Abstract
This study aimed to explore the changes of the vaginal microbiota and enzymes in the women with high-risk human papillomavirus (HR-HPV) infection and cervical lesions. A total of 448 participants were carried out HPV genotyping, cytology tests, and microecology tests, and 28 participants were treated as sub-samples, in which vaginal samples were characterized by sequencing the bacterial 16S V4 ribosomal RNA (rRNA) gene region. The study found the prevalence of HR-HPV was higher in patients with BV (P = 0.036). The HR-HPV infection rate was 72.73% in G. vaginalis women, which was significantly higher than that of women with lactobacillus as the dominant microbiota (44.72%) (P = 0.04). The positive rate of sialidase (SNA) was higher in women with HR-HPV infection (P = 0.004) and women diagnosed with cervical intraepithelial neoplasia (CIN) (P = 0.041). In HPV (+) women, the α-diversity was significantly higher than that in HPV (−) women. The 16S rRNA gene-based amplicon sequencing results showed that Lactobacillus was the dominant bacteria in the normal vaginal microbiota. However, the proportion of Gardnerella and Prevotella were markedly increased in HPV (+) patients. Gardnerella and Prevotella are the most high-risk combination for the development of HPV (+) women. The SNA secreted by Gardnerella and Prevotella may play a significant role in HPV infection progress to cervical lesions.
Similar content being viewed by others
Introduction
Human papillomavirus (HPV) infections gave rise to over 600,000 cases of cancer in a year1. Most women will have been infected with HPV by intercourse during their lives, most HPV infections fade away by themselves in a few months, a few HPV infections persist and cause lesions2. Although most instantaneous HPV infections are cleared by the immune system, persistent infections can cause viral gene integration into the host genome and lead to HPV-related cancer3. Previous studies have found that local microbiota, epithelial surface integrity, immune regulation were synergistic factors in the progression of HPV to cancer. Nevertheless, little is known about the functional composition of the local microbiota and how it varies by cervicovaginal syndromes, infections, and diseases4.
The vaginal microenvironment can be categorized into five kinds of community state types (CSTs), Lactobacillus spp. was the dominant microbiota in CST I, II, III, and V. And the CSTs have different Lactobacillus species types, such as Lactobacillus-crispatus, Lactobacillus-iners, Lactobacillus-jensenii, Lactobacillus-gasseri, and so on5. Notably, The features of CST IV are higher vaginal pH (> 4.5), lack of Lactobacillus, and abundance of Gardnerella. These features are also features of bacterial vaginosis (BV)6. BV is a kind of mixed infection characterized by the reduction of Lactobacillus and the multiplication of pathogen, mainly Gardnerella vaginalis (G. vaginalis), accompanied by increased vaginal pH. It has been reported that BV infection in the Chinese population ranges from 10.5 to 51.6%7. Previous studies indicate that there is a close correlation between BV infection and HR-HPV persistence. A study by Gillet8 showed that patients with BV are more prone to HR-HPV infections, while Guo9 further found that a BV infection prolongs the duration and the regression time of HR-HPV infections.
As the main pathogen of BV, the detection rate of Gardnerella was significantly increased in HR-HPV-positive women10. In a 2-year longitudinal prospective study, the presence of specific anaerobic groups, including Gardnerella, was associated with the persistence and slow degradation of CIN211. Meanwhile, different dominant bacterial communities produce different metabolomes. Gardnerella secretes SNA while elevated SNA concentration was associated with increased risk for cervical lesion12. The vaginal metabolome of HPV (−) women differed from HPV (+) women in terms of several metabolites, including biogenic amines, glutathione, and lipid-related metabolites13. L. crispatus producing hydrogen peroxide (H2O2) show the strongest associations with vaginal health and are depleted in dysbiosis14. With the development of high-throughput sequencing, there is still a lack of systematic and comprehensive studies to investigate the types and enzymes of vaginal microbiota and their relationship with HPV infection and cervical lesions.
To solve the above problems, this study started from clinical data, with the help of high throughput sequencing, aimed to explore the changes of the vaginal microbiota and enzymes in the HR-HPV infection progress to cervical lesions and provide ideas for further exploration of the interaction mechanism between vaginal microbiota and HPV infection.
Results
Socio-demographic characteristics of participants
Characteristics of 448 participants were analyzed. Participants with normal cervical pathology or negative cytology and HPV (−) were defined as the normal group. Our study indicated that there were no significant differences in terms of age, nationality, marital status, and reproductive history among the groups (P > 0.05; Table 1).
Distribution of HPV genotypes in patients with different cervical intraepithelial neoplasias
The HR-HPV infection rates in women with NILM and CIN were 22.17% (88/397) and 72.55% (37/51), and the difference was statistically significant (P < 0.001). Among all participants, the most susceptible HPV types were HPV52 (6.7%), HPV58 (4.7%), and HPV16 (4.2%). Among the women diagnosed with NILM, the most susceptible HPV types were HPV52 (5.5%), HPV58 (2.9%), and HPV51 (2.9%). Among women diagnosed with CIN I, the most susceptible HPV genotypes were HPV52 (27.3%), HPV16 (18.2%), and HPV18 (18.2%). The most susceptible HPV types were HPV58 (20.7%), HPV16 (17.2%), and HPV18 (10.3%) in patients diagnosed with CIN II and above. The detailed results are shown in Table 2.
The relationship between vaginal microenvironment and HPV infection, cervical lesions
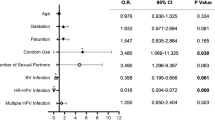
In this study, the infection rate of BV was the highest, accounting for 41.96% (188/448), followed by vulvovaginal candidiasis (VVC), aerobic vaginitis (AV), cytolytic vaginosis (CV), and trichomonal vaginitis (TV), accounting for 12.05% (54/448), 5.13% (23/448) and 1.78% (8/448) and 0.89% (4/448). BV and HR-HPV infections were associated with each other. The difference was statistically significant (P = 0.036). The HR-HPV infection rate was 72.73% in G. vaginalis women, which was significantly higher than that of women with lactobacillus as the dominant microbiota (44.72%) (P = 0.04). However, no obvious correlation with HR-HPV infection was found between other dominant microbiota. We also found that the positive rate of SNA was higher in HR-HPV infection women (P = 0.004). The results are shown in Table 3.
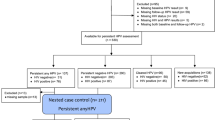
Among the 448 participants in this study, 18 were excluded because they did not undergo cytological or pathological examination. The remaining participants were in a ratio of approximately 1:2 according to HR-HPV infection or not. The pathological results of NILM and ≥ CIN I patients were matched, and they were divided into a case group (≥ CIN I) and a control group. Ultimately, a total of 177 patients were included in the second part of this study, including 51 cases and 126 controls. In women diagnosed with CIN, the positive rate of SNA increased (31.37% vs 17.46%, P = 0.041). However, catalase or leukocyte esterase (LE) were not significantly associated with cervical lesions (Table 4).
Changes of vaginal microbial diversity and microbiota in HPV infection women
In our study, 28 samples were carried out high-throughput sequencing. Twenty-three HPV (+) and five HPV (−) samples were collected for the study and the control groups, respectively. There was no significant difference in age between HPV (+) and HPV (−) groups (P = 0.666). The curve plateaued (Fig. 1a,b) when the sample size was approximately 20, indicating that although 28 cases seem small, it was enough for data analysis. Therefore, the sample size was ample in this study. The evolutionary classification tree of the top 100 species with their abundance, and the corresponding phylum or genus of the top 20 species with their abundance (marked with asterisks) are marked in different colors (Fig. 1c–f).
(a) COG function cumulative curve: The species accumulation curve can be used to judge whether the sample quantity is sufficient. The sharp rise of the curve indicates that the sample quantity is insufficient and the sampling quantity should be increased. When the curve flattens out, it indicates that the sampling is sufficient for data analysis. (b) Species abundance heat map in the phylum horizontal: species abundance heat map, drawn with a species abundance matrix, each column in the figure represents a sample, the row represents the community structure, the color block represents the relative species abundance value, the redder the color, the higher the relative abundance. (c–f) Visualization of classification and phylogeny information drawn by GraPhlAn and iTOL, According to the taxonomic comparison results of each sample, the dominant species were selected, and the species abundance information was combined to display in a ring-shaped tree diagram. (c) The visualization of the phylum level in HPV-negative group. (d) The visualization of the phylum level in HPV-positive group. (e) The visualization of the genus level in HPV-negative group. (f) The visualization of the genus level in HPV-positive group.
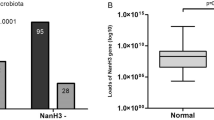
The distribution of phylum, class, order and genus of vaginal microbiota in HPV infected women were shown in Fig. 2. Most of the vaginal microbial communities in the overall samples belong to the following phylum: Firmicutes, Actinobacteria, Bacteroidetes, Fusobacteria, Proteobacteria (Fig. 2a). Firmicutes accounted for 97.38% among total microbiota, which was the main vaginal microbiota in the HPV (−) women. However, the proportion of Firmicutes decreased (68.26%), and the proportions of Actinobacteria and Bacteroides increased (respectively 15.45%, 4.58%) in the HPV (+) women. The composition and differences of vaginal microbiota between the two groups were further analyzed at the genus level (Fig. 2d). In the HPV (−) women, Lactobacillus was the dominant bacteria (95.73%), with a small amount of Gardnerella (1.39%), Atopobium (0.03%) and other genera. In the HPV (+) women, the composition of the vaginal bacterial community structure had significant changes, mainly reflected in the decrease of Lactobacillus (62.91%), and the increase of Gardnerella (12.22%) and Prevotella (6.78%).
Vaginal microbiota distribution in HPV infectious women. (a) Vaginal microbiota in the phylum level of the HPV-negative group and the HPV-positive group. (b) Vaginal microbiota in the class level of the HPV-negative group and the HPV-positive group. (c) Vaginal microbiota in the order level of the HPV-negative group and the HPV-positive group. (d) Vaginal microbiota in the genus level of the HPV-negative group and the HPV-positive group.
The diversity indicators of the two group samples were shown in Fig. 3a–d. In HPV (+) women, the Shannon index was higher than the HPV (−) women (F = 6.14, P = 0.023), indicating that the number of microbiota in HPV (+) women was more. In HPV (+) women, the Simpson index was lower (F = 9.494, P = 0.006), suggesting that the complexity of the vaginal microbiota was increased in patients with HPV infection and decreased in cases of vaginal health. However, the Chao index or ACE index were not related to HPV infection (P > 0.5).
(a–d) Microbial α diversity between HPV-negative group and HPV-positive group. (e) LEfSE analysis ring tree diagram shows the two groups’ microbial distribution: nodes of different colors in the branches indicate the groups of microorganisms that play an important role in the corresponding group of the color. Yellow nodes indicate groups of microorganisms that have not played an important role. The species names represented by the English letters in the picture are shown in the legend on the right. (f) LDA scores distribution histogram shows the two groups’ dominant microbial distribution: the X-axis is the LDA score obtained after LDA analysis, and the Y-axis is the group of microorganisms with significant effects.
To discover the changes of vaginal microbiota in HPV infection women, LEfSe analysis was performed on the two groups (Fig. 3e,f). The results showed that Lactobacillus was the main dominant bacteria in the HPV (−) women, and Prevotella was a distinct microbiota that plays a significant role in the HPV (+) women according to the Linear discriminant analysis (LDA) scores.
Discussion
The emerging studies suggest that vaginal microenvironment plays an essential role in women’s health, specifically in sexually transmitted diseases. This is a cross-sectional study to explore the changes of the vaginal microbiota and enzymes in the HPV infection and cervical lesions. Overall, a significantly higher microbiota diversity was observed in HPV (+) women than that in HPV (−) women. The increase of Gardnerella and Prevotella and the decrease of Lactobacillus are closely associated with HPV infection.
Previous studies indicated Lactobacillus is the dominant bacteria, which play important role in protecting the health of women’s lower reproductive tract15. The vaginal microbiota is primarily dominated by one of the four most common Lactobacillus species: Lactobacillus crispatus, Lactobacillus iners, Lactobacillus gasseri, and Lactobacillus jensenii5. Lee et al.16 found the proportion of Lactobacillus was lower in HPV (+) patients. On the one hand, Lactobacillus maintains the weak acid environment of the vagina through its own lactic acid. On the other hand, a large quantity of Lactobacillus can reduce and inhibit the planting and growth of some opportunistic pathogenic bacteria to protect the lower reproductive tract from infection17,18. The presence of the signature “abnormal vaginal microbiota” in CIN was found by a laboratory culture in 1992 and confirmed in subsequent studies19. When the vagina’s protective microbiota was destroyed, the defense against pathogen infection was weakened.
In this study, We have identified vaginal microenvironment disorder was bound up with HPV infection. Compared with BV (−) women, the HR-HPV infection rate in BV (+) patients increased. BV is associated with an increased risk of detection of HPV, and HPV infections are associated with an increased risk of BV20. Vaginal microenvironment disturbance was associated with increased inflammatory cytokines, mucosal injury and chronic inflammation. In order to investigate the mechanism of BV and HPV infection, Rodriguez-Cerdeira et al. suggested that G. vaginalis is one of the common microbiota in HPV (+) women21, and this has been reported in other researches that using next-generation sequencing22,23. Women with BV had higher levels of the cytokine interleukin (IL)-1β and lower levels of IL-1724,25.
As an important component of vaginal microenvironment, metabolomes play an important role in the pathogenicity of microbiota. The H2O2 produced by Lactobacillus can catalyze peroxidase and further produce hypochlorite. It is a process that can prevent HPV from invading into cervical epithelial cells and prevent cervical lesions26. LE is an intracellular enzyme. When vaginal inflammation occurs, a large number of white blood cells can gather to engulf pathogens, resulting in the destruction of white cell membrane, and thus LE can be detected. However, no studies have linked LE to any type of vaginal inflammation. Similarly, this study also showed that there was no statistical significance between LE and HPV infection or cervical intraepithelial neoplasia. Gardnerella adheres tightly to the surface of vaginal epithelial cells, forms a dense biofilm, and can release vaginal cytolysin, which may inhibit the effect of vaginal mucosal barrier immunoglobulin A. Gardnerella can produce SNA, which can degrade mucosal protective factors (such as mucin) and causes vaginal epithelial cells to dissolve and expel27. SNA is an enzyme that cleaves terminal sialic acid residues and is associated with tissue destruction, immune response evasion, bacterial invasion, and access to bacterial-associated nutrients28. In addition to Gardnerella bacteria, such as Prevotella bacteria, Bacteroides bacteria and Mobiluncus bacteria also produce SNA29. SNA usually occupies terminal positions attached to mucosal defense factors, such as secretory IgA, secretory components, lactoferrin, and secretory leukocyte protease inhibitors30.
In order to investigate the mechanism of cervical lesions caused by vaginal microbiota and metabolomes, Zariffard et al. found that31 the expression level of Toll-like receptor—(TLR) 4 mRNA in vaginal and cervical epithelial cells was significantly increased in patients with G. vaginalis-infected BV. Previous studies showed that TLR9 recognizes HPV infection and initiates immune response. Experiments at the transcriptional level confirmed that E6 and E7 oncoproteins directly downregulate TLR932. However, whether Gardnerella activates the TLRs-related pathways through SNA to cause HPV infection is still unknown and needs to be assessed. All of these mucosal, bacterial, and immune activations associated with BV and G. vaginalis may lead to progression of HPV infection to cervical cancer.
This study also has some limitations. First, this is a cross-sectional study to explore the changes of the vaginal microbiota and enzymes in women with HPV infection and cervical lesions, further experiments are needed to confirm that SNA secreted by Gardnerella and Prevotella contributes to HPV causing cervical lesions. Secondly, our study only focused on the distribution of different microbiota, ignoring the identification of Lactobacillus species. Recent researches suggest that Lactobacillus iners is a transitional species that colonize after the vaginal environment is disturbed and leads to BV, sexually transmitted infections, and adverse pregnancy outcomes33. Further studies are necessary to identify the exact role of different Lactobacillus species in larger samples.
Our research demonstrates vaginal microenvironment, especially BV was closely related to HPV infection. So more attention should be paid to the prevention, discovery and proper management of BV in HPV infection women. Particularly, compared to HPV (−) women, Gardnerella and Prevodella are the most high-risk combination for the development of HPV (+) women. The SNA secreted by Gardnerella and Prevodella may play a significant role in HPV infection progress to cervical lesions. This finding provides ideas for further exploration of the interaction mechanism between vaginal microbiota and HPV infection.
Materials and methods
Study population
The study participants were selected from the Fujian Cervical Lesions Screening Cohorts (FCLSCs), China. A total of 448 participants have carried out microecology tests, HPV genotyping and cytology tests, and 28 participants were treated as sub-samples, in which vaginal microecological samples were characterized by sequencing the region of bacterial 16S V4 ribosomal RNA (rRNA) gene (Fig. 4). All participants came from Fujian Maternity and Child Health Hospital, affiliated hospital of Fujian Medical University cohort which was established from June 2018 to March 2020. The participants were eligible when the following inclusion criteria were satisfied: history of sexual activity, aged 20–74 years, no history of cervical lesion treatment or chemoradiotherapy, no severe immune system diseases, no sexually transmitted diseases. The exclusion criteria are shown below: washed the vagina within 48 h, used drug in vagina or had sexual intercourse within the last 3 days, oraled antibiotics within 1 month. The Ethics Committees of the Fujian Maternity and Child Health Hospital approved this study (2020KY015), and all individuals in this study signed informed consent. All experiments in the text were carried out in compliance with the relevant rules and regulations and under the supervision and guidance of the Ethics Committee.
Sample collection
The vaginal samples were collected from the upper third of vaginal walls by rotating for 10–15 s by study participants using cotton swabs (Santai, Jiangsu, China). The cotton swabs were inserted into a tube (Vaginal micro-microbiota diagnostic unit-700, Shtars, China) carefully avoiding skin contamination. The samples were stored at − 20 °C as soon as possible after collection for the vaginal microbiota analysis.
The researchers used plastic brushes to collect cervical cells from all participants’ cervix, elutioned in ThinPrep PreservCyt Solution (Hologic Inc., Madison, WI, USA), and stored specimens at 4 °C in laboratory immediately.
Vaginal microbiological metabolites detection
The Vaginal secretions were obtained on 1/3 of the vaginal sidewall. Check whether there is trichomonad, mycelium, clue cells under the microscope after daubing on clean slide. H2O2, LE and SNA in secretions were detected by bPR-2014A vaginitis automatic detector and supporting detection kit (Master Biotechnology Co., Ltd, Jiangsu, China). Vaginal PH value was determined by color strips. If the PH value was no more than 4.5 (pH ≤ 4.5), the result was defined as normal. On the contrary, pH > 4.5 was defined as abnormal. Vagina cleanness was diagnosed in accordance with the standard of National Clinical Laboratory Practice Guideline 18: I–II were defined as normal vagina cleanness, and vagina cleanness III–IV defined as abnormal. AV, BV, CV, TV and VVC were all negative or positive. The Nugent scoring method was used to diagnose BV. The Nugent score was calculated by assessing the numbers of Lactobacillus morphotypes (scored as 0–4), G. vaginalis morphotypes (scored as 0–4), and Mobiluncus morphotypes (scored as 0–2). A Nugent score of 7–10 was interpreted as consistent with BV and a score of 4–6 as intermediate, while a score of 0–3 was interpreted as negative for BV. SNA colorless is normal (−), red or purple is positive (+). LE colorless is normal (−), and green or blue is positive (+). H2O2 > 2 mmol/L is red or purple, negative (−), H2O2 < 2 mmol/L is positive (+), blue. All laboratory procedures were conducted according to the manufacturer’s instructions.
HPV genotyping
The HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82, 83, 6, 11, 42, 43, 81) were detected by Polymerase chain reaction-reverse dot blot (PCR-RDB) HPV genotyping kit (YaNeng Biosciences, Shenzhen, China)34. This method and kit have been approved by China Food and Drug Administration (Approval number 20020515). The procedures were conducted according to the manufacturer’s instructions.
Liquid-based cytology
The cytological samples were blinded and independently evaluated by two experienced cytopathologists and re-evaluated until reach a consensus when the diagnoses were different. Samples were classified as NILM, atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), atypical squamous cells, and it was not possible to exclude high-grade squamous intraepithelial lesions (ASC-H), squamous cervical cancer (SCC) and atypical glandular cells (AGC).
Histology
According to the cervical cancer screening procedure, women with HR-HPV infection or abnormal cytology results may be referred for colposcopy or biopsy. When biopsy diagnosis results in ≥ HSIL, patients underwent a loop electrosurgical excision procedure cone or conization by cold knife to biopsy. Formalin (10%) was used to fix specimens, which were routinely processed for paraffin embedding. Then, 4 µm thick histological sections were cut and stained with hematoxylin and eosin using standard methods. Cervical biopsy specimens were examined and diagnosed according to the CIN system. If the review reading is inconsistent, conduct a second histological review. If two-thirds of the diagnoses are the same, the result is considered the final result.
16S rRNA gene-based amplicon sequencing
Genomic DNA of vaginal secretions samples was extracted by E.Z.N.A Mag-Bind Soil DNA Kit (Omega Bio-Tek, GA, USA) according to the manufacturer’s protocol. DNA samples were quantified by the Qubit 3.0 DNA Kit (Invitrogen, Waltham, MA, USA) and transferred to Sangon Biotech Testing Center (Shanghai, China) for high-throughput sequencing.
Bacterial DNA was amplified by 16S V4F primers (5′ CCTACGGGNGGCWGCAG 3′) and 16S V4R primers (5′ GACTACHVGGGTATCTAATCC 3′) complementary to the V4 region of 16S rRNA gene. This variable region has been verified can accurately amplify and resolve DNAs of vaginal microbiome. PCR reaction system consisted of 9–12 µL of nuclease-free water, 15 µL of 2 × Hieff Robust PCR Master Mix, 5 µM of each primer and 20–30 ng of genomic DNA. The cycling conditions include: initial denaturation at 95 °C for 3 min, then 94 °C for 30 s, 45 °C for 20 s, 65 °C for 30 s, then 94 °C for 20 s, 55 °C for 20 s, 72 °C for 5 min. The samples were analyzed using Roche LightCycler 480 PCR system (Roche, Switzerland).
Purified PCR products were accurately quantified by Qubit 3.0 Fluorometric High-Sensitivity dsDNA Assay (Invitrogen, Waltham, MA, USA) and then constructed library by KAPA LTP Library Kit (Kapa Biosystems, USA). High-throughput sequencing of 2 × 300 paired-end reads was performed on an Illumina MiSeq platform (Illumina, California, USA) at Sangon Biotech (Shanghai, China). Raw FASTQ files were obtained and merged.
The version of mother was v1.30.1. SILVA release 132 was used as database. R v3.6.3 was used for statistical analyses. The similarity truncation rate of the operational taxonomic units cluster was 97%. The diversity analysis of the sample (α or β diversity) could indicate the diversity or abundance of microbial communities, including the Chao and ACE (http://www.mothur.org/wiki/Chao; http://www.mothur.org/wiki/Ace) indices, for calculating the abundance of community distribution were used. Furthermore, the Shannon and Simpson (http://www.mothur.org/wiki/Shannon; http://www.mothur.org/wiki/Simpson) indices were used to calculate the diversity of community distribution. Biomarker discovery analysis was carried out by the LEfSe tool and LDA scores higher than 2.0 were considered statistically significant.
Statistical analysis
The measurement data were counted as mean ± standard deviation in this study. The significance of BV associated with the states of HPV infection or cervical lesions was assessed by Chi-squared test or Fisher’s exact test. The data were calculated using the IBM SPSS statistical package version 22.0 (IBM, Corporation, Armonk, USA) in this study. The significance level was set at a two-tailed p-value < 0.05.
Ethics approval and consent to participate
The study was approved approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (2020KY015).
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- ASCUS:
-
Atypical squamous cells of undetermined significance
- AV:
-
Aerobic vaginitis
- BV:
-
Bacterial vaginosis
- CIN:
-
Cervical intraepithelial neoplasia
- CST:
-
Community state type
- CV:
-
Cytolytic vaginosis
- GV:
-
Gardnerella vaginalis
- H2O2 :
-
Hydrogen peroxide
- HPV:
-
Human papillomavirus
- HR-HPV:
-
High-risk human papillomavirus
- HSIL:
-
High-grade squamous intraepithelial lesion
- LE:
-
Leukocyte esterase
- LEfSe:
-
Linear discriminant analysis effect size
- TLRs:
-
Toll-like receptors
- LSIL:
-
Low-grade squamous intraepithelial lesion
- NILM:
-
Negative for intraepithelial lesion or malignancy
- TV:
-
Trichomonas vaginitis
- TCT:
-
Thinprep cytologic test
- OTU:
-
Operational-Taxonomy Units
- RT-qPCR:
-
Real-time quantitative PCR
- SNA:
-
Sialidase
- VVC:
-
Vulvovaginal candidiasis
References
Arbyn, M. et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int. J. Cancer 131, 1969–1982. https://doi.org/10.1002/ijc.27650 (2012).
Usyk, M. et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog. 16, e1008376. https://doi.org/10.1371/journal.ppat.1008376 (2020).
Bosch, F. et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. https://doi.org/10.1016/j.vaccine.2013.10.003 (2013).
Schiffman, M. et al. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primer 2, 16086. https://doi.org/10.1038/nrdp.2016.86 (2016).
Borgogna, J. et al. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: A cross-sectional analysis. BJOG 127, 182–192. https://doi.org/10.1111/1471-0528.15981 (2020).
Łaniewski, P. et al. Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci. Rep. 9, 7333. https://doi.org/10.1038/s41598-019-43849-5 (2019).
Zhang, D. et al. Epidemiological investigation of the relationship between common lower genital tract infections and high-risk human papillomavirus infections among women in Beijing, China. PLoS ONE 12, e0178033. https://doi.org/10.1371/journal.pone.0178033 (2017).
Gillet, E. et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: A meta-analysis. BMC Infect. Dis. 11, 10. https://doi.org/10.1186/1471-2334-11-10 (2011).
Guo, Y., You, K., Qiao, J., Zhao, Y. & Geng, L. Bacterial vaginosis is conducive to the persistence of HPV infection. Int. J. STD AIDS 23, 581–584. https://doi.org/10.1258/ijsa.2012.011342 (2012).
Gao, W., Weng, J., Gao, Y. & Chen, X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: A cross-sectional study. BMC Infect. Dis. 13, 271. https://doi.org/10.1186/1471-2334-13-271 (2013).
Mitra, A. et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat. Commun 11, 1999. https://doi.org/10.1038/s41467-020-15856-y (2020).
Govinden, G. et al. Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch. Microbiol. 200, 1129–1133. https://doi.org/10.1007/s00203-018-1520-4 (2018).
Borgogna, J. C. et al. The vaginal metabolome and microbiota of cervical HPV-positive and HPV-negative women: A cross-sectional analysis. BJOG 127(2), 182–192. https://doi.org/10.1111/1471-0528.15981 (2020).
Lagenaur, L. et al. Connecting the dots: Translating the vaginal microbiome into a drug. J. Infect. Dis. 223, S296–S306. https://doi.org/10.1093/infdis/jiaa676 (2021).
Anahtar, M., Gootenberg, D., Mitchell, C. & Kwon, D. Cervicovaginal microbiota and reproductive health: The virtue of simplicity. Cell Host Microbe 23, 159–168. https://doi.org/10.1016/j.chom.2018.01.013 (2018).
Lee, J. et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE 8, e63514. https://doi.org/10.1371/journal.pone.0063514 (2013).
Martin, D. & Marrazzo, J. The vaginal microbiome: Current understanding and future directions. J. Infect. Dis. https://doi.org/10.1093/infdis/jiw184 (2016).
Nunn, K. & Forney, L. Unraveling the dynamics of the human vaginal microbiome. Yale Le J. Biol. Med. 89, 331–337 (2016).
Guijon, F. et al. Vaginal microbial flora as a cofactor in the pathogenesis of uterine cervical intraepithelial neoplasia. Int. J. Gynecol. Obstet. 37, 185–191. https://doi.org/10.1016/0020-7292(92)90379-w (1992).
Watts, D. H. et al. Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-infected and HIV-uninfected women. J. Infect. Dis. 191, 1129–1139. https://doi.org/10.1086/427777 (2005).
Rodriguez-Cerdeira, C., Sanchez-Blanco, E. & Alba, A. Evaluation of association between vaginal infections and high-risk human papillomavirus types in female sex workers in Spain. ISRN Obstetr. Gynecol. 2012, 240190. https://doi.org/10.5402/2012/240190 (2012).
Zhang, C. et al. The direct and indirect association of cervical microbiota with the risk of cervical intraepithelial neoplasia. Cancer Med. 7, 2172–2179. https://doi.org/10.1002/cam4.1471 (2018).
Di Paola, M. et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 7, 10200. https://doi.org/10.1038/s41598-017-09842-6 (2017).
Murphy, K. & Mitchell, C. The interplay of host immunity, environment and the risk of bacterial vaginosis and associated reproductive health outcomes. J. Infect. Dis. https://doi.org/10.1093/infdis/jiw140 (2016).
Gosmann, C., Mattarollo, S., Bridge, J., Frazer, I. & Blumenthal, A. IL-17 suppresses immune effector functions in human papillomavirus-associated epithelial hyperplasia. J. Bacteriol. 193, 2248–2257. https://doi.org/10.4049/jimmunol.1400216 (2014).
Castelão, C. et al. Association of myeloperoxidase polymorphism (G463A) with cervix cancer. Mol. Cell. Biochem. 404, 1–4. https://doi.org/10.1007/s11010-015-2359-5 (2015).
Gelber, S., Aguilar, J., Lewis, K. & Ratner, A. Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. Am. J. Obstet. Gynecol. 190, 3896–3903. https://doi.org/10.1128/jb.01965-07 (2008).
Lewis, W., Robinson, L., Gilbert, N., Perry, J. & Lewis, A. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. J. Biol. Chem. 288, 12067–12079. https://doi.org/10.1074/jbc.M113.453654 (2013).
Briselden, A., Moncla, B., Stevens, C. & Hillier, S. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J. Clin. Microbiol. 30, 663–666. https://doi.org/10.1128/jcm.30.3.663-666.1992 (1992).
Cauci, S., Culhane, J., Di Santolo, M. & McCollum, K. Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1beta. Am. J. Obstet. Gynecol. 198, 132. https://doi.org/10.1016/j.ajog.2007.05.035 (2008).
Zariffard, M. et al. Induction of tumor necrosis factor- alpha secretion and toll-like receptor 2 and 4 mRNA expression by genital mucosal fluids from women with bacterial vaginosis. J. Infect. Dis. 191, 1913–1921. https://doi.org/10.1086/429922 (2005).
Wakabayashi, R., Nakahama, Y., Nguyen, V. & Espinoza, J. The host-microbe interplay in human papillomavirus-induced carcinogenesis. Microorganisms. https://doi.org/10.3390/microorganisms7070199 (2019).
Zheng, N., Guo, R., Wang, J., Zhou, W. & Ling, Z. Contribution of Lactobacillus iners to vaginal health and diseases: A systematic review. Front. Cell Infect. Microbiol. 11, 792787. https://doi.org/10.3389/fcimb.2021.792787 (2021).
Dong, B. et al. Cost-effectiveness and accuracy of cervical cancer screening with a high-risk HPV genotyping assay vs a nongenotyping assay in China: An observational cohort study. Cancer Cell Int. 20, 421. https://doi.org/10.1186/s12935-020-01512-4 (2020).
Acknowledgements
We would like to thank the participants for their patience and kindness.
Funding
The study was supported by Grants from the Fujian Maternity and Child Health Hospital Technology Innovation Project (Grant No. YCXM-19-07 and Grant No. YCXM-20-04).
Author information
Authors and Affiliations
Contributions
W.L. contributed to the acquisition, analysis, and interpretation of data; and to drafting the article. Q.Z. contributed to the conception and design of the study. Y.C. contributed to the analysis and interpretation of data and to draw the figure. B.D. contributed to the methods and performed the laboratory analyses. H.X., Y.L. and H.L. contributed to Sample collection. X.W. and P.S. contributed to the acquisition of data, critically revised the article for important intellectual content, and supervised the study. All authors gave their final approval of the version to be submitted. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, W., Zhang, Q., Chen, Y. et al. Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: a cross-sectional analysis. Sci Rep 12, 2812 (2022). https://doi.org/10.1038/s41598-022-06731-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06731-5
This article is cited by
-
Analyses of human papillomavirus, Chlamydia trachomatis, Ureaplasma urealyticum, Neisseria gonorrhoeae, and co-infections in a gynecology outpatient clinic in Haikou area, China
BMC Women's Health (2023)
-
Characteristics of vaginal microbiota in various cervical intraepithelial neoplasia: a cross-sectional study
Journal of Translational Medicine (2023)
-
Vaginal Microbiome Dysbiosis is Associated with the Different Cervical Disease Status
Journal of Microbiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.