Abstract
While the signaling pathways and transcription factors involved in the differentiation of thyroid follicular cells, both in embryonic and adult life, are increasingly well understood, the underlying mechanisms and potential crosstalk between the thyroid transcription factors Nkx2.1, Foxe1 and Pax8 and inductive signals remain unclear. Here, we focused on the transcription factor Sox9, which is expressed in Nkx2.1-positive embryonic thyroid precursor cells and is maintained from embryonic development to adulthood, but its function and control are unknown. We show that two of the main signals regulating thyroid differentiation, TSH and TGFβ, modulate Sox9 expression. Specifically, TSH stimulates the cAMP/PKA pathway to transcriptionally upregulate Sox9 mRNA and protein expression, a mechanism that is mediated by the binding of CREB to a CRE site within the Sox9 promoter. Contrastingly, TGFβ signals through Smad proteins to inhibit TSH-induced Sox9 transcription. Our data also reveal that Sox9 transcription is regulated by the thyroid transcription factors, particularly Pax8. Interestingly, Sox9 significantly increased the transcriptional activation of Pax8 and Foxe1 promoters and, consequently, their expression, but had no effect on Nkx2.1. Our study establishes the involvement of Sox9 in thyroid follicular cell differentiation and broadens our understanding of transcription factor regulation of thyroid function.
Similar content being viewed by others
Introduction
The thyroid is an endoderm-derived gland whose main function is the synthesis and secretion of the thyroid hormones T3 (triiodothyronine) and T4 (thyroxine), which are essential for the normal growth, differentiation and metabolism of several organs. Unlike other endoderm-derived organs, such as pancreas and liver, some aspects of the embryonic development of the thyroid gland remain unclear. During embryogenesis in the mouse, a small group of cells from the primitive pharyngeal endoderm simultaneously begin to express the so-called thyroid transcription factors Nkx2.1, Pax8 and Foxe1 at embryonic day (E) 8.5, a process known as specification. After annexing cells from the adjacent endoderm, these precursor cells migrate to their final destination just above the trachea, where they form follicles and express the necessary thyroid-specific genes for thyroid hormone production1,2,3,4. Studies in animal models and in pluripotent stem cells have contributed to a better understanding of the thyroid specification control. Besides leaded to the identification of several secreted growth factors important for thyroid development, such as Sonic Hedgehog5, fibroblast growth factor (Fgf)2, bone morphogenic protein 46 and Fgf107, which are derived mostly from the surrounding cardiogenic mesoderm8,9. Likewise, new transcription factors have been identified that act upstream or cooperate with the thyroid transcription factors during embryonic thyroid development, supporting the existence of a complex network of pathways involved in thyroid differentiation2. Among them, the transcription factor Sox9 has recently emerged as a critical regulator of early thyroid development7.
Sox9 belongs to the Sox family of transcriptional regulators (Sry-related HMG Box)10,11,12 and is involved in the development of several endoderm-derived organs including pancreas and liver4,13,14,15. Together with Fgf10, Sox9 controls thyroid morphogenesis by regulating the branching process during the expansion of the thyroid anlage7. This process is conserved in other organs such as the lung and exocrine glands13,16,17,18,19. These findings have provided a more complete picture of the mechanisms controlling the growth and expansion of thyroid precursors during thyroid development. Sox9 is expressed at E9.5 in mice, and remains expressed even at later stages of thyroid embryogenesis where it marks a group of proliferating thyroid precursors involved in thyroid branching growth. It has been reported that various tissues including liver, pancreas and intestine contain a subset of Sox9-expressing cells with regenerative capacity during adulthood20, reinforcing the important role of Sox9 in sustaining the undifferentiated state of cell precursors during embryonic development and adulthood, including possibly the thyroid7,21. Furthermore, given that Sox9 is expressed before terminal differentiation of the thyroid follicular cells, and that its expression is maintained in the adult thyroid, it can be hypothesized that this factor likely functions in the transcriptional network that regulates thyroid differentiation and function.
In addition to the thyroid transcription factors, several hormones and growth factors regulate follicular cell differentiation. Among them, thyrotropin (TSH)22 and transforming gowth factor (TGFβ)23,24 appear to play critical roles. Acting through their respective main signaling pathways, cAMP/CREB and Smad, both ligands regulate the expression of thyroid differentiation markers such as Pax825,26, Foxe127, thyroglobulin (Tg)28,29, thyroperoxidase (TPO)24,30 and the sodium iodide symporter (NIS)31.
Given the expression of Sox9 in the embryonic thyroid and its potential to regulate the differentiation of thyroid follicular cells, we studied the role of Sox9 in thyroid follicular cell development, its control by the main regulators of thyroid function, TSH and TGFβ, and its involvement in the transcriptional network that controls thyroid differentiation and homeostasis.
Results
Sox9 is expressed both in embryonic thyroid precursors and in adult thyroid follicular cells
We first surveyed the expression of Sox9 in mouse thyroid sagittal sections at E9.5, E10.5 and E11.5 by immunofluorescence microscopy (Fig. 1A). We used Nkx2.1, a thyroid-specific marker32, to visualize the thyroid precursors emerging from the pharyngeal endoderm labeled with the epithelial-specific marker E-cadherin (Fig. 1A). We found that Sox9 was expressed weakly in a limited number of these thyroid precursors during early development, but was expressed at higher levels in the mesenchyme adjacent to the thyroid primordium. Sox9 expression increased in the thyroid primordium at later stages of embryonic development, and marked all the thyroid epithelial cells at E11.5. Sox9 expression in the mesenchyme surrounding the thyroid primordium was maintained along the different stages of embryonic development (Fig. 1A).
Immunolocalization of Sox9 in embryonic and adult mouse thyroid. (A) Sagittal sections of thyroid primordia at different stages of embryonic development immunolabeled for the transcription factors Nkx2.1 and Sox9, together with the epithelial marker cadherin (E-cadherin). The white arrows indicate the location of the thyroid primordium. (B) Thyroid sections from Sox9-GFP transgenic mouse embryos at E16.5 immunolabeled for Sox9 and GFP. Nuclei were counterstained with Hoechst. (C) Thyroid gland sections from Sox9-GFP transgenic adult mice immunolabeled for Sox9 and GFP. Nuclei were counterstained with Hoechst. (D) Thyroid gland sections from 2-month-old mice immunostained for Sox9 and counterstained with hematoxylin.
Because different members of the Sox family show a high similarity, especially Sox9 and Sox1033, we repeated the experiment in transgenic mice expressing green fluorescent protein (GFP), under the control of Sox9 regulatory elements34,35 to unequivocally demonstrate that the signal detected during embryonic development is indeed Sox9. As shown in Fig. 1B, an intense GFP signal was evident in the thyroid of E16.5 embryos. Furthermore, immunofluorescence analysis of adult thyroid sagittal sections, from the same reporter mice, revealed robust Sox9 nuclear expression in the GFP+ epithelial cells forming follicles (Fig. 1C). These data demonstrate that Sox9 expression starts to be detected in the embryonic thyroid from the early budding stage, progressively increases as embryonic development continues, and is specifically found in follicular epithelial cells, confirming previous results7. Finally, we observed expression of Sox9 in the adult thyroid, as demonstrated by immunohistochemistry of thyroid sagittal sections in 2-month-old adult mice (Fig. 1D).
Overall, our results show that Sox9 is expressed in thyroid follicular cells during the middle and late embryonic stages, and is retained in the adult.
TSH induces Sox9 expression in thyroid follicular cells via the cAMP/PKA signaling pathway
The finding that Sox9 expression is retained in the adult thyroid prompted us to study its function in follicular cell differentiation. We analyzed its expression in PCCl3 thyroid cells precultured in starvation medium and then treated with physiological doses of TSH. We found that hormone treatment markedly increased nuclear Sox9 protein levels visualized by confocal microscopy (Supplementary Fig. 1) and total protein determined by western blotting (Fig. 2A).
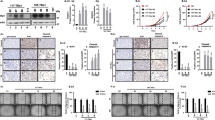
Analysis of the cAMP/CREB signaling pathway during thyrotropin (TSH)-mediated regulation of Sox9 expression. (A) PCCl3 cells were maintained for 48 h in starvation medium (–) and then treated with different stimuli for 24 h. The H89 inhibitor was added 1.5 h before the treatment with TSH (1 nM) or forskolin (10 µM). Total protein extracts were analyzed by western blotting for the detection of Sox9. β-actin was used as a loading control. (B) PCCl3 cells were transfected with pSox9 and luciferase/Renilla-encoding pRL-CMV vectors. After transfection, cells were grown for 24 h in complete medium and then for 48 h in starvation medium (–) before treatment with TSH or forskolin for 24 h. The H89 inhibitor was added 1.5 h before the treatment, when necessary. Relative luciferase activity is represented as fold induction over the value in starved cells. (C) Electrophoretic mobility shift assays (EMSAs) were performed with a labeled cAMP-response element (CRE) sequence derived from the Sox9 promoter. The labeled probe (R*) was incubated without protein or with 7 µg of CREB (TNT-CREB) recombinant protein. For competition, a 100-fold excess of the same related (R) or non-related (NR) cold oligonucleotide was used. (D) HeLa cells were transfected with pSox9 and co-transfected with a CREB expression vector. Results represent fold induction with respect to the control. (E and F) PCCl3 cells were transfected with control (scrambled) or CREB (siCREB) small interfering RNAs (siRNAs). (E) Total protein extracts were obtained 24 h after transfection and analyzed by western blotting for the detection of Sox9 and CREB. β-actin was used as a loading control. (F) PCCl3 cells were maintained for 48 h in starvation medium (–) after transfection and then treated with TSH or forskolin for 16 h. Sox9 mRNA expression levels were detected by RT-qPCR and normalized to those of β-actin. Values represent the mean ± standard error of the mean (SEM; n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. Original blots/gels are presented in Supplementary Fig. 5.
To test the involvement of the TSH-stimulated cAMP/PKA pathway, we treated PCCl3 cells with forskolin, an adenylate cyclase activator, or with the PKA inhibitor H89, prior to TSH/forskolin stimulation. We found that forskolin mimicked the effect of TSH on Sox9 expression whereas H89 inhibited both TSH- and forskolin-induced Sox9 expression (Fig. 2A). Inhibitors of the PKC or MAPK pathway failed to abolish the TSH-mediated effect on Sox9 expression (Supplementary Fig. 2A). The regulation of by TSH occurred at the transcriptional level, as TSH stimulated the activity of a transiently-transfected construct containing Sox9 regulatory regions fused to the luciferase reporter gene36 (Fig. 2B). Altogether, these data suggest that TSH transcriptionally regulates Sox9 expression via cAMP/PKA signaling.
Consistent with the involvement of the cAMP/PKA pathway in Sox9 expression, in silico analysis of the Sox9 gene regulatory region (~ 1 kb upstream from transcription initiation site) identified a putative cAMP response element (CRE) at position -202/-209 from the transcriptional start site (Supplementary Fig. 3A). We therefore studied the role of CREB, the major mediator of cAMP/PKA signaling, on Sox9 transcription. Electrophoretic mobility shift assay (EMSA) revealed that recombinant CREB protein could specifically bind to the putative CRE (Fig. 2C) and was functional when co-transfected with a Sox9-Luc promoter construct (including the putative CRE site), as shown by a significant increase in luciferase activity (Fig. 2D).
We next silenced CREB gene expression in PCCl3 cells using siRNA, and this resulted in a marked decrease in Sox9 protein levels (Fig. 2E). Likewise, silencing CREB expression blunted TSH- and forskolin-induced Sox9 expression (Fig. 2F). Overall, these results indicate that TSH/cAMP-dependent stimulation of Sox9 expression is mediated, at least in part, by the CREB transcription factor.
TGFβ inhibits the stimulating effect of TSH on Sox9 expression
TGFβ signaling is operative in thyroid follicular cells and is known to be involved in the differentiation and pathophysiology of thyroid follicular cells23,24. Indeed, we previously demonstrated that TGFβ prevents TSH-dependent induction of thyroid differentiation gene expression26,27. Based on this evidence, we sought to determine the potential role of TGFβ in the regulation of Sox9 expression.
We first treated starved PCCl3 cells with exogenous TSH and/or TGFβ. We found that while TGFβ alone had no effect on Sox9 expression, it blocked the TSH-dependent increase of Sox9 mRNA (Fig. 3A) and protein (Fig. 3B) levels. These effects occurred at the transcriptional level, as TGFβ also inhibited the TSH-dependent induction of Sox9 promoter activity in transient transfections assays (Fig. 3C). TGFβ signaling is critically dependent on Smad signaling proteins, and we identified a putative Smad-binding site within the Sox9 promoter (Supplementary Fig. 3A). EMSA showed that recombinant Smad3 bound specifically to an oligonucleotide containing the Smad-binding site located at -25/-17 from the transcription initiation site of the Sox9 gene (Fig. 3D). Furthermore, Smad3 overexpression in PCCl3 cells mimicked the inhibitory effect of TGFβ on the TSH-dependent activation of the Sox9 promoter (Fig. 3E). Taken together, these data demonstrate that TGFβ inhibition of TSH-dependent stimulation of Sox9 promoter activity occurs via Smad proteins.
Role of Smad proteins in the inhibitory effect of TGFβ on TSH-induced Sox9 expression. PCCl3 cells were maintained for 48 h in starvation medium (–) and then treated or not with TSH + TGFβ for (A) 16 h or (B) 24 h. Sox9 mRNA expression levels were detected by RT-qPCR and normalized to those of β-actin. Total protein extracts were analyzed by western blotting for the detection of Sox9. β-actin was used as a loading control. pSmad2 was used as a control of Smad signaling activation. (C) PCCl3 cells were transfected with pSox9-firefly luciferase and with Renilla luciferase-encoding pRL-CMV vectors. After transfection, cells were grown for 24 h in complete medium and then maintained 48 h in starvation medium before treatment with TSH or TSH + TGFβ for 24 h. Relative luciferase activity is represented as fold induction over the value of starved cells. (D) An electrophoretic mobility shift assay (EMSA) was performed with a labeled oligonucleotide corresponding to the Smad binding site identified in the Sox9 promoter. The probe was incubated without protein or with 7 µg Smad3 (TNT-Smad3) recombinant protein, as indicated. For competition, a 100-fold excess of related (R) or non-related (NR) cold oligonucleotide was used. (E) Sox9 promoter activity was analyzed in PCCl3 cells co-transfected with pSox9 and a Smad3 expression vector and treated with TSH and TGFβ, as described in (C). Results show the fold induction of pSox9 with respect to the control (–). Values represent means ± SEM (n = 3). ns not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Original blots/gels are presented in Supplementary Fig. 5.
Sox9 is involved in the transcriptional network that regulates thyroid differentiation
Thyroid differentiation is regulated by the simultaneous expression of the thyroid transcription factors Nkx2.1, Foxe1 and Pax837. In silico analysis identified putative binding sites for all three transcription factors within the ~ 1-kb Sox9 regulatory region (Supplementary Fig. 3A). We therefore postulated that Sox9 might play a role in the differentiation of thyroid follicular cells.
As a first approach, we analyzed the ability of the thyroid transcription factors to bind to the Sox9 promoter elements. EMSAs were performed using oligonucleotides containing the putative binding sites for Nkx2.1 (at -280/-271 and at -7/-15), Pax8 (at + 35/ + 43) and Foxe1 (at -652/-647) identified in silico and using proteins translated in vitro to generate recombinant transcription factors. Results showed a clear and specific band shift for Pax8 and Foxe1 (Fig. 4A,B), suggesting that both thyroid transcription factors can bind to the Sox9 promoter. While Nkx2.1 was also able to bind to its putative -7/-15 site, the binding was not specific since the non-related oligonucleotide competed as efficiently as the related oligonucleotide (Supplementary Fig. 4A). A similar lack of specificity for Nkx2.1 binding was observed when the putative -280/-271 site was assessed (data not shown). We next questioned whether the specific binding of Pax8 and Foxe1 was transcriptionally functional, and would result in the regulation of Sox9 promoter activity. To do this, we co-transfected HeLa cells with the Sox9-Luc construct (pSox9 in Fig. 4C) and expression vectors for Pax8, Nkx2.1, and Foxe1 alone or in all possible combinations. Results revealed that Pax8 had the greatest and most significant positive effect on Sox9 reporter activity (Fig. 4C). Despite the non-specific binding determined by EMSA, Nkx2.1 modestly increased Sox9 reporter activity (Fig. 4C), reflecting its weak binding to the Sox9 promoter (Supplementary Fig. 4A). Notably, Nkx2.1 cooperated with Pax8 to further stimulate Sox9 reporter activity, having an additive effect (Fig. 4C). By contrast, Foxe1 overexpression, alone or in combination with Nkx2.1 and Pax8, decreased Sox9 reporter activity, suggesting a repressive effect on Sox9 transcription.
Involvement of Sox9 in the thyroid transcriptional regulatory network. (A and B) Electrophoretic mobility shift assays (EMSAs) were performed with labeled oligonucleotides derived from the Sox9 regulatory regions. The probes were incubated without protein or with 7 µg (A) Pax8 (TNT-Pax8) or (B) Foxe1 (TNT-Foxe1) recombinant proteins. For competition, a 100-fold excess of related (R) or nonrelated (NR) cold oligonucleotide was used. (C) HeLa cells were co-transfected with pSox9 and the indicated expression vectors (Pax8, Nkx2.1, or Foxe1). Results show the fold induction of pSox9 with respect to the control (–). (D) EMSAs were performed with labeled oligonucleotides derived from the Pax8 and Foxe1 promoters. The probes were incubated without protein or with 7 µg Sox9 (TNT-Sox9) recombinant protein. For competition, a 100-fold excess of related (R) or non-related (NR) cold oligonucleotide was used. (E) HeLa cells were co-transfected with pFoxe1, pNkx2.1 or pPax8 and the Sox9 expression vector. Results show the fold induction of Foxe1, Nkx2.1 and Pax8 promoters with respect to the control (–). (F, G, H) PCCl3 cells were transfected with control (scrambled) or Sox9 (siSox9) siRNAs. Total protein extracts were obtained 24 h after transfection and analysed by western blotting for Sox9 and Foxe1 (F), Sox9 and Pax8 (G) and Sox9 and Nkx2.1 (G). Tubulin or β-actin was used as a loading control. Values represent means ± SEM (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001. Original blots/gels are presented in Supplementary Fig. 5.
Our findings highlight Sox9 as a transcription factor that is finely tuned in the thyroid, suggesting it could be part of the transcriptional network of genes regulating thyroid differentiation. We therefore examined whether Sox9 controls the expression of the thyroid transcription factors Nkx2.1, Pax8 and Foxe1. Putative binding sites for Sox9 were identified in the promoters of all three genes by in silico analyses. EMSA with recombinant Sox9 indicated that it bound strongly and specifically to putative sites located -389 and -2268 bp from the transcription initiation site of Pax8 and Foxe1, respectively (Fig. 4 D). Weaker and non-specific binding of Sox9 to the -274 site of the Nkx2.1 regulatory region was also observed (Supplementary Fig. 4B). Binding of Sox9 was functional, as co-transfection of the Sox9 expression vector in Hela cells stimulated the activity of all three promoters, albeit to a different extent (Fig. 4E). The maximum activity (12-fold) was elicited on the Pax8 promoter. Sox9 stimulated the Foxe1 and Nkx2.1 promoters four- and two-fold, respectively. These data confirm that Sox9 is directly involved in the transcriptional regulation of the three thyroid-specific transcription factors, with a major effect on Pax8 expression. To validate these finding we used a Sox9-specific siRNA to silence its expression in PCCl3 cells. Western blotting of Sox9-silenced PCCl3 cells caused a concomitant decrease in the steady-state levels of Foxe1 and Pax8 (Fig. 4F,G), but not of Nkx2.1 (Fig. 4H).
Overall, these data suggest that Sox9 might be part of the network of transcription factors that control thyroid cell differentiation as revealed by the observed in vitro inter-relationship between Pax8 and Sox9. Moreover, our findings on the extrinsic regulation of Sox9 expression by TSH and TGFβ support the potential importance of this transcription factor in thyroid follicular cells, not only during development but also in adulthood.
Discussion
Thyroid differentiation begins during embryonic development and the differentiated state persists throughout adult life to maintain the production of T3 and T4 by the thyroid gland. Our knowledge of the mechanisms that govern this process has advanced considerably in recent years, resulting in the identification of many new actors2,4. Among these is the transcription factor Sox9, which is involved in thyroid branching morphogenesis, a fundamental mechanism in the embryonic development of various organs7. In adults, Sox9 expression appears to be exclusive to follicular cells, as it is undetectable in C cells and connective tissue cells7. Here we show that the expression of Sox9, observed in precursor cells of the thyroid primordium, persists in adult follicular cells with an evident nuclear localization. The finding that Sox9 expression is more robust in adult thyroid follicle cells than in thyroid embryonic cells suggests that it plays a role in the maintenance of thyroid follicle cell differentiation. Likewise, the discovery that TSH finely regulates Sox9 might indicate a role for this transcription factor in chronically challenging thyroid states, such as hypo- and hyper-thyroidism. In addition to TGFβ, other molecular signals controlling thyroid differentiation also control Sox9 expression. This resembles the embryonic situation where Fgf10, through its receptor Fgfr2b, regulates Sox9 expression during thyroid branching7. Sox9 is therefore likely at the center of an important regulatory network of signals that govern thyroid differentiation both in embryos and in adults. This potentially important role for Sox9 is reinforced by the finding that its promoter is conserved between rodents and humans (Supplementary Fig. 3B).
The finding that forskolin mimics the action of TSH on Sox9 together with the positive action of PKA inhibition and the lack of effect of both PKC and the MAPK inhibitors over TSH, convincingly demonstrates that the effects of TSH on Sox9 are mediated by cAMP/PKA. We would note that the present study was performed using physiological doses of TSH that do not activate the aforementioned pathways22,38 (Supplementary Fig. 2B). Finally, the influence of TSH/cAMP/PKA occurs at the transcriptional level through the binding of CREB to a CRE site in the Sox9 promoter. Silencing of CREB expression dramatically reduced the abundance of Sox9 mRNA and protein, and silenced cells lost their responsiveness to TSH and forskolin, overall demonstrating that the CREB transcription factor is essential for the regulation of Sox9 by TSH via cAMP.
Similar to other thyroid differentiation genes27,31, TGFβ inhibited the inductive effect of TSH on Sox9 expression, a transcriptional mechanism mediated by Smad proteins. Indeed, overexpression of Smad3 inhibited TSH-induced Sox9 promoter activity independently of TGFβ addition. The finding that Sox9 is negatively regulated by TGFβ confirms the existence of a homeostatic balance between the TSH/cAMP and TGFβ/Smads pathways to control thyroid follicular cell differentiation. Indeed, in a recent study designed to improve in vitro protocols for functional thyroid organoid cultures, the authors, guided by single-cell RNA sequencing, found that the addition of cAMP activators and TGFβ inhibitors to the differentiation medium improved thyroid maturation and functional activity39. Thus, the positive action of cAMP and the blocking negative action of TGFβ are likely crucial factors for thyroid maturation, and perhaps for Sox9-induced branching.
Our results suggest that Sox9 is involved in the hormonal and transcriptional regulatory network that controls the maintenance of thyroid differentiation. As previously demonstrated32, thyroid transcription factors are linked in an integrated regulatory system. In this context, we identified DNA binding sites for the thyroid transcription factors Nkx2.1, Foxe1 and Pax8 within the Sox9 promoter, and all appeared to bind, albeit to a different extent. A possible explanation for the different binding ability of each factor might be the absence of coactivators, as EMSA studies are in vitro experiments that analyze transcription factors/DNA interactions. Functional experiments would suggest that this is the case, as the three transcription factors increased the activity of Sox9 promoter individually in transient transfection experiments, but performed better in combination (Fig. 4C). Accordingly, Pax8 had a greater stimulatory effect than Nkx2.1 on Sox9 expression, but simultaneous binding of both factors were additive. The functional cooperation between Nkx2.1 and Pax8 has been previously described for other thyroid differentiation genes such as Tg, and this effect is mediated respectively by their N- and C-terminal domains40. The superior performance of Pax8 in the regulation of Sox9 artificial reporter construct confirms its fundamental role in thyroid differentiation41. Interestingly, Foxe1 elicited a repressive effect on Sox9 promoter activity, confirming a previous report42. Moreover, Foxe1 also repressed the inducing activity of Nkx2.1 and Pax8 alone or together. This observation supports the study of Perrone et al. (2000), which showed that Foxe1 inhibits the activity of Nkx2.1 and Pax8 only on certain promoters42. The repressor activity of Foxe1 is borne by its C-terminal region, which contains an alanine-rich domain. Foxl2, which is another forkhead transcription factor, has been shown to inhibit ovarian expression of Sox9 by synergistic binding with the estrogen receptor to an enhancer present in the regulatory region of Sox9. The absence of Foxl2 leads to enhanced Sox9 expression and ovary cell transdifferentiation43. Future studies should examine the mechanisms through which Foxe1 inhibits Sox9 promoter activity, and their relationship in early development. This would provide information on whether Foxe1, through its inhibitory actions, functions as a temporary modulator of Sox9 activity during thyroid follicular cell differentiation.
The involvement of Sox9 in thyroid follicular cell transcriptional regulation networks was also supported by the finding of Sox9-binding sites in Nkx2.1, Foxe1 and Pax8 promoters, which appeared to be functional. It is currently accepted that Sox9 activity depends not only on the consensus binding sequence in target promoters, but also on its interaction with other transcription factors. For example, Sox9 forms homo- and hetero-dimers with other transcription factors such as the Sonic Hedgehog mediator Gli44 or CREB45. Thus, it will be important in the future to study whether homodimerization of Sox9 and its heterodimerization with other Sox factors or proteins such as CREB or thyroid transcription factors, differentially controls the expression of thyroid differentiation genes.
The present study provides new data on the reciprocal regulation of Pax8, Foxe1 and Sox9 expression and opens new avenues to better understand the complex network controlling thyroid differentiation. We speculate that Sox9 might play an important role in the regulation of thyroid differentiation genes, such as Tg and NIS. Our preliminary results point in this direction and future work will be aimed at understanding these mechanisms of regulation. Along this line, a crucial yet poorly understood mechanism is thyroid folliculogenesis. Based on our previous results demonstrating a role for Pax8 in thyroid folliculogenesis46 and our new data describing the regulation of Sox9 by Pax8, we believe that its role should be studied in 3D follicle formation assays, which better mimic the in vivo environment.
Materials and methods
Bioinformatic analysis
We utilized two bioinformatic tools to search for transcription factor binding sites in human, mouse and rat gene promoters: TRANSFAC (www.gene-regulation.com/pub/databases.html) and the ECR Browser (www.ecrbrowser.dcode.org). We used the NCBI/BLAST server (www.blast.ncbi.nlm.nih.gov) to compare different species promoter sequences from the NCBI/Gene database.
Animals
The experimentation in mice was performed in compliance with the European Community Law (86/609/EEC). Studies in mice embryos were performed according to the guidelines of laboratory animal care of the University Animal Welfare Committee, Université Catholique de Louvain; the corresponding experiments in adult mice followed the guidelines of the Spanish law (R.D. 1201/2005), with the approval of the ethics committee of the Consejo Superior de Investigaciones Científicas (CSIC, Spain).
Cell cultures
PCCl3 rat thyroid follicular cells are an extensively used thyroid model system, as they express thyroid-specific genes47. Cells were cultured in Coon’s modified Ham’s F-12 medium supplemented with 5% donor calf serum (Life Technologies, Inc., Gaithersburg, MD). The following 6-hormone mixture, 1 nM TSH, 10 µg/ml insulin, 10 ng/ml somatostatin, 5 µg/ml transferrin, 10 nM hydrocortisone, and 10 ng/ml glycil-L-histidyl-L-lysine acetate, as well as H89, forskolin and PDBU were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). The effect of hormones and growth factors was studied after starving near confluent cells for TSH and insulin in the presence of 0.1% donor calf serum (starvation medium) for 2 days. Subsequently, the following agents were added at the final concentrations: TSH (1 nM), forskolin (10 µM), TGFβ (10 ng/ml), phorbol 12,13-dibutyrate (PDBU, 500 nM) and epidermal growth factor (EGF (10 ng/µl). The PKA inhibitor H89 (10 µM)48, the PKC inhibitor GF1 (3.5 µM)49 and the MEK1/2 inhibitor U0126 (10 µM)50 were added 90, 30 and 60 min, respectively, before treatment with the ligands. HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, glutamine, antibiotics and sodium pyruvate. TGFβ was obtained from Peprotech (Rocky Hill, NJ), EGF from R&D Systems (Minneapolis, MN) and GF1 and U0126 from Calbiochem (San Diego, CA).
RT-qPCR
Total RNA was extracted using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA) and 2 µg of RNA were used to obtain cDNA through a reverse transcription reaction (MMLV; Promega, Madison, WI). Quantitative RT-PCR was performed using specific primers (see Supplementary Table 1) using the KAPA Sybr Fast qPCR Master Mix (Merck KGaA, Darmstadt, Germany) for 40 cycles. The specificity of the reactions was determined by melting curve analysis. The number of cycles needed to reach the threshold level (Ct) for each sample was used to calculate the amount of each PCR product with the 2−ΔΔCt method51. Relative levels of the PCR products were expressed as a function of β-actin. RT-qPCR was performed on a Mx3000P QPCR platform (Agilent Technologies).
Western blotting
Total proteins were extracted and prepared as described52. Equal amounts of protein (20–30 µg) were separated by SDS-PAGE, transferred to nitrocellulose membranes, blocked in PBS-T buffer (PBS + 0.1% Tween 20 pH 7.5) containing 5% nonfat dry milk, and incubated overnight at 4ºC with the primary antibodies (described in Supplementary Table 2). After washing three times with PBS-T buffer, the membranes were incubated with the appropriate species-specific-HRP-conjugated secondary antibody for one hour. After another washing step, immunoreactive bands were detected with the Luminol Western blot detection reagent (Thermo Fisher Scientific).
Transient transfection
200,000 cells were seeded in 6-well plates and transiently transfected with calcium chloride, after 24 hours53. Sox936, Nkx2.154, Pax855 and Foxe127 promoters fused to the luciferase reporter gene were transiently transfected with the following expression vectors: hCREB56, hSMAD3/457, hPAX858, rFoxe159, rNkx2.160 and hSOX961 or the corresponding empty vector. Transfections were performed with 1.5 µg/well of the promoter construct and 1.5 µg/well of the expression vector. To check the transfection efficiency, 0.1 µg of the CMV-Renilla promoter construct was added to each well. After 48 h, cells were harvested, lysed and analyzed using the luciferase reporter dual assay system (Promega) to measure luciferase and Renilla expression. Promoter activity was determined as the ratio of luciferase and Renilla levels and is represented as relative luciferase activity.
Inmunohistochemistry
Paraffin-embedded thyroid sections were prepared for immunohistochemistry using the EnVision + System-HRP (DAB) Kit (Dako, Carpinterio, CA). Samples were deparaffined, rehydrated and treated with citrate buffer (0.01 M pH 6) for antigen retrieval. Endogenous peroxidase activity was inhibited with hydrogen peroxide (3%) and slides were incubated with the primary antibody (Supplementary Table 2) for one hour. After washing, sections were incubated with an HRP-conjugated secondary antibody for one hour and a deaminobenzine (DAB) solution was used for staining and detection of the antibody. Subsequently, sections were stained with hematoxylin, dehydrated and mounted on coverslips with DPX. Finally, the stained sections were examined using a Nikon 90i fluorescence microscope (Nikon, Japan).
Inmunofluorescence
Embryonic tissues were obtained from mice at E9.5, E10.5 and E11.5 and were processed as in Villacorte, 201662. Antigen–antibody complexes were visualized using Alexa 488-, 568- or 647-conjugated secondary antibodies (Invitrogen). Finally, nuclei were stained with Hoechst fluorescent dye (Hoechst 33,258; Sigma) and sections examined with a spinning disk confocal microscope using a Plan Apochromat 100x/1.4 Oil DIC objective (Cell Observer Spinning Disk; Zeiss).
Transgenic mice expressing GFP under the control of Sox9 regulatory elements34,35 were also used for immunofluorescence studies. Tissue samples were extracted at E16.5 or from adult mice, embedded in gelatin and processed as described above. Tissues were also analyzed using a spinning disk confocal microscope (Cell Observer Spinning Disk; Zeiss).
When using cell lines for immunofluorescence, cells were seeded on coverslips and fixed with 4% paraformaldehyde before blocking and incubation with antibodies. Samples were examined with a Leica TCS SP5 confocal microscope (Leica Systems GmbH, Watzlar, Germany).
Gene silencing
PCCl3 cells were silenced using an ON-TARGET plus SMARTpool small interfering RNA (siRNA) against Sox9 or CREB (Dharmacon, GE Healthcare, Lafayette, CO). Cells were harvested 24 and 48 h post-transfection, and total RNA and protein were extracted as described. A non-targeting siRNA (scrambled siRNA) was used as a negative control.
Electrophoretic mobility shift assays
Oligonucleotides corresponding to the binding sites of CREB, Smad3, Pax8, Foxe1 and Nkx2.1 in the Sox9 promoter and of Sox9 in the Pax8, Foxe1 and Nkx2.1 promoters (see Supplementary Table 3) were labeled with 25 µCi of ɣ32P-ATP using T4 polynucleotide kinase (Promega) and were purified on QuickSpin G-25 Sephadex columns (Roche Life Sciences, Mannheim, Germany). Recombinant proteins were produced with the in vitro transcription-translation (TNT) kit (Promega) and incubated with the labeled probes. For electrophoretic mobility shift assay (EMSA), binding reactions were performed in a buffer containing 40 mM Hepes pH 7.9, 75 mM KCl, 0.2 mM EDTA, 0.5 mM dithiothretiol, 150 ng/µl poly (dI-dC) and 5% Ficoll at room temperature for 30 min. Samples were electrophoresed on 5% polyacrylamide geld in 0.5 × Tris–borate EDTA. To evaluate the specificity of the DNA–protein interactions, labeled probes were incubated with an excess (50-fold) of the same (related) or different (unrelated) unlabeled oligonucleotides.
Statistical analysis
Results are represented as fold induction ± standard error of the mean from at least three different experiments. Student’s two-tailed t-test was used to assess the differences between measurements. A P-value of ˂0.05 was considered statistically significant.
References
Fernández, L. P., López-Márquez, A. & Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 11, 29–42. https://doi.org/10.1038/nrendo.2014.186 (2015).
López-Márquez, A., Carrasco-López, C., Fernández-Méndez, C. & Santisteban, P. Unraveling the complex interplay between transcription factors and signaling molecules in thyroid differentiation and function, from embryos to adults. Front. Endocrinol. (Lausanne) 12, 654569. https://doi.org/10.3389/fendo.2021.654569 (2021).
Nilsson, M. & Fagman, H. Development of the thyroid gland. Development 144, 2123–2140. https://doi.org/10.1242/dev.145615 (2017).
Pierreux, C. E. Shaping the thyroid: from peninsula to de novo lumen formation. Mol. Cell Endocrinol. 531, 111313. https://doi.org/10.1016/j.mce.2021.111313 (2021).
Fagman, H., Grande, M., Gritli-Linde, A. & Nilsson, M. Genetic deletion of sonic hedgehog causes hemiagenesis and ectopic development of the thyroid in mouse. Am. J. Pathol. 164, 1865–1872. https://doi.org/10.1016/S0002-9440(10)63745-5 (2004).
Kurmann, A. A. et al. Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17, 527–542. https://doi.org/10.1016/j.stem.2015.09.004 (2015).
Liang, S. et al. A branching morphogenesis program governs embryonic growth of the thyroid gland. Development 145, 102. https://doi.org/10.1242/dev.146829 (2018).
Serls, A. E., Doherty, S., Parvatiyar, P., Wells, J. M. & Deutsch, G. H. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development 132, 35–47. https://doi.org/10.1242/dev.01570 (2005).
Wendl, T. et al. Early developmental specification of the thyroid gland depends on han-expressing surrounding tissue and on FGF signals. Development 134, 2871–2879. https://doi.org/10.1242/dev.02872 (2007).
Gubbay, J. et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346, 245–250. https://doi.org/10.1038/346245a0 (1990).
Sinclair, A. H. et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244. https://doi.org/10.1038/346240a0 (1990).
Kamachi, Y. & Kondoh, H. Sox proteins: regulators of cell fate specification and differentiation. Development 140, 4129–4144. https://doi.org/10.1242/dev.091793 (2013).
Seymour, P. A. et al. A Sox9/Fgf feed-forward loop maintains pancreatic organ identity. Development 139, 3363–3372. https://doi.org/10.1242/dev.078733 (2012).
Shih, H. P. et al. A gene regulatory network cooperatively controlled by Pdx1 and Sox9 governs lineage allocation of foregut progenitor cells. Cell Rep. 13, 326–336. https://doi.org/10.1016/j.celrep.2015.08.082 (2015).
McDonald, E. et al. SOX9 regulates endocrine cell differentiation during human fetal pancreas development. Int. J. Biochem. Cell Biol. 44, 72–83. https://doi.org/10.1016/j.biocel.2011.09.008 (2012).
Chang, D. R. et al. Lung epithelial branching program antagonizes alveolar differentiation. Proc. Natl. Acad. Sci. U S A 110, 18042–18051. https://doi.org/10.1073/pnas.1311760110 (2013).
Bellusci, S., Grindley, J., Emoto, H., Itoh, N. & Hogan, B. L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867–4878 (1997).
Abler, L. L., Mansour, S. L. & Sun, X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev. Dyn. 238, 1999–2013. https://doi.org/10.1002/dvdy.22032 (2009).
Chatzeli, L., Gaete, M. & Tucker, A. S. Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144, 2294–2305. https://doi.org/10.1242/dev.146019 (2017).
Furuyama, K. et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 43, 34–41. https://doi.org/10.1038/ng.722 (2011).
Jo, A. et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 1, 149–161. https://doi.org/10.1016/j.gendis.2014.09.004 (2014).
Kimura, T. et al. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr. Rev. 22, 631–656. https://doi.org/10.1210/edrv.22.5.0444 (2001).
Carneiro, C., Alvarez, C. V., Zalvide, J., Vidal, A. & Dominguez, F. TGF-beta1 actions on FRTL-5 cells provide a model for the physiological regulation of thyroid growth. Oncogene 16, 1455–1465. https://doi.org/10.1038/sj.onc.1201662 (1998).
Nicolussi, A., D’Inzeo, S., Santulli, M., Colletta, G. & Coppa, A. TGF-beta control of rat thyroid follicular cells differentiation. Mol. Cell Endocrinol. 207, 1–11 (2003).
Medina, D. L. et al. Role of insulin and serum on thyrotropin regulation of thyroid transcription factor-1 and pax-8 genes expression in FRTL-5 thyroid cells. Thyroid 10, 295–303. https://doi.org/10.1089/thy.2000.10.295 (2000).
Costamagna, E., García, B. & Santisteban, P. The functional interaction between the paired domain transcription factor Pax8 and Smad3 is involved in transforming growth factor-beta repression of the sodium/iodide symporter gene. J. Biol. Chem. 279, 3439–3446. https://doi.org/10.1074/jbc.M307138200 (2004).
Lopez-Marquez, A., Fernandez-Mendez, C., Recacha, P. & Santisteban, P. Regulation of Foxe1 by thyrotropin and transforming growth factor beta depends on the interplay between thyroid-specific, CREB and SMAD transcription factors. Thyroid https://doi.org/10.1089/thy.2018.0136 (2019).
Colletta, G., Cirafici, A. M. & Di Carlo, A. Dual effect of transforming growth factor beta on rat thyroid cells: inhibition of thyrotropin-induced proliferation and reduction of thyroid-specific differentiation markers. Cancer Res. 49, 3457–3462 (1989).
Christophe, D. et al. Identification of a cAMP-responsive region in thyroglobulin gene promoter. Mol. Cell Endocrinol. 64, 5–18 (1989).
Damante, G. et al. Thyrotropin regulation of thyroid peroxidase messenger ribonucleic acid levels in cultured rat thyroid cells: evidence for the involvement of a nontranscriptional mechanism. Endocrinology 124, 2889–2894. https://doi.org/10.1210/endo-124-6-2889 (1989).
Costamagna, E., Garcia, B. & Santisteban, P. The functional interaction between the paired domain transcription factor Pax8 and Smad3 is involved in transforming growth factor-beta repression of the sodium/iodide symporter gene. J. Biol. Chem. 279, 3439–3446. https://doi.org/10.1074/jbc.M307138200 (2004).
Parlato, R. et al. An integrated regulatory network controlling survival and migration in thyroid organogenesis. Dev. Biol. 276, 464–475. https://doi.org/10.1016/j.ydbio.2004.08.048 (2004).
Weider, M. & Wegner, M. SoxE factors: transcriptional regulators of neural differentiation and nervous system development. Semin. Cell Dev. Biol. 63, 35–42. https://doi.org/10.1016/j.semcdb.2016.08.013 (2017).
Gong, S. et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925. https://doi.org/10.1038/nature02033 (2003).
Formeister, E. J. et al. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1108-1118. https://doi.org/10.1152/ajpgi.00004.2009 (2009).
Piera-Velazquez, S. et al. Regulation of the human SOX9 promoter by Sp1 and CREB. Exp. Cell Res. 313, 1069–1079. https://doi.org/10.1016/j.yexcr.2007.01.001 (2007).
Fernandez, L. P., Lopez-Marquez, A. & Santisteban, P. Thyroid transcription factors in development, differentiation and disease. Nat. Rev. Endocrinol. 11, 29–42. https://doi.org/10.1038/nrendo.2014.186 (2015).
García-Jiménez, C. & Santisteban, P. TSH signalling and cancer. Arq. Bras. Endocrinol. Metabol. 51, 654–671. https://doi.org/10.1590/s0004-27302007000500003 (2007).
Romitti, M. et al. Single-cell trajectory inference guided enhancement of thyroid maturation. Front. Endocrinol. (Lausanne) 12, 657195. https://doi.org/10.3389/fendo.2021.657195 (2021).
Di Palma, T. et al. The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J. Biol. Chem. 278, 3395–3402. https://doi.org/10.1074/jbc.M205977200 (2003).
di Pasca, M. M., Di Lauro, R. & Zannini, M. Pax8 has a key role in thyroid cell differentiation. Proc. Natl. Acad. Sci. U S A 97, 13144–13149. https://doi.org/10.1073/pnas.240336397 (2000).
Perrone, L., di Pasca, M. M., Zannini, M. & Di Lauro, R. The thyroid transcription factor 2 (TTF-2) is a promoter-specific DNA-binding independent transcriptional repressor. Biochem. Biophys. Res. Commun. 275, 203–208. https://doi.org/10.1006/bbrc.2000.3232 (2000).
Uhlenhaut, N. H. et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139, 1130–1142. https://doi.org/10.1016/j.cell.2009.11.021 (2009).
Leung, V. Y. et al. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 7, e1002356. https://doi.org/10.1371/journal.pgen.1002356 (2011).
Zhao, L., Li, G. & Zhou, G. Q. SOX9 directly binds CREB as a novel synergism with the PKA pathway in BMP-2-induced osteochondrogenic differentiation. J. Bone Miner. Res. 24, 826–836. https://doi.org/10.1359/jbmr.081236 (2009).
Koumarianou, P., Goméz-López, G. & Santisteban, P. Pax8 controls thyroid follicular polarity through cadherin-16. J. Cell Sci. 130, 219–231. https://doi.org/10.1242/jcs.184291 (2017).
Fusco, A. et al. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol. Cell Biol. 7, 3365–3370. https://doi.org/10.1128/mcb.7.9.3365 (1987).
Marunaka, Y. & Niisato, N. H89, an inhibitor of protein kinase A (PKA), stimulates Na+ transport by translocating an epithelial Na+ channel (ENaC) in fetal rat alveolar type II epithelium. Biochem. Pharmacol. 66, 1083–1089. https://doi.org/10.1016/s0006-2952(03)00456-8 (2003).
Bousquet, S. M., Monet, M. & Boulay, G. Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J. Biol. Chem. 285, 40534–40543. https://doi.org/10.1074/jbc.M110.160051 (2010).
Favata, M. F. et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273, 18623–18632. https://doi.org/10.1074/jbc.273.29.18623 (1998).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. https://doi.org/10.1006/meth.2001.1262 (2001).
Zaballos, M. A., Garcia, B. & Santisteban, P. Gbetagamma dimers released in response to thyrotropin activate phosphoinositide 3-kinase and regulate gene expression in thyroid cells. Mol. Endocrinol. 22, 1183–1199. https://doi.org/10.1210/me.2007-0093 (2008).
Chen, C. A. & Okayama, H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques 6, 632–638 (1988).
Oguchi, H. & Kimura, S. Multiple transcripts encoded by the thyroid-specific enhancer-binding protein (T/EBP)/thyroid-specific transcription factor-1 (TTF-1) gene: evidence of autoregulation. Endocrinology 139, 1999–2006. https://doi.org/10.1210/endo.139.4.5933 (1998).
Sastre-Perona, A. & Santisteban, P. Wnt-independent role of β-catenin in thyroid cell proliferation and differentiation. Mol. Endocrinol. 28, 681–695. https://doi.org/10.1210/me.2013-1377 (2014).
Mendez-Pertuz, M., Sanchez-Pacheco, A. & Aranda, A. The thyroid hormone receptor antagonizes CREB-mediated transcription. EMBO J. 22, 3102–3112. https://doi.org/10.1093/emboj/cdg295 (2003).
Sanchez-Elsner, T. et al. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J. Biol. Chem. 276, 38527–38535. https://doi.org/10.1074/jbc.M104536200 (2001).
Vilain, C. et al. Autosomal dominant transmission of congenital thyroid hypoplasia due to loss-of-function mutation of PAX8. J. Clin. Endocrinol. Metab. 86, 234–238. https://doi.org/10.1210/jcem.86.1.7140 (2001).
Zannini, M. et al. TTF-2, a new forkhead protein, shows a temporal expression in the developing thyroid which is consistent with a role in controlling the onset of differentiation. EMBO J. 16, 3185–3197. https://doi.org/10.1093/emboj/16.11.3185 (1997).
Guazzi, S. et al. Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J. 9, 3631–3639 (1990).
Lefebvre, V., Huang, W., Harley, V. R., Goodfellow, P. N. & de Crombrugghe, B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell Biol. 17, 2336–2346 (1997).
Villacorte, M. et al. Thyroid follicle development requires Smad1/5- and endothelial cell-dependent basement membrane assembly. Development 143, 1958–1970. https://doi.org/10.1242/dev.134171 (2016).
Acknowledgements
We are grateful to Dr. Kenneth McCreath for helpful comments on the manuscript. To Dr. Teresa Iglesias (Instituto de Investigaciones Biomédicas) for kindly providing material for analysis of PKC pathway activation and to Javier Pérez (Instituto de Investigaciones Biomédicas) for the artwork.
Funding
This work was supported by grants PID2019-105303RB-I00/AEI/10.13039/501100011033 from Ministerio de Ciencia e Innovación (MICIN), Spain; B2017/BMD-3724 from Comunidad de Madrid, and GCB14142311CRES from Fundación Española Contra el Cáncer (AECC) to PS, and the Actions de Recherche concertées-UCLouvain (ARC 15/20-065), the Fonds pour la Recherche Scientifique (F.R.S-FNRS, # J.0126.16), and Fondation Roi Baudouin to CEP. C.C-L holds a FPI fellowship associated to Grant SAF2016-75531-R/FEDER from MICIN.
Author information
Authors and Affiliations
Contributions
A.L.-M., C.C.-L. conducted the experiments and acquired and analyzed the data. A.M.-C. performed transient transfection analysis. P.L. and C.P. contributed to embryonic mice study and C.P. contributed to critical discussion. A.L.-M., C.C.-L. and P.S. designed research studies and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
López-Márquez, A., Carrasco-López, C., Martínez-Cano, A. et al. Sox9 is involved in the thyroid differentiation program and is regulated by crosstalk between TSH, TGFβ and thyroid transcription factors. Sci Rep 12, 2144 (2022). https://doi.org/10.1038/s41598-022-06004-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-06004-1
This article is cited by
-
Role of Sox9 in BPD and its effects on the Wnt/β-catenin pathway and AEC-II differentiation
Cell Death Discovery (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.