Abstract
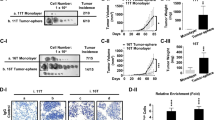
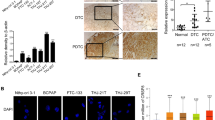
Thyroid hormone receptor α1 (TRα1) mediates the genomic actions of thyroid hormone (T3). The biology of TRα1 in growth and development has been well studied, but the functional role of TRα1 in cancers remains to be elucidated. Analysis of the human thyroid cancer database of The Cancer Genome Atlas (TCGA) showed that THRA gene expression is lost in highly dedifferentiated anaplastic thyroid cancer (ATC). We, therefore, explored the effects of TRα1 on the progression of ATC. We stably expressed TRα1 in two human ATC cell lines, THJ-11T (11T-TRα1 #2, #7, and #8) and THJ-16T (16T-TRα1 #3, #4, and #8) cells. We found that the expressed TRα1 inhibited ATC cell proliferation and induced apoptosis. TCGA data showed that THRA gene expression was best correlated with the paired box gene 8 (PAX8). Consistently, we found that the PAX8 expression was barely detectable in parental 11T and 16T cells. However, PAX8 gene expression was elevated in 11T- and 16T-TRα1-expressing cells at the mRNA and protein levels. Using various molecular analyses, we found that TRα1 directly regulated the expression of the PAX8 gene. Single-cell transcriptomic analyses (scRNA-seq) demonstrated that TRα1 functions as a transcription factor through multiple signaling pathways to suppress tumor growth. Importantly, scRNA-seq analysis showed that TRα1-induced PAX8, via its transcription program, shifts the cell landscape of ATC toward a differentiated state. The present studies suggest that TRα1 is a newly identified regulator of thyroid differentiation and could be considered as a potential therapeutic target to improve the outcome of ATC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
scRNA-seq data have been submitted to NCBI GEO. The GEO accession number is GSE235216 for accessing the data.
References
Sap J, Munoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, et al. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986;324:635–40.
Benbrook D, Pfahl M. A novel thyroid hormone receptor encoded by a cDNA clone from a human testis library. Science. 1987;238:788–91.
Thompson CC, Weinberger C, Lebo R, Evans RM. Identification of a novel thyroid hormone receptor expressed in the mammalian central nervous system. Science. 1987;237:1610–4.
Hodin RA, Lazar MA, Wintman BI, Darling DS, Koenig RJ, Larsen PR, et al. Identification of a thyroid hormone receptor that is pituitary-specific. Science. 1989;244:76–9.
Murray MB, Zilz ND, McCreary NL, MacDonald MJ, Towle HC. Isolation and characterization of rat cDNA clones for two distinct thyroid hormone receptors. J Biol Chem. 1988;263:12770–7.
Pascual A, Aranda A. Thyroid hormone receptors, cell growth and differentiation. Biochim Biophys Acta. 2013;1830:3908–16.
Sun G, Roediger J, Shi YB. Thyroid hormone regulation of adult intestinal stem cells: Implications on intestinal development and homeostasis. Rev Endocr Metab Disord. 2016;17:559–69.
Giolito MV, Plateroti M. Thyroid hormone signaling in the intestinal stem cells and their niche. Cell Mol Life Sci. 2022;79:476.
Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. The development of the adult intestinal stem cells: Insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci. 2011;1:30.
Lee YK, Ng KM, Chan YC, Lai WH, Au KW, Ho CY, et al. Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway. Mol Endocrinol. 2010;24:1728–36.
Chen C, Zhou Z, Zhong M, Zhang Y, Li M, Zhang L, et al. Thyroid hormone promotes neuronal differentiation of embryonic neural stem cells by inhibiting STAT3 signaling through TRalpha1. Stem Cells Dev. 2012;21:2667–81.
Deng C, Zhang Z, Xu F, Xu J, Ren Z, Godoy-Parejo C, et al. Thyroid hormone enhances stem cell maintenance and promotes lineage-specific differentiation in human embryonic stem cells. Stem Cell Res Ther. 2022;13:120.
Fernandez M, Pirondi S, Manservigi M, Giardino L, Calza L. Thyroid hormone participates in the regulation of neural stem cells and oligodendrocyte precursor cells in the central nervous system of adult rat. Eur J Neurosci. 2004;20:2059–70.
Gothie JD, Sebillot A, Luongo C, Legendre M, Nguyen Van C, Le Blay K, et al. Adult neural stem cell fate is determined by thyroid hormone activation of mitochondrial metabolism. Mol Metab. 2017;6:1551–61.
Liu R, Bishop J, Zhu G, Zhang T, Ladenson PW, Xing M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2017;3:202–8.
Pasca di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci USA. 2000;97:13144–9.
Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–90.
Tomás G, Tarabichi M, Gacquer D, Hebrant A, Dom G, Dumont JE, et al. A general method to derive robust organ-specific gene expression-based differentiation indices: application to thyroid cancer diagnostic. Oncogene 2012;31:4490–8.
Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31:139–70.
Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122:3035–43.
Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20:163–72.
Mabbott NA, Baillie JK, Brown H, Freeman TC, Hume DA. An expression atlas of human primary cells: inference of gene function from coexpression networks. BMC Genomics. 2013;14:632.
Thompson B, Davidson EA, Liu W, Nebert DW, Bruford EA, Zhao H, et al. Overview of PAX gene family: analysis of human tissue-specific variant expression and involvement in human disease. Hum Genet. 2021;140:381–400.
Dupain C, Ali HM, Mouhoub TA, Urbinati G, Massaad-Massade L. Induction of TTF-1 or PAX-8 expression on proliferation and tumorigenicity in thyroid carcinomas. Int J Oncol. 2016;49:1248–58.
Laury AR, Perets R, Piao H, Krane JF, Barletta JA, French C, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–26.
Sangoi AR, Cassarino DS. PAX-8 expression in primary and metastatic Merkel cell carcinoma: an immunohistochemical analysis. Am J Dermatopathol. 2013;35:448–51.
Tacha D, Zhou D, Cheng L. Expression of PAX8 in normal and neoplastic tissues: a comprehensive immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19:293–9.
Ma R, Latif R, Davies TF. Human embryonic stem cells form functional thyroid follicles. Thyroid. 2015;25:455–61.
Chu Y, Zhu C, Wang Q, Liu M, Wan W, Zhou J, et al. Adipose-derived mesenchymal stem cells induced PAX8 promotes ovarian cancer cell growth by stabilizing TAZ protein. J Cell Mol Med. 2021;25:4434–43.
Ozcan A, Shen SS, Hamilton C, Anjana K, Coffey D, Krishnan B, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24:751–64.
Fu DJ, De Micheli AJ, Bidarimath M, Ellenson LH, Cosgrove BD, Flesken-Nikitin A, et al. Cells expressing PAX8 are the main source of homeostatic regeneration of adult mouse endometrial epithelium and give rise to serous endometrial carcinoma. Dis Model Mech. 2020;13.
Kaminski MM, Tosic J, Kresbach C, Engel H, Klockenbusch J, Muller AL, et al. Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol. 2016;18:1269–80.
Futreal PA, Soderkvist P, Marks JR, Iglehart JD, Cochran C, Barrett JC, et al. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992;52:2624–7.
Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, et al. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2:283–7.
Dayton AI, Selden JR, Laws G, Dorney DJ, Finan J, Tripputi P, et al. A human c-erbA oncogene homologue is closely proximal to the chromosome 17 breakpoint in acute promyelocytic leukemia. Proc Natl Acad Sci USA. 1984;81:4495–9.
Huang ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–72.
Degos L, Dombret H, Chomienne C, Daniel MT, Miclea JM, Chastang C, et al. All-trans-retinoic acid as a differentiating agent in the treatment of acute promyelocytic leukemia. Blood. 1995;85:2643–53.
Warrell RP Jr., de The H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329:177–89.
de The H. Differentiation therapy revisited. Nat Rev Cancer. 2018;18:117–27.
Hong CM, Ahn BC. Redifferentiation of Radioiodine Refractory Differentiated Thyroid Cancer for Reapplication of I-131 Therapy. Front Endocrinol (Lausanne). 2017;8:260.
Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–32.
Rothenberg SM, McFadden DG, Palmer EL, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21:1028–35.
Forrest D, Hallbook F, Persson H, Vennstrom B. Distinct functions for thyroid hormone receptors alpha and beta in brain development indicated by differential expression of receptor genes. EMBO J. 1991;10:269–75.
Gothe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, et al. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13:1329–41.
Dellovade TL, Chan J, Vennstrom B, Forrest D, Pfaff DW. The two thyroid hormone receptor genes have opposite effects on estrogen-stimulated sex behaviors. Nat Neurosci. 2000;3:472–5.
Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther. 2017;173:135–45.
Forrest D, Vennstrom B. Functions of thyroid hormone receptors in mice. Thyroid. 2000;10:41–52.
Kim WG, Zhu X, Kim DW, Zhang L, Kebebew E, Cheng SY. Reactivation of the silenced thyroid hormone receptor beta gene expression delays thyroid tumor progression. Endocrinology. 2013;154:25–35.
Martinez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, et al. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009;69:501–9.
Davidson CD, Gillis NE, Carr FE.Thyroid Hormone Receptor Beta as Tumor Suppressor: Untapped Potential in Treatment and Diagnostics in Solid Tumors. Cancers (Basel). 2021;13:4254.
Lee WK, Zhu X, Park S, Zhu YJ, Zhao L, Meltzer P, et al. Regulation of cancer stem cell activity by thyroid hormone receptor beta. Oncogene. 2022;41:2315–25.
Park S, Han CR, Park JW, Zhao L, Zhu X, Willingham M, et al. Defective erythropoiesis caused by mutations of the thyroid hormone receptor alpha gene. PLoS Genet. 2017;13:e1006991.
Acknowledgements
This research was supported by the Intramural Research Programs of the Center for Cancer Research of the National Cancer Institute, National Institutes of Health. We thank Dr. Michael Kelly and his team for carrying out single-cell RNA sequencing at the Single Cell Analysis Facility, Cancer Research Technology Program, Frederick National Laboratory (Leidos Biomed), NCI. We also thank Joelle Mornini, NIH Library, for manuscript editing assistance.
Author information
Authors and Affiliations
Contributions
Conception and designs were performed by S-YC, EH, WKLD, YJZ and XZ. Development of methodology and acquisition of data were carried out by EH, WKLD, YJZ, XZ, LZ, and YY. Analysis and interpretation of data were performed by EH, WKLD, YJZ, XZ, YY and S-YC. Administrative, technical, or material support were performed by S-YC and LZ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hwang, E., Doolittle, W.K.L., Zhu, Y.J. et al. Thyroid hormone receptor α1: a novel regulator of thyroid cancer cell differentiation. Oncogene 42, 3075–3086 (2023). https://doi.org/10.1038/s41388-023-02815-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02815-2