Abstract
Background/objectives
The choroid, mostly composed of vascular structures, can be directly affected by systemic hemodynamic changes. Blood pressure variability (BPV) may factor into choroidal dysfunction, which can be associated with the pathogenesis of central serous chorioretinopathy (CSCR). The aim of our study was to investigate short-term BPV over 24 h in patients with acute CSCR versus healthy controls.
Subjects/methods
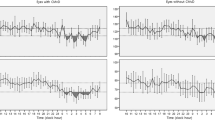
Our cross-sectional comparative study included 50 patients with CSCR (i.e., patient group) and 60 healthy individuals (i.e., control group). In all participants, 24-h ambulatory blood pressure was monitored every 15 min during the day and every 30 min at night. Mean variability index (VI), systolic blood pressure (SBP), and diastolic blood pressure (DBP) during the day, at night, and across the 24-h period were subjected to statistical analyses.
Results
Mean 24-h, daytime, and night-time SBP and DBP did not significantly differ between the groups. The mean 24-h and daytime VI values for SBP and DBP were significantly higher in the patient group than in the control group, whereas the mean night-time VI values for SBP and DBP between the groups were similar. Multivariate logistic regression models revealed that the VI values for 24-h and daytime SBP and DBP emerged as independent risk factors for developing CSCR.
Conclusion
Our study revealed that variabilities in 24-h, daytime SBP and DBP were significantly higher in patients with CSCR than in controls. Our results thus suggest that increased BPV may be a new risk factor for the development of CSCR.
Similar content being viewed by others
Introduction
An idiopathic chorioretinal disease, central serous chorioretinopathy (CSCR) is characterized by the spontaneous serous detachment of the neurosensory retina and is often associated with focal detachments of retinal pigment epithelium [1, 2]. Even though its initial episode usually suggests a benign, self-limited disease, CSCR becomes chronic or recurrent in 30–45% of patients and can result in permanent visual loss due to foveal atrophy [3,4,5,6]. CSCR typically occurs in men in their fourth and fifth decades of life [7].
Although the exact pathogenetic mechanism of CSCR remains unclear, different but not mutually exclusive models have been proposed to explain how serous subretinal fluid accumulates at the posterior pole [8, 9]. Increased choroidal vascular permeability and the dilatation of choroidal vessels suggest that changes in the choroid play a key role in the leakage of fluid into the interstitial or stromal space [10, 11]. All imaging methods used in diagnosing CSCR also indicate that the primary source of serous subretinal fluid is the choroid [12,13,14].
The choroid, in being mostly composed of vascular structures, can be directly affected by systemic hemodynamic changes [15, 16]. Indeed, a close relationship between hypertension and CSCR has been detected [17]. Recent studies have also suggested that blood pressure variability (BPV) is an independent risk factor for vascular disorders [18,19,20]. Abnormal BPV causes hemodynamic changes in the eye’s vascular structures and increases the risk of various ocular diseases, including branch retinal vein occlusion, primary open-angle glaucoma, and normal-tension glaucoma [21, 22].
Considering those findings, we hypothesized that BPV is involved in choroidal vascular dysfunction, which can be associated with the pathogenesis of CSCR. To test that hypothesis, we investigated short-term BPV over 24 h in patients with CSCR versus controls. To the best our knowledge, no study to date has involved evaluating BPV in patients with CSCR.
Materials and methods
Design and participants
We conducted our cross-sectional comparative study in the Department of Ophthalmology at Dünya Eye Hospital in Gaziantep, Turkey, after obtaining approval from the institutional review board and ethics committee. In compliance with the tenets of the Declaration of Helsinki, all participants provided their oral and written informed consent to participate prior to the outset of the study.
Our sample consisted of 50 patients with CSCR (i.e., patient group) and 60 healthy individuals (i.e., control group). Between the groups, participants were matched in terms of age and sex. For the patient group, only patients newly diagnosed with acute CSCR and without any prior ocular or systemic treatment were included, whereas participants in the control group were selected from among healthy individuals who requested a routine ocular examination at the ophthalmology clinic where the study was conducted.
An experienced ophthalmologist diagnosed each patient for acute CSCR according to the findings of a retinal fundus examination, fundus fluorescein angiography (FFA), and optical coherence tomography (OCT). Acute CSCR was diagnosed in patients exhibiting the loss of visual acuity and visual symptoms (e.g., micropsia, metamorphopsia, chromatopsia, and central scotomata) within 3 months and who demonstrated serous subretinal fluid in spectral domain OCT images. To confirm the diagnosis, FFA and OCT imaging using the Spectralis HRA + OCT Spectral Domain OCT and Fluorescein Angiography system (Heidelberg Engineering, Heidelberg, Germany) were performed by a retina specialist in each patient.
Exclusion criteria for participants were the presence of glaucoma, retinal disease other than CSCR (e.g., diabetic retinopathy, retinal vein occlusion, and macular degeneration), systemic disease other than hypertension (e.g., diabetes mellitus, chronic inflammatory disease, renal disease, and endocrinological impairment), arterial hypotension, or the current use of systemic medication other than antihypertensive drugs. Hypertension was defined as systolic blood pressure (SBP) of at least 140 mmHg or diastolic blood pressure (DBP) of at least 90 mmHg at the time of the examination at the clinic. Patients currently taking antihypertensive medication were also considered to have hypertension.
Each participant received a comprehensive ophthalmic evaluation involving an assessment of visual acuity, slit lamp biomicroscopy, intraocular pressure measurement with Goldmann applanation tonometry, and SD-OCT imaging. FFA was not performed in the controls. Each participant’s medical history, history of smoking, body mass index (BMI), and history of systemic diseases (e.g., diabetes mellitus and hypertension) were recorded.
Evaluation of blood pressure variability
In all participants, 24-h ambulatory blood pressure was monitored using a Mobil-O-Graph NG (IEM GmbH, Stolberg, Germany), a device approved by the European Society of Hypertension [23]. First, the appropriate cuff size was determined for each participant, and the cuff was placed on the nondominant arm. The device was programmed to take measurements every 15 min during the day and every 30 min during the night. Daytime and night-time were defined according to each participant’s self-reported sleeping and waking hours. Participants were instructed to continue performing their daily activities during the daytime but to refrain from any physically demanding tasks. During the measurement period, each participant kept a diary of his or her sleep and wake periods as well as any activities that could influence blood pressure. Following the 24-h monitoring of ambulatory blood pressure, the data of participants who subsequently modified their reported night-time interval were corrected during data transfer. All records of measurements were transferred to HMS Client-Server version 5.1 (IEM GmbH, Stolberg, Germany) data management software.
BPV was evaluated in terms of a variability index (VI) automatically calculated by the HMS Client-Server software. VI was defined as the standard deviation (SD) of each blood pressure measurement taken during the day and night [24]. In addition to VI, data gathered from the HMS Client-Server software were mean SBP and DBP during the day, at night, and across the 24-h period. Ambulatory blood pressure measurements (ABPM) with a total valid number of <90% were not included in the analyzes and measurements were repeated.
Statistical analyses
Data analysis was performed using the Statistical Package for the Social Sciences version 25.0 (IBM, Armonk, NY, USA). Values were recorded as n (%) M ± SD. A post-power analysis was conducted with VI values obtained for 24-h SBP and DBP between the groups. When Cronbach’s α equalled 0.05, the observed power was 100%, meaning that the sample size was adequate for our study. The normality of data was gauged with the Shapiro–Wilk test, categorical data between the groups were analyzed using the chi-squared test, and the independent samples t test was used to compare variables for normally distributed data between the groups. Multiple logistic regression analyses were performed to evaluate significant independent factors. Any p value < 0.05 was considered to indicate statistical significance.
Results
The demographics and other characteristics of the participants are summarized in Table 1. The mean age and sex distribution between the groups did not differ significantly (p = 0.408 and p = 0.709, respectively), nor did the number of participants with hypertension and who reported smoking (p = 0.235 and p = 0.156, respectively). Mean BMI was also similar between the groups (p = 0.214).
Mean ABPMs are summarized in Table 2. Mean 24-h, daytime, and night-time SBP and DBP values did not significantly differ between the groups. By contrast, as shown in Table 3, the mean VI values for 24-h, daytime, and night-time SBP and DBP did vary somewhat. Whereas the mean 24-h and daytime VI values for SBP and DBP were significantly higher in the patient group than in the control group, the mean night-time VI for SBP and DBP were similar between the groups. A comparison of the VI values of patients with and without hypertension appears is presented in Table 4, which shows that no statistically significant difference was detected.
Multivariate logistic regression models were created using VI values to determine independent variables that predict CSCR (Table 5). In Model 1, the VI values for 24-h SBP and DBP emerged as independent risk factors for developing CSCR, OR = 1.12, 95% CI [1.02, 1.23] and OR = 1.48, 95% CI [1.07, 2.04], respectively. In Model 2, the VI values for daytime SBP and DBP remained independently associated with CSCR, OR = 1.76 95% CI [1.05, 2.58] and OR = 1.82, 95% CI [1.23, 2.64], respectively.
Discussion
Although ample research has identified that CSCR originates in a disorder of the choroidal vascular system, its relationship with BPV has not been investigated. The study reported here is the first to examine short-term BPV using 24-h ambulatory blood pressure monitoring in patients with CSCR. Among the results, the VI values for 24-h and daytime SBP and DBP were impaired in patients with CSCR, which indicates an association between CSCR and BPV.
Blood pressure measurement values show marked variations over time in response to various physical and emotional stimuli. Such variation can be evaluated as both long- and short-term BPV. In long-term BPV, blood pressure fluctuates over days, weeks, months, or even years, whereas it fluctuates from beat to beat, minute to minute, hour to hour, or day to night in short-term BPV. In our study, short-term BPV was investigated over the course of 24 h [25].
BPV has recently been identified as a risk factor for many vascular diseases, including stroke, hypertension, carotid artery damage, and atherosclerosis [18, 26, 27]. Sander et al. have reported that high systolic BPV during the day is a strong risk factor for the development and early progression of atherosclerosis [28]. According to other research, increased BPV may occur due to abnormal arterial stiffness, renal disturbances, and autonomic or hormonal dysfunction. However, because the exact mechanisms of BPV remain unknown, further investigations into its relationships with those conditions are necessary [29, 30].
High BPV has been associated with not only systemic vascular diseases but some ocular disorders as well [21, 31, 32]. Veloudi et al. reported that retinal arteriolar diameter was positively associated with daytime SBP variability among patients with type 2 diabetes [33]. In another study, long-term SBP variability was significantly associated with moderate diabetic retinopathy [34]. Meanwhile, Gulmez et al., who examined short-term BPV in patients with branch retinal vein occlusion, observed that daytime, night-time, and 24-h BPV in SBP and DBP were independent predictors of BRVO [20]. Such findings suggest that BPV-related mechanisms underlying retinal vascular diseases may be critical for the development and progression of retinal diseases. In our study, the VI values for 24-h and daytime SBP and DBP were significantly higher in patients with CSCR than in healthy controls, as well as significantly associated with CSCR in our regression models.
BPV may be decisive in CSCR’s development due to certain pathophysiological mechanisms. Because the arterial wall of blood vessels is more susceptible to intermittent stress than to continuous stress, it is conceivable that wide oscillations in blood pressure contribute to the development of choroidal vascular damage and, in turn, cause CSCR [35]. Changes in the tension of vessel walls may prompt chronic strain on the vessels, such that consequent vascular damage results in pronounced hyperpermeability. Indocyanine green angiography of patients with CSCR has revealed hyperpermeability with evidence of ischemia and the congestion of choroidal lobules [8]. Those findings indicate that BPV may affect choroidal circulation and contribute to the development of CSCR.
CSCR has also been reported to be associated with several systemic vascular diseases, including cardiovascular diseases, hypertension, ischemic stroke, and coronary heart disease [36, 37]. By extension, patients with CSCR were found to be more likely to have hypertension [37]. In previous studies, BPV has also been reported to be associated with those diseases [18, 26, 27]. Thus, increased BPV as a common risk factor may explain the relationship between CSCR and the systemic diseases mentioned above.
Although potential sources of error were minimized in our study, a few limitations merit attention. For one, though patients were instructed to continue living their normal lives during the measurement period, they may have engaged in unrecorded activities that affected the measurements. Second, all participants submitted to blood pressure monitoring for a 24-h period. Had we monitored participants for a longer period, then we could have obtained more reliable results.
Practical difficulties of conducting the study included monitoring ABPMs for 24 h. Because no reliable results (e.g., total valid number of blood pressure measurements less than 90% in 24 h) were obtained in the first measurements of ABPM in 14 participants, the measurements were repeated.
In sum, our study demonstrated that variability in 24-h and daytime SBP and DBP were significantly higher in patients with CSCR than in controls. Our results thus suggest that increased BPV may be another risk factor for the development of CSCR. Patients with CSCR should therefore be warned about their increased risk of other manifestations of vascular disorders associated with BPV. Further prospective investigations with larger samples and longer follow-up periods are necessary to clarify the association between BPV and CSCR.
Summary
What was known before
-
Abnormal blood pressure variability causes hemodynamic changes in the eye’s vascular structures and raises the risk of various ocular diseases, including branch retinal vein occlusion, primary open-angle glaucoma, and normal-tension glaucoma.
What this study adds
-
The variability in 24-h and daytime systolic blood pressure and diastolic blood pressure were significantly higher in patients with central serous chorioretinopathy than in controls.
-
Increased blood pressure variability may be a new risk factor for the development of central serous chorioretinopathy.
References
Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967;63:1–139.
Liegl R, Ulbig MW. Central serous chorioretinopathy. Ophthalmologica. 2014;232:65–76.
Piccolino FC, de la Longrais RR, Ravera G, Eandi CM, Ventre L, Abdollahi A, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87–99.
Castro-Correia J, Coutinho MF, Rosas V, Maia J. Long-term follow-up of central serous retinopathy in 150 patients. Doc Ophthalmol. 1992;81:379–386.
Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology. 1984;91:1554–1572.
Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol. 1984;68:815–820.
Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Ophthalmol. 2010;149:361–363.
Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34.
Piccolino FC, Borgia L, Zinicola E, Zingirian M. Indocyanine green angiographic findings in central serous chorioretinopathy. Eye. 1995;9(Pt 3):324–332.
Klufas MA, Yannuzzi NA, Pang CE, Srinivas S, Sadda SR, Freund KB, et al. Feasibility and clinical utility of ultra-widefield indocyanine green angiography. Retina. 2015;35:508–520.
Spaide RF, Hall L, Haas A, Campeas L, Yannuzzi LA, Fisher YL, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16:203–213.
Guyer DR, Yannuzzi LA, Slakter JS, Sorenson JA, Ho A, Orlock D. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–1062.
Chan SY, Wang Q, Wei WB, Jonas JB. Optical coherence tomographic angiography in central serous chorioretinopathy. Retina. 2016;36:2051–2058.
Giovannini A, Scassellati-Sforzolini B, D’Altobrando E, Mariotti C, Rutili T, Tittarelli R. Choroidal findings in the course of idiopathic serous pigment epithelium detachment detected by indocyanine green videoangiography. Retina. 1997;17:286–293.
Sansom LT, Suter CA, McKibbin M. The association between systolic blood pressure, ocular perfusion pressure and subfoveal choroidal thickness in normal individuals. Acta Ophthalmol. 2016;94:e157–158.
Reiner A, Fitzgerald MEC, Del Mar N, Li C. Neural control of choroidal blood flow. Prog Retin Eye Res. 2018;64:96–130.
Venkatesh P, Gadia R, Tewari HK, Kumar D, Garg S. Prehypertension may be common in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1101–1103.
Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, et al. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2001;19:1981–1989.
Ferrari A, Buccino N, Di Rienzo M, Pedotti A, Mancia G, Zanchetti A. Labetalol and 24-hour monitoring of arterial blood pressure in hypertensive patients. J Cardiovasc Pharmacol. 1981;3:S42–52.
Gulmez M, Tekce A. Blood pressure variability in patients with branch retinal vein occlusion. Retina. 2019.
Kochkorov A, Gugleta K, Katamay R, Flammer J, Orgul S. Short-term variability of systemic blood pressure and submacular choroidal blood flow in eyes of patients with primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2010;248:833–837.
Sodhi PK, Chawla AK. Relationship between daytime variability of blood pressure or ocular perfusion pressure and glaucomatous visual field progression. Am J Ophthalmol. 2015;160:844–845.
Franssen PM, Imholz BP. Evaluation of the Mobil-O-Graph new generation ABPM device using the ESH criteria. Blood Press Monit. 2010;15:229–231.
Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension. 2012;60:512–517.
Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003;289:2363–2369.
Parati G, Pomidossi G, Albini F, Malaspina D, Mancia G. Relationship of 24-hour blood pressure mean and variability to severity of target-organ damage in hypertension. J Hypertens. 1987;5:93–98.
Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke. 2000;31:463–468.
Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3-year follow-up study. Circulation. 2000;102:1536–1541.
Whittle J. Blood pressure variability predicts clinical outcomes: now what? Hypertension. 2017;69:584–586.
Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens. 2018;20:1133–1137.
Lee J, Choi J, Jeong D, Kim S, Kook MS. Relationship between daytime variability of blood pressure or ocular perfusion pressure and glaucomatous visual field progression. Am J Ophthalmol. 2015;160:522–537.e521.
Plange N, Kaup M, Daneljan L, Predel HG, Remky A, Arend O. 24-h blood pressure monitoring in normal tension glaucoma: night-time blood pressure variability. J Hum Hypertens. 2006;20:137–142.
Veloudi P, Blizzard L, Srikanth VK, McCartney P, Lukoshkova EV, Hughes AD, et al. Associations of blood pressure variability and retinal arteriolar diameter in participants with type 2 diabetes. Diab Vasc Dis Res. 2016;13:299–302.
Foo V, Quah J, Cheung G, Tan NC, Ma Zar KL, Chan CM, et al. HbA1c, systolic blood pressure variability and diabetic retinopathy in Asian type 2 diabetics. J Diabetes. 2017;9:200–207.
O’Rourke MF. Basic concepts for the understanding of large arteries in hypertension. J Cardiovasc Pharmacol. 1985;7:S14–21.
Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016;36:9–19.
Dada T. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 2000;130:379.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karadağ, M.F. A new potential risk factor for central serous chorioretinopathy: blood pressure variability. Eye 35, 2190–2195 (2021). https://doi.org/10.1038/s41433-020-01222-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01222-1
This article is cited by
-
Pathomechanisms in central serous chorioretinopathy: A recent update
International Journal of Retina and Vitreous (2023)