes with and without CMvD (all P > 0.05). Nighttime DBP dip “%” (19.7% vs. 12.8%, P = 0.009), dip “time” (1.7 h and 1.1 h, P = 0.041), and dip “area” (16.1 mmHg h vs. 8.7 mmHg h, P = 0.037) were significantly greater in eyes with CMvD that in those without CMvD, whereas other daytime and nighttime DBP parameters did not show significant differences between the 2 groups (all P > 0.05). Among 24-h IOP parameters, eyes with CMvD showed significantly higher daytime peak IOP than eyes without CMvD. Figure 1 shows the overall 24-h BP patterns of eyes with and without CMvD in the entire patient group. Nighttime DBP in eyes with CMvD showed a BP dip with a longer duration and greater magnitude (i.e., at least 10 mmHg below mean daytime BP) than those in eyes without CMvD. However, nighttime SBP did not differ in patterns of duration and magnitude of BP dip between eyes with and without CMvD.
Overall 24-h blood pressure (BP) patterns of eyes with and without choroidal microvasculature dropout (CMvD) in the entire patient group (n = 88). Nighttime diastolic BP (DBP) in eyes with CMvD showed a longer duration and greater magnitude of BP dip (black area; at least 10 mmHg below the mean daytime BP) than those in eyes without CMvD. However, nighttime systolic BP (SBP) did not show differences in patterns of duration and magnitude of BP dip between eyes with and without CMvD.
In VF MD- and age-matched subgroup analyses (Table 2), nighttime SBP range (24.7 mmHg vs. 17.1 mmHg, P = 0.011) and dip “%” (15.9% vs. 10.9%, P = 0.033), nighttime DBP trough (62.9 mmHg vs. 69.2 mmHg, P = 0.023), range (17.8 mmHg vs. 12.9 mmHg, P = 0.034), dip “%” (18.8% vs. 8.6%, P = 0.001), dip “time” (1.7 h vs. 0.5 h, P < 0.001), and dip “area” (15.3 mmHg h vs. 2.6 mmHg h, P = 0.001) were significantly greater in eyes with CMvD that in those without CMvD. Daytime SBP and DBP parameters did not show differences between the 2 subgroups (all P > 0.05). Eyes with CMvD showed a wider daytime IOP range than eyes without CMvD in subgroup analysis (P = 0.048).
The factors associated with the presence of CMvD were determined using logistic regression analyses (Tables 3, 4, and 5). In all subjects, worse VF MD, lower cpVD, lower cpRNFLT, larger β-PPA area, greater nighttime DBP dip (“%”, “time”, and “area”), and higher daytime peak IOP were significantly associated with the presence of CMvD, in univariate analyses (all P < 0.05, Table 3). In multivariate analyses, worse VF MD (odds ratio [OR], 0.786; P = 0.001), greater nighttime DBP dip “%” (OR 1.051; P = 0.034), and higher daytime peak IOP (OR 1.459; P = 0.013) were significantly associated with the presence of CMvD (Table 4). In VF MD- and age-matched subgroup analyses, greater nighttime SBP range and dip “%”, lower nighttime DBP trough, and greater nighttime DBP range and dip (“%”, “time”, and “area”) were significantly associated with the presence of CMvD in univariate analyses (P < 0.05, Table 3). In multivariate analyses with the subgroup, greater nighttime DBP dip “time” (OR 4.418; P = 0.031) and “area” (OR 1.182; P = 0.048) showed significant associations with the presence of CMvD (Table 5).
Table 6 shows the results of logistic regression analyses for determining clinical factors associated with the presence of extreme nighttime DBP dip. In all subjects, the presence of CMvD and lower office-hour DBP were associated with the presence of extreme nighttime DBP dip, in univariate analyses (P < 0.05). In VF MD- and age-matched subgroups, the presence of CMvD was associated with the presence of an extreme nighttime DBP dip, in univariate analyses (P < 0.05). Multivariate analyses revealed that the presence of CMvD was a significant factor associated with the presence of an extreme nighttime DBP dip, after adjusting for office-hour DBP in all subjects (OR 2.488; P = 0.047) and in the VF MD- and age-matched subgroup (OR 7.087; P = 0.019).
Representative cases are shown in Fig. 2. A 52-year-old woman with CMvD as indicated by red arrow in the β-PPA (Fig. 2A) and 56-year-old woman without CMvD (Fig. 2B) had similar severity of glaucomatous damage (VF MD − 7.17 dB vs. − 6.23 dB, respectively; cpRNFLT, 79 μm vs. 76 μm, respectively). Nevertheless, 24-h BP patterns showed differences, depending on the presence of CMvD. Nighttime DBP in the eye with CMvD showed a longer duration and greater magnitude of BP dip (i.e., at least 10 mmHg below the mean daytime BP) than those in the eye without CMvD. However, nighttime SBP showed similar patterns of BP dips in eyes with and without CMvD.
Representative cases of normal-tension glaucoma eyes with and without choroidal microvasculature dropout (CMvD). A 52-year-old woman with CMvD as indicated by red outline in the β-zone parapapillary atrophy shown by yellow outlines (A) and a 56-year-old woman without CMvD (B) had similar circumpapillary retinal nerve fibre layer loss (thickness, 79 μm vs. 76 μm) in the inferotemporal area and visual field defect (mean deviation, − 7.17 dB vs. − 6.23 dB, respectively) in the superior hemifield. Nevertheless, 24-h blood pressure (BP) patterns showed differences depending on the presence of CMvD. Nighttime diastolic BP (DBP) in the eye with CMvD showed a longer duration and greater magnitude of BP dip (black area; at least 10 mmHg below the mean daytime BP) than those in the eye without CMvD. However, nighttime systolic BP (SBP) showed similar patterns of BP dip in eyes with and without CMvD.
Discussion
The presence of CMvD has been reported to be associated with systemic vascular insufficiency, such as lower office-hour DBP, MAP, and OPP13,15, and may represent a regional filling defect of the parapapillary choroid in glaucoma patients12. These findings suggest that CMvD may be a sign of ischemia in the parapapillary choroid and ONH, rather than being secondary to glaucomatous damage13,15. Since BP is a dynamic biologic parameter, a single snap-shot measurement of BP during office hours may not accurately reflect the true relationship between circadian BP readings and CMvD in glaucomatous patients. Using 24-h ABPM, Hayreh et al.22 demonstrated that significant nocturnal DBP dip may reduce the ONH blood flow to below a critical level, and thereby may contribute significantly to the pathogenesis of NTG. In the current study, nighttime DBP dip parameters were significantly associated with the presence of CMvD in the entire group, as well as in subgroup analyses in NTG eyes with and without CMvD. Moreover, the presence of CMvD was also an independent predictor of an extreme nighttime DBP dip in both analyses. Our findings, therefore, suggest that nocturnal hypotension, as expressed in the magnitude and duration of DBP dip (“%”, “area”, and “time”), may be independently linked to CMvD, a parapapillary choroidal perfusion defect. To our knowledge, no previous study has assessed the association between nocturnal BP dip and CMvD detection in NTG eyes using 24-h ABPM data; our findings may provide additional knowledge regarding the clinical implications of CMvD in glaucoma.
Nocturnal hypotension has been regarded as a significant predictor of glaucoma progression4,5,6,7,8,9,23. Charlson et al.7 reported that the duration and magnitude of nocturnal MAP dip were associated with progressive VF loss in NTG eyes. Our group recently reported that the nocturnal DBP dip, in terms of its duration and magnitude, had a significant impact on glaucomatous VF progression in NTG eyes9. Since DBP has been considered as a major determinant of tissue perfusion in various end organs including eye9,24,25,26,27,28,29,30,31, chronic ischemia on the ONH was proposed as a possible mechanism for the relationship between nocturnal hypotension and future glaucoma progression5,32. Of note, we found that eyes with CMvD had a significant association with nighttime DBP dip, as expressed by “%”, “time”, and “area” in both the entire group and subgroup analyses, irrespective of differences in the glaucoma severity (Tables 4 and 5). Therefore, it is not surprising that the presence of CMvD may be a significant predictor of future VF and RNFL progression, as reported in previous studies17,18,33.
Contrary to previous studies13,15, none of the office-hour BP parameters showed significant association with CMvD in both the entire group and the VF MD- and age-matched subgroup analyses in the current study. Since short- and long-term variabilities are important characteristics of systemic BP, different study results can be derived from snapshot BP readings, depending on when BP is measured during office hours. For this reason, 24-h ABPM data is considered a better predictor of end-organ disease, such as glaucoma, than single daytime BP readings7,8,9. In a study of 93 participants from the Maracaibo Aging Study8, extreme nocturnal BP dip, defined as a decrease of more than 20% of the nocturnal BP levels relative to daytime BP levels in 24-h ABPM outcomes, was found to be a more useful indicator of glaucomatous damage than once-off measurement of office-hour BP. Consequently, various nighttime and daytime BP dip parameters, derived from 24-h ABPM as outlined in our study, may provide more accurate information regarding the true association between systemic BP dip and CMvD in NTG patients.
Although vascular autoregulation preserves constant blood flow in the ONH against BP instability or fluctuation, this adaptive mechanism cannot compensate for extreme nocturnal BP dip, which can be detected by 24-h ABPM in some patients4. This may explain our finding that nighttime DBP dip parameters (“%”, “time”, and “area”) were significantly associated with the presence of CMvD in a multivariate model in both the entire group and subgroup analyses, while office-hour BP did not show any association. Our findings suggest that a large magnitude or long duration of DBP dip at nighttime may reduce the blood flow below a critical level, impairing parapapillary choroid perfusion in the form of CMvD. Therefore, 24-h ABPM may have an advantage in assessing the risk of a regional perfusion defect in the ONH, represented by CMvD. Future prospective and longitudinal studies are needed to elucidate a temporal relationship between nocturnal hypotension and development or enlargement of a CMvD in NTG patients.
Although nocturnal hypotension, particularly a nocturnal DBP dip, is associated with ONH ischemia and progressive VF loss in NTG eyes9,23, the association between extreme nighttime DBP dip and CMvD has not been studied. In the current study, the presence of CMvD was an independent predictor of extreme nighttime DBP dip, irrespective of the patient group analysed (Table 6). DBP has been regarded as a major determinant of perfusion status in various end organs, including the eye9,24,25,26,27,28,29,30,31. Although it remains to be clarified, an explanation for the association of CMvD with extreme nocturnal DBP dip is that nighttime DBP dip may lead to compromised perfusion in the posterior ciliary arteries (PCAs), which supply both the parapapillary choroid and the deep optic nerve tissues19,34. This may, in turn, lead to regional perfusion deficiency, such as CMvD, at the location of the watershed zone, which is vulnerable to ischemic conditions, such as a systemic DBP dip. Fluorescein angiography showed that non-filling of the watershed zone in the human eye is found at the temporal part of ONH and the parapapillary choroid between the lateral and medial PCAs34. As such, the temporal parapapillary choroid within the β-PPA is the most common location of CMvD in glaucomatous eyes14,15,16.
IOP has a significant impact on the development and progression of NTG. Crichton et al.35 reported that inter-eye differences in IOP do occur in patients with NTG, and that VF damage was greater in the eye with the higher mean IOP. Both daytime and nighttime IOP peaks have been linked to glaucomatous progression36,37. In the current study, daytime IOP peak was significantly associated with the presence of CMvD in the multivariate model using the entire patient group, in addition to VF MD and nighttime DBP dip “%” (Table 4). In contrast, previous studies have not found significant association between IOP parameters and the presence of CMvD13,15. Although the reasons for this discrepancy remain unclear, it may be related to whether IOP lowering agents were used in the study subjects. Our treatment-naïve patients reflect the actual relationship between IOP and the presence of CMvD, since higher baseline IOP prior to treatment may induce mechanical stress in the vulnerable zone of the ONH, such as the temporal part of the ONH and the parapapillary choroid, independent of ischemic compromise. Interestingly, however, the impact of the daytime IOP peak was no longer significant in the multivariate model of VF MD- and age-matched subgroups (Table 5).
Systemic vascular risk factors, such as systemic hypotension, nocturnal BP dip, and large BP variability, have been reported to be associated with parafoveal scotoma in NTG38,39. It remains unclear how parafoveal scotoma is related to eyes with these systemic vascular risk factors. Recent OCT-A studies showed that parafoveal scotoma is related to the presence of CMvD15,16. Interestingly, Lee et al.15 reported that only CMvD was a significant predictor for parafoveal scotoma in multivariate analyses, although BP measurements, such as office-hour MAP and OPP, showed significant association with parafoveal scotoma in univariate analyses. Considering the significant relationship between nighttime DBP dip and the presence of CMvD in the current study, we hypothesize that nocturnal hypotension may induce ischemia in the parapapillary choroid (e.g., CMvD). This, in turn contributes to the development of parafoveal scotoma, since CMvD is frequently observed at the inferotemporal region12,15,16, nearby the macular vulnerability zone, which is a narrow region of the ONH typically associated with parafoveal scotoma40.
Our study had several limitations. First, we had a relatively small sample size for the matched subgroup analysis (n = 60 in total). In the current study, subgroup analysis was performed for the eyes with early-to-moderate severity of VF damage, matched by VF MD (≤ 1 dB) and age (≤ 10 years), in eyes with and without CMvD, since the presence of CMvD is significantly related to the severity of glaucomatous damage and age13,14. The small number of patients in each group may also limit the generalizability of our findings to the general population with glaucoma. Second, although there was no significant difference in the proportion of antihypertensive medication use between groups with and without CMvD, information regarding different types of oral antihypertensive medications were not available, due to a lack of self-reported information from patients. Calcium channel blockers can induce a steal phenomenon and cause a sudden and extreme reduction of BP41, although there was no association between extreme nocturnal DBP dip and use of antihypertensive medication in our logistic regression analysis (Table 6). Further studies including large numbers of glaucoma patients, stratified by use of different types of oral antihypertensive agents, are needed to evaluate the impact of these agents on 24-h ABPM readings and its association with CMvD. Third, subjectivity can intervene in the determination of CMvD, although we attempted to minimize this by using 2 independent examiners and a 3rd adjudicator. In addition, eyes with partially reduced microvasculature may have been classified as not having CMvD, because we defined CMvD as a complete loss of the choroidal microvasculature, with a size of ≥ 200 μm in diameter. Fourth, projection artifacts or shadow effects may lead to under- or over-estimation of CMvD. Thus, our results should be interpreted with caution considering the technical limitation of OCT-A imaging. Quantitative measure of overall parapapillary choroidal vessel density, followed by examination of the relationship between 24-h ABPM and choroidal vessel density, may mitigate this issue42, and provide an alternative means to study the influence of BP dip on CMvD or parapapillary choroidal ischemia. All participants in the current study were Korean NTG patients with β-PPA, thus preventing generalization of our results to those without β-PPA or other types of glaucoma with different racial/ethnic groups. Various IOP-independent factors, such as vascular dysregulation or systemic hypotension, may be more closely associated with Koreans or the pathogenesis of NTG43,44,45. Selection bias may have been present as our patients were enrolled at a large university practice rather than in population-based settings; thus, they may not possess the same characteristics as similar patients in the general population. Finally, because of the cross-sectional study design, we could not assess the longitudinal relationship between various nocturnal BP dip parameters and CMvD detection. Longitudinal studies are required to evaluate the temporal relationship between nocturnal hypotension and the emergence of or change in CMvD.
In conclusion, NTG eyes with CMvD had nighttime DBP dip of greater magnitude and longer duration than eyes without CMvD. Moreover, nighttime DBP dip parameters (“time”, “area”, and “%”) were significantly associated with the presence of CMvD in NTG eyes. Despite that 24-h ABPM provides clinicians with valuable information regarding nighttime DBP dip and parapapillary/ONH ischemia, it may be too burdensome to perform 24-h ABPM in all glaucoma patients. Since our study demonstrates that the eyes with CMvD are closely associated with having nighttime DBP dip, NTG patients with CMvD should be recommended to undergo 24-h ABPM. Based on the results of 24-h ABPM, the modulation of nighttime DBP dips may prevent or slow down future glaucoma progression.
Methods
Study subjects
This cross-sectional study enrolled consecutive NTG patients from the cohort of an ongoing prospective study evaluating the relationship between NTG and systemic BP, which began recruitment from January 2013 at the glaucoma clinic of Asan Medical Center, Seoul, Korea. The study protocol was approved by the institutional review board of Asan Medical Center, and all procedures were carried out in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
All subjects underwent complete ophthalmologic examinations, including best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, gonioscopy, Goldmann applanation tonometry, a manifest refraction test, AL (IOLMaster; Carl Zeiss Meditec, Dublin, CA) and CCT measurements (DGH-550; DGH Technology, Exton, PA), dilated color fundus photography, stereoscopic optic disc photography, red-free fundus photography (AFC-210; Nidek, Aichi, Japan), standard automated perimetry (Humphrey Field Analyzer with Swedish Interactive Threshold Algorithm standard 24-2 test; Carl Zeiss Meditec), cpRNFLT measurement using Cirrus HD spectral-domain optical coherence tomography (SD-OCT, Carl Zeiss Meditec), and OCT-A (Angiovue; Optovue Inc, Fremont, CA). SBP and DBP were measured once during office clinic hours.
NTG was defined as the presence of an open anterior chamber angle on gonioscopy, the absence of an identifiable secondary cause of glaucoma, a maximum bilateral untreated IOP < 22 mmHg in the outpatient clinic at 9:00 AM, 12:00 PM, and 4:00 PM, signs of glaucomatous optic neuropathy (i.e., vertical cup-to-disc [C/D] ratio > 0.7; asymmetry in the vertical C/D ratio between the eyes exceeding 0.2, and not explained by optic disc size; focal or generalized neuroretinal rim thinning; or RNFL defect), and compatible glaucomatous VF loss9. Eyes were considered to have glaucomatous VF loss if the glaucoma hemifield test results were outside normal limits and the PSD had a P value < 5%, confirmed on 2 consecutive reliable VF tests. When the first VF result showed glaucomatous defects, the first perimetric result was excluded from analysis, to obviate learning effects. All VF tests had to be reliable, defined as false-positive and false-negative error rates < 15% each, and fixation loss < 20%.
To be included in the current study, all NTG subjects had to meet the following criteria: no prior glaucoma treatment, age > 18 years, BCVA of 20/30 or better, a spherical equivalent between − 8.0 and + 3.0 diopters (D), cylinder correction within + 3 D, and visible β-PPA on fundus photography. All included participants underwent in-hospital 24-h monitoring of IOP and BP in the habitual position (sitting during the daytime [8 AM–10 PM] and supine at nighttime [12 AM–6 AM]). The affected eye was selected in patients with unilateral disease. If both eyes of a patient were eligible, 1 eye was selected at random.
Patients were excluded from the study if they had outpatient IOP > 21 mmHg; a history of intraocular or refractive surgery; pathologic myopia (patchy chorioretinal atrophy, lacquer crack, intrachoroidal cavitation, or choroidal neovascularization) or other evidence of retinal pathology that could affect the VF test; opaque media, such as visually significant cataract or BCVA < 20/30; previous or current use of systemic or topical steroid; and any history of neurologic or ophthalmic disease that could lead to VF abnormality9. Individuals who smoked or had irregular daily sleep patterns were also excluded. However, individuals taking systemic antihypertensive agents were not excluded9.
In-hospital 24-h intraocular pressure and ambulatory blood pressure monitoring
The method of measuring in-hospital 24-h IOP an ABPM has been extensively described in our previous studies9,10,46,47. Briefly, all subjects were instructed to abstain from alcohol and caffeine for 3 days before hospital admission. IOP in both eyes of each patient was measured using the TonoPen XL (Mentor Ophthalmics, Santa Barbara, CA) every 2 h, from 8 AM to 10 PM, in a sitting position (daytime IOP) and every 3 h from 12 to 6 AM, in a supine position (nighttime IOP). This schedule was used to provide the best tradeoff between the maximal number of IOP readings over 24 h and minimal nonphysiological responses during in-hospital IOP measurement9,10,46. The IOP was measured 3 times in each eye, and average IOP was used for analysis. Various IOP parameters, including the mean, peak, and range of daytime and nighttime IOPs were calculated separately.
In-hospital 24-h SBP, DBP, and heart rate were measured every 30 min using a fully automated ABPM device (Spacelabs Healthcare, Issaquah, WA). This automated device minimized the variability among operators and measured BP in the most physiological environment, while patients continued their routine 24-h activities. The mean, peak, trough, and range of daytime and nighttime SBP and DBP were calculated separately, since short-term (daytime or nighttime) BP data and its variability over 24 h independently contribute to end-organ damage, including glaucoma, while concurrently avoiding the use of single daytime and nighttime BP readings4,5,6,48,49,50,51,52. Patients were encouraged to continue their normal indoor activities during the day and were asked to refrain from any physical activities that could affect BP at night.
Nighttime and daytime blood pressure dip parameters
The magnitude and duration of the nighttime BP dip relative to the mean daytime BP level (i.e., nocturnal BP trough > 10 mmHg below the average daytime BP) is predictive of future glaucoma progression7,9,10. Therefore, we investigated the magnitude and duration of the nighttime and daytime BP dip relative to the mean daytime BP value, using the following 3 variables: (1) “%”, (2) “time”, and (3) “area.”
The “%” of the nighttime and daytime BP dip was calculated as9,10:
The “time” (in h) of the nighttime and daytime BP dip was defined as the total time spent at least 10 mmHg below the mean daytime BP during nighttime and daytime, respectively7,9. The “area” (mmHg h) of the nighttime and daytime BP dip was defined as the time multiplied by the magnitude of the nighttime and daytime BP that was at least 10 mmHg below the mean daytime BP, respectively7,9. In the current study, the “%”, “time”, and “area” of the SBP and DBP dip during daytime and nighttime were calculated separately. Finally, the extreme nocturnal BP dip was defined as an abnormal decrease in the nighttime BP trough exceeding 20% in relation to the mean daytime BP10.
Choroidal microvasculature dropout and circumpapillary vessel density measurement using optical coherence tomography angiography
The AngioVue OCT-A imaging system (Software version 2017.1.0.144; Optovue Inc.) facilitates noninvasive visualization of the ophthalmic microvasculature. This OCT-A system has a light source with a center wavelength of 840 nm, a scanning speed of 70,000 A-scans per second, an axial resolution of 5 μm, and a transverse resolution of 15 μm. A split-spectrum amplitude-decorrelation angiography algorithm was used to identify perfused vessels by capturing the dynamic motion of moving particles, such as red blood cells (RBCs)53. All NTG participants underwent OCT-A imaging of a 4.5 × 4.5 mm2 region centered on the optic disc. Only qualified images with a signal strength index (SSI) > 45, no motion artifacts, and segmentation error were analysed.
The choroidal microvasculature was evaluated within the β-PPA area, based on the choroidal layer of the OCT-A image. The β-PPA was defined as an atrophic region of the retinal pigment epithelium resulting in a distinctive appearance of scleral and large choroidal vessels54. The β-PPA area was outlined and calculated on scanning laser ophthalmoscopic images by 2 observers (J.W.S. and Y.J.) using ImageJ software (version 1.51; National Institutes of Health, Bethesda, MD, USA). The Littmann formula was used for correction of AL-related ocular magnification effects in measuring the β-PPA area55,56. Average values of the β-PPA area measurements from the 2 examiners were used in analyses. The CMvD within the β-PPA was defined as a complete loss of the choriocapillaris and choroidal microvasculature, without any visible microvasculature network, as defined in previous studies12,13. The minimum width of the CMvD at the smallest part of the lesion was required to be 200 μm or greater than the width of the central retinal vein, to avoid false positive findings13,16,57. 2 observers (J.W.S. and Y.J.) independently determined the presence of CMvD, blinded to the clinical information of participants. Disagreements between these 2 observers were resolved by a third adjudicator (M.S.K.).
The cpVD was evaluated within the radial peripapillary capillary slab, from the internal limiting membrane to the nerve fibre layer. The cpVD was calculated as the percentage of measured area occupied by vessels with flowing RBCs in a region defined as a 1000-µm-wide elliptical annulus extending from the optic disc boundary.
Spectral-domain optical coherence tomography evaluation
Optic disc cube scans, obtained using the Cirrus HD-OCT (software version 10.0), measured RNFL thickness at peripapillary regions (6 × 6 mm2) centered on the optic disc. The average cpRNFLT was measured in a 3.46-mm diameter circle. Poor quality SD-OCT images, with motion artifacts, segmentation failure, or signal strength < 7, were excluded.
Statistical analysis
Interobserver agreement regarding the presence of CMvD was calculated by kappa statistics. The interobserver reproducibility for β-PPA area measurement was assessed by calculating the ICC. Clinical characteristics and in-hospital 24-h IOP and ABPM parameters were compared between eyes with and without CMvD. Continuous variables were compared using independent Student’s t-tests or Mann–Whitney U tests, depending on the result of the normality test (Shapiro–Wilk test). Categorical variables were compared using the chi-square test. Univariate logistic regression analysis was performed to determine potential clinical variables (i.e., office BPs, in-hospital daytime and nighttime SBP, DBP dip, and IOP parameters) associated with the presence of the CMvD. A backward elimination process was used to build a multivariate logistic regression model incorporating variables with P < 0.10 in univariate analysis. Multicollinearity among independent variables in the multivariate model was assessed by quantifying the variance inflation factor (VIF), with high multicollinearity defined as a VIF > 1058.
Subgroup analysis was performed for eyes with early-to-moderate severity of VF damage (MD > -12 dB). Subgroups were matched by VF MD (≤ 1 dB) in eyes with and without CMvD, since the presence of CMvD is significantly related to the severity of glaucomatous damage13,14. In addition, 2 subgroups with and without CMvD were also matched by patient age (≤ 10 years). Finally, the factors associated with extreme nocturnal DBP dip were also determined using logistic regression analyses, as a profound DBP dip is significantly linked to ONH ischemia and NTG3,9. P values < 0.05 (two-tailed) were considered statistically significant. All statistical analyses were performed using SPSS software (ver. 20.0; IBM Corp., Armonk, NY) and the R statistical computing package (ver. 3.1.2; R Foundation, Vienna, Austria).
References
Leske, M. C. et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 114(11), 1965–1972 (2007).
Leske, M. C. et al. Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology 115(1), 85–93 (2008).
Hayreh, S. S. Role of nocturnal arterial hypotension in the development of ocular manifestations of systemic arterial hypertension. Curr. Opin. Ophthalmol. 10(6), 474–482 (1999).
Graham, S. L. et al. Ambulatory blood pressure monitoring in glaucoma. Nocturnal Dip. Ophthalmol. 102(1), 61–69 (1995).
Collignon, N., Dewe, W., Guillaume, S. & Collignon-Brach, J. Ambulatory blood pressure monitoring in glaucoma patients: The nocturnal systolic dip and its relationship with disease progression. Int. Ophthalmol. 22(1), 19–25 (1998).
Tokunaga, T. et al. Association between nocturnal blood pressure reduction and progression of visual field defect in patients with primary open-angle glaucoma or normal-tension glaucoma. Jpn. J. Ophthalmol. 48(4), 380–385 (2004).
Charlson, M. E. et al. Nocturnal systemic hypotension increases the risk of glaucoma progression. Ophthalmology 121(10), 2004–2012 (2014).
Melgarejo, J. D. et al. Glaucomatous optic neuropathy associated with nocturnal dip in blood pressure: Findings from the maracaibo aging study. Ophthalmology 125(6), 807–814 (2018).
Kwon, J. et al. Baseline systolic versus diastolic blood pressure dip and subsequent visual field progression in normal-tension glaucoma. Ophthalmology 126(7), 967–979 (2019).
Lee, J. et al. Relationship between daytime variability of blood pressure or ocular perfusion pressure and glaucomatous visual field progression. Am. J. Ophthalmol. 160(3), 522–37.e1 (2015).
Sung, K. R. et al. Twenty-four hour ocular perfusion pressure fluctuation and risk of normal-tension glaucoma progression. Invest. Ophthalmol. Vis. Sci. 50(11), 5266–5274 (2009).
Lee, E. J., Lee, K. M., Lee, S. H. & Kim, T. W. Parapapillary choroidal microvasculature dropout in glaucoma: A comparison between optical coherence tomography angiography and indocyanine green angiography. Ophthalmology 124(8), 1209–1217 (2017).
Suh, M. H. et al. Deep retinal layer microvasculature dropout detected by the optical coherence tomography angiography in glaucoma. Ophthalmology 123(12), 2509–2518 (2016).
Shin, J. W., Kwon, J., Lee, J. & Kook, M. S. Choroidal microvasculature dropout is not associated with myopia, but is associated with glaucoma. J. Glaucoma 27(2), 189–196 (2018).
Lee, E. J., Kim, T. W., Kim, J. A. & Kim, J. A. Central visual field damage and parapapillary choroidal microvasculature dropout in primary open-angle glaucoma. Ophthalmology 125(4), 588–596 (2018).
Kwon, J., Shin, J. W., Lee, J. & Kook, M. S. Choroidal microvasculature dropout is associated with parafoveal visual field defects in glaucoma. Am. J. Ophthalmol. 188, 141–154 (2018).
Park, H. L., Kim, J. W. & Park, C. K. Choroidal microvasculature dropout is associated with progressive retinal nerve fiber layer thinning in glaucoma with disc hemorrhage. Ophthalmology 125(7), 1003–1013 (2018).
Kwon, J. M., Weinreb, R. N., Zangwill, L. M. & Suh, M. H. Parapapillary deep-layer microvasculature dropout and visual field progression in glaucoma. Am. J. Ophthalmol. 200, 65–75 (2019).
Hayreh, S. S. Posterior ciliary artery circulation in health and disease: The Weisenfeld lecture. Invest. Ophthalmol. Vis. Sci. 45(3), 749–757 (2004).
Anderson, D. R. & Braverman, S. Reevaluation of the optic disk vasculature. Am. J. Ophthalmol. 82(2), 165–174 (1976).
Suh, M. H. et al. Deep-layer microvasculature dropout by optical coherence tomography angiography and microstructure of parapapillary atrophy. Invest. Ophthalmol. Vis. Sci. 59(5), 1995–2004 (2018).
Hayreh, S. S., Zimmerman, M. B., Podhajsky, P. & Alward, W. L. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am. J. Ophthalmol. 117(5), 603–624 (1994).
Graham, S. L. & Drance, S. M. Nocturnal hypotension: Role in glaucoma progression. Surv. Ophthalmol. 43(Suppl 1), S10–S16 (1999).
Varsos, G. V. et al. Cessation of diastolic cerebral blood flow velocity: The role of critical closing pressure. Neurocrit. Care 20(1), 40–48 (2014).
Fokkema, D. S. et al. Diastolic time fraction as a determinant of subendocardial perfusion. Am. J. Physiol. Heart Circ. Physiol. 288(5), H2450–H2456 (2005).
McEvoy, J. W. et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: Implications for blood pressure control. J. Am. Coll. Cardiol. 68(16), 1713–1722 (2016).
Protogerou, A. D. et al. Diastolic blood pressure and mortality in the elderly with cardiovascular disease. Hypertension 50(1), 172–180 (2007).
Senthong, V. et al. Low diastolic blood pressure is associated with a high atherosclerotic burden in patients with obstructive coronary artery disease. Cardiol. J. 25(3), 345–352 (2018).
Legrand, M. et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: A retrospective observational study. Crit. Care 17(6), R278 (2013).
Saito, S. et al. Postoperative blood pressure deficit and acute kidney injury progression in vasopressor-dependent cardiovascular surgery patients. Crit. Care 20, 74 (2016).
Sato, R., Luthe, S. K. & Nasu, M. Blood pressure and acute kidney injury. Crit. Care 21(1), 28 (2017).
Cioffi, G. A. Ischemic model of optic nerve injury. Trans. Am. Ophthalmol. Soc. 103, 592–613 (2005).
Jo, Y. H. et al. Rapid central visual field progression rate in eyes with open-angle glaucoma and choroidal microvasculature dropout. Sci. Rep. 9(1), 8525 (2019).
Hayreh, S. S. The blood supply of the optic nerve head and the evaluation of it—myth and reality. Prog. Retin. Eye Res. 20(5), 563–593 (2001).
Crichton, A., Drance, S. M., Douglas, G. R. & Schulzer, M. Unequal intraocular pressure and its relation to asymmetric visual field defects in low-tension glaucoma. Ophthalmology 96(9), 1312–1314 (1989).
De Moraes, C. G. et al. Visual field change and 24-hour IOP-related profile with a contact lens sensor in treated glaucoma patients. Ophthalmology 123(4), 744–753 (2016).
Konstas, A. G. et al. Peak intraocular pressure and glaucomatous progression in primary open-angle glaucoma. J. Ocul. Pharmacol. Ther. 28(1), 26–32 (2012).
Park, S. C. et al. Initial parafoveal versus peripheral scotomas in glaucoma: Risk factors and visual field characteristics. Ophthalmology 118(9), 1782–1789 (2011).
Jin, S. W., Seo, H. R., Rho, S. S. & Rho, S. H. The effects of nocturnal dip and blood pressure variability on paracentral scotoma in early open-angle glaucoma. Semin. Ophthalmol. 32(4), 504–510 (2017).
Hood, D. C. et al. Glaucomatous damage of the macula. Prog. Retin. Eye Res. 32, 1–21 (2013).
Sodhi, P. K., Garg, E. & Vidula, ,. Association between nocturnal blood pressure dips and optic disc hemorrhage in patients with normal-tension glaucoma. Am. J. Ophthalmol. 181, 176–177 (2017).
Park, H. Y., Shin, D. Y., Jeon, S. J. & Park, C. K. Association between parapapillary choroidal vessel density measured with optical coherence tomography angiography and future visual field progression in patients with glaucoma. JAMA Ophthalmol. 137(6), 681–688 (2019).
Flammer, J. et al. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye. Res. 21(4), 359–393 (2002).
Kim, C. S., Seong, G. J., Lee, N. H. & Song, K. C. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology 118(6), 1024–1030 (2011).
Kim, J. M. et al. Comparison of clinical characteristics between Korean and Western normal-tension glaucoma patients. Am. J. Ophthalmol. 155(5), 852–857 (2013).
Kwon, J. et al. Association between nocturnal blood pressure dips and optic disc hemorrhage in patients with normal-tension glaucoma. Am. J. Ophthalmol. 176, 87–101 (2017).
Jeong, D. W. et al. Circadian pattern of intraocular pressure fluctuations in young myopic eyes with open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 55(4), 2148–2156 (2014).
Frattola, A. et al. Prognostic value of 24-hour blood pressure variability. J. Hypertens. 11(10), 1133–1137 (1993).
Rothwell, P. M. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet 375(9718), 938–948 (2010).
Verdecchia, P. et al. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am. J. Hypertens. 20(2), 154–161 (2007).
Pierdomenico, S. D. et al. Blood pressure variability and cardiovascular risk in treated hypertensive patients. Am. J. Hypertens. 19(10), 991–997 (2006).
Palatini, P. et al. Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch. Intern. Med. 152(9), 1855–1860 (1992).
Jia, Y. et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express 20(4), 4710–4725 (2012).
Jonas, J. B., Nguyen, X. N., Gusek, G. C. & Naumann, G. O. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest. Ophthalmol. Vis. Sci. 30(5), 908–918 (1989).
Bennett, A. G., Rudnicka, A. R. & Edgar, D. F. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch. Clin. Exp. Ophthalmol. 232(6), 361–367 (1994).
Aykut, V. et al. Influence of axial length on peripapillary retinal nerve fiber layer thickness in children: A study by RTVue spectral-domain optical coherence tomography. Curr. Eye Res. 38(12), 1241–1247 (2013).
Mitchell, P. et al. Retinal vessel diameter and open-angle glaucoma: The Blue Mountains Eye Study. Ophthalmology 112(2), 245–250 (2005).
Dormann, C. F. et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 36(1), 27–46 (2013).
Author information
Authors and Affiliations
Contributions
Study design (M.S.K. and J.W.S.), data collection (M.K.S. and H.J.W), analysis and interpretation of the data (M.S.K. and J.W.S.), writing the article (M.S.K. and J.W.S.), final approval of the article (M.K.S.), literature search (Y.J. and M.K.S.). All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shin, J.W., Jo, Y.H., Song, M.K. et al. Nocturnal blood pressure dip and parapapillary choroidal microvasculature dropout in normal-tension glaucoma. Sci Rep 11, 206 (2021). https://doi.org/10.1038/s41598-020-80705-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80705-3
This article is cited by
-
Clinical characteristics of open-angle glaucoma progression with peripapillary microvasculature dropout in different locations
Eye (2024)
-
Empty-Sella-Syndrom und/oder Normaldruckglaukom?
Die Ophthalmologie (2023)
-
Association of choroidal blood flow with autonomic dysfunction in patients with normal tension glaucoma
Scientific Reports (2022)
-
Clinical characteristics of choroidal microvasculature dropout in normal-tension glaucoma versus nonarteritic anterior ischemic optic neuropathy: an optical coherence tomography angiography study
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.