Abstract
HLA-B*52 is an established genetic factor in Takayasu arteritis (TAK). Recently, single nucleotide polymorphisms (SNPs) in IL12B (rs6871626) and LILRA3 (rs103294) were newly identified as non-HLA susceptibility loci in TAK. Here, we examined how these SNPs contribute to clinical characteristics and vascular damage in TAK. We retrospectively reviewed the medical records of 99 TAK patients enrolled in our previous genome-wide association study, and whose genotypes for IL12B rs6871626, LILRA3 rs103294, and HLA-B*52 were available. Incidence of aortic regurgitation (AR) was significantly associated with the A allele (risk allele) of IL12B rs6871626 (CC 42%, AC 61%, AA 81%; p = 0.0052; odds ratio [OR] 2.45), as well as with the incidence of hypertension (p = 0.049; OR 1.82) and the proportion of patients who underwent aortic valve replacement (p = 0.023; OR 3.64). Regarding vascular damage, there was positive correlation between the Takayasu Arteritis Damage Score and the A allele of IL12B rs6871626 (CC 3.42 ± 2.71, AC 4.06 ± 3.25, AA 6.00 ± 2.81; p = 0.0035; β = 1.35) and between the Vasculitis Damage Index and the A allele (CC 3.47 ± 1.98, AC 4.33 ± 2.40, AA 5.37 ± 2.22; p = 0.0054; β = 0.96). Contrarily, no correlation was found between LILRA3 rs103294 and vascular damage. In the present study, IL12B rs6871626 was associated with vascular damage in TAK, whereas LILRA3 rs103294 was not. Genotyping of IL12B rs6871626 may help to identify patients at risk of disease progression.
Similar content being viewed by others
Introduction
Takayasu arteritis (TAK) is an idiopathic, chronic, inflammatory disease, characterized by granulomatous panarteritis of the aorta and its major branches, and typically presents before the age of 40 years1. TAK is a potentially devastating disease, often causing substantial vascular damage in affected patients. Stenoses and occlusions of major aortic branches can result in serious ischemic complications such as cerebral infarction, visual loss, and claudication. In addition, patients can develop rapidly expanding aneurysms resulting in aortic rupture. Cardiac complications are common, and 30–60% of patients develop aortic regurgitation (AR)1,2.
HLA-B*52 is a well-established genetic component associated with TAK worldwide3. As a non-HLA susceptibility locus of TAK, we previously reported a single nucleotide polymorphism (SNP), rs6871626, in the IL12B region4,5. IL12B rs6871626 was positively associated with AR and inflammatory marker levels4. In addition, Matsumura et al. identified the association between IL12B rs6871626 and disease severity defined by early onset, steroid resistance, and/or relapsing disease course6. However, it has not been elucidated whether IL12B rs6871626 is associated with vascular damage. In addition to IL12B rs6871626, we recently identified four unreported susceptibility loci for TAK5. Among these, rs103294 in the LILRA3 region showed a significant epistasis effect with HLA-B*525. LILRA3 belongs to the LILR family expressed on various leukocytes, and its detailed functions and mechanisms are still not known5,7,8. The association between LILRA3 rs103294 and clinical characteristics in TAK, including vascular damage, has never been examined.
Damage to systemic vasculitides is defined as irreversible scars in the involved organs, which may be caused by either disease activity or therapeutic approaches9. The damage measures for TAK, such as the Takayasu arteritis damage score (TADS)10 and Vasculitis Damage Index (VDI) have been developed11. The former is a TAK-specific damage measure focusing mainly on disease-related damage in the cardiovascular system, whereas the latter is a validated damage score for use in all types of systemic vasculitides, which also captures treatment-related damage well9,12. In addition, as an imaging-based scoring system to evaluate systemic angiographic damages in large vessel vasculitis, Arteritis Stenosis Score (ASS), Arteritis Dilation Score (ADS), and Arteritis Composite Score (ACS) have been developed and validated13. ASS and ACS were reported to correlate with TADS and reflect vascular damage rather than disease activity. These are novel vascular outcome measures with wide applicability and utility for clinical trials.
Here, we evaluated the association of IL12B rs6871626 and LILRA3 rs103294 with vascular damage and other clinical characteristics of TAK.
Results
Demographic characteristics and organ involvements
A summary of demographic characteristics and organ involvements stratified by genotypes of IL12B rs6871626 and LILRA3 rs103294 genotypes are shown in Table 1. Age at onset and male–female ratio were not associated with the A allele (risk allele) of IL12 rs6871626; however, the incidence of AR was positively associated with this allele in the additive model (CC 8/19 [42%], AC 31/51 [61%], AA 22/27 [81%]; p = 0.0052; OR 2.45, 95% CI 1.27–4.73). No association was found between the prevalence of AR and the genotypes of LILRA3 rs103294 (Fig. 1). The incidence of hypertension was also associated with the A allele of IL12B rs6871626 in the additive model (CC 10/19 [53%], AC 23/53 [45%], AA 21/27 [78%]; p = 0.049; OR 1.82; 95% CI 0.99–3.36); however, no association was found between the prevalence of hypertension and genotypes of LILRA3 rs103294. Other organ involvements were not associated with either IL12B rs6871626 or LILRA3 rs103294.
In the dominant model, we did not observe significant associations between IL12B rs6871626 and AR or hypertension, although the incidence tended to be higher in risk genotypes of IL12B rs6872626 (Supplementary Table).
Treatment profiles
Treatment profiles stratified by genotypes of IL12B rs6871626 and LILRA3 rs103294 are summarized in Table 2. No association was found between IL12B rs6871626 or LILRA3 rs103294 and the use of glucocorticoids, oral immunosuppressants, or biologics, although the proportion of biologic users tended to be higher in the risk genotypes of IL12B rs6871626.
Regarding surgery or catheter intervention, the proportion of patients who had undergone aortic valve replacement was associated with the A allele of IL12B rs6871626 (CC 1/19 [5%], AC 2/53 [4%], AA 6/27 [22%]; p = 0.023; OR 3.64, 95% CI 1.08–12.24), whereas no association was found with genotypes of LILRA3 rs103294. The overall proportion of surgery or catheter interventions and the proportion of patients who had undergone other specific vascular interventions, such as coronary artery bypass graft and percutaneous coronary intervention, were not associated with either IL12B rs6871626 or LILRA3 rs103294.
Arterial involvement
Figure 2A,B summarizes the distribution of arterial involvement. No association was found between arterial involvement and genotypes of IL12B rs6871626 or LILRA3 rs103294, except for a lower incidence of right subclavian artery involvement in patients with the T allele (risk allele) of LILRA3 rs103294 (CC 1/1 [100%], CT 22/34 [65%], TT 22/56 [39%]; p = 0.010; OR 0.36, 95% CI 0.14–0.79) (Fig. 2A,B).
Association of genotypes with arterial involvement. (A,B) The association of IL12B rs6871626 and LILRA3 rs103294 with the distribution of arterial involvement. (C) The association of IL12B rs6871626 and LILRA3 rs103294 with Arteritis Composite Score. Rt right, Lt left, As ascending, Des thoracic descending, Abd abdominal descending, NS not significant. *P < 0.05.
To quantify systemic vascular damage on imaging, we calculated ASS, ADS, and ACS. We did not observe the association between these scores and genotypes of IL12B rs6871626 or LILRA3 rs103294 (Fig. 2C, Supplementary Figure).
Vasculitis-associated damage measures
Then, we evaluated vasculitis-associated damage measures using TADS and VDI. There was a positive correlation between TADS and the A allele of IL12B rs6871626 in the additive model (CC 3.42 ± 2.71, AC 4.06 ± 3.25, AA 6.00 ± 2.81; p = 0.0035; β = 1.35) (Fig. 3A). In addition, VDI was positively correlated with the A allele in the additive model (CC 3.47 ± 1.98, AC 4.33 ± 2.40, AA 5.37 ± 2.22; p = 0.0054; β = 0.96) (Fig. 3A). When adjusted for disease duration, the correlation of TADS and VDI with the A allele of IL12B rs6871626 remained statistically significant (p = 0.013, β = 1.11 for TADS; p = 0.025, β = 0.72 for VDI). No correlation was found between LILRA3 rs103294 and TADS or VDI (Fig. 3B).
In the dominant model, we did not observe significant associations between IL12B rs6871626 and TADS or VDI, although their scores tended to be higher in risk genotypes of IL12B rs6872626 (Supplementary Table).
Association between clinical characteristics and LILRA3 rs103294 genotypes in HLA-B*52-positive patients
We previously reported a significant epistasis effect between HLA-B*52 and LILRA3 rs1032945. The TT genotype with HLA-B*52 was associated with TAK susceptibility, whereas the TT genotype without HLA-B*52 was not. In other words, LILRA3 rs103294 functions as a TAK susceptibility gene only in the presence of HLA-B*52. Therefore, we hypothesized that the association between clinical characteristics and LILRA3 rs103294 is found only in HLA-B*52-positive patients. However, as summarized in Table 3, there was no significant association between clinical characteristics and LILRA3 rs103294 even in HLA-B*52-positive patients. In addition, the association between clinical characteristics and LILRA3 rs103294 was not observed in HLA-B*52-negative patients (data not shown).
Discussion
In the present study, we examined for the first time the impact of IL12B rs6871626 and LILRA3 rs103294, which were newly identified susceptibility loci, on clinical characteristics and vascular damage in TAK patients. Our study demonstrated that IL12B rs6871626, but not LILRA3 rs103294, was associated with organ involvement and vascular damage assessed by TADS and VDI. Our study results suggest that patients harboring risk alleles of IL12B rs6871626 may be at risk of disease progression, requiring close monitoring. In addition, we detected for the first time an association between IL12B rs6871626 and hypertension in TAK, which was not attributed to renal artery involvement. Low compliance of arterial walls in patients with risk alleles may lead to systemic hypertension.
Our results are compatible with previous reports showing the association of IL12B rs6871626 with AR, inflammatory marker levels, and disease severity4,6. IL12B encodes IL-12p40, a common subunit of IL-12 and IL-2314. More specifically, IL-12p40-dependent cytokines include IL-12p70, IL-12p80, and IL-2315. IL-12p70 is composed of IL-12p40 and IL-12p35 subunits, while IL-12p80 is a homodimer of IL-12p40 and considered as an antagonist of IL-12p70 function15. Although there have been conflicting results regarding whether IL-12p7016,17,18,19 and IL-2319,20,21,22 participate in the pathogenesis of TAK, our recent work has demonstrated that both IL-12p40 and IL-12p70 were enriched in the plasma of TAK patients, and was more pronounced in patients with the risk allele of IL12B rs687162623. Taken together, the risk genotype of IL12B rs6871626 may contribute to the severe phenotype of TAK through subsequent overproduction of IL-12p40 and IL-12p70.
This hypothesis is supported by our pilot study using ustekinumab (UST), a monoclonal antibody targeting IL-12p40, for treating TAK. We confirmed the improvement of symptoms, inflammatory marker levels, and safety in the first three months of treatment with UST24. We have also conducted an observational study evaluating long-term outcomes of refractory TAK patients treated with biologics, and found that UST has effects on reducing inflammatory marker levels and glucocorticoid dose25. IL12B rs6871626 has also been reported as a susceptibility locus in inflammatory bowel disease26,27 and ankylosing spondylitis28. Co-occurrence of TAK and ulcerative colitis is not uncommon, and the two diseases are reported to share a significant proportion of genetic overlap29,30. IL-12p40 blockade is an approved treatment of choice for inflammatory bowel disease31,32. In light of this, further studies are warranted to assess the clinical efficacy of IL-12p40 blockade in TAK.
In this study, we did not identify any influence of LILRA3 rs103294 on clinical phenotypes of TAK, except for a lower incidence of right subclavian artery involvement in the risk genotype. In our study population, the genotype of LILRA3 rs103294 was strongly biased towards risk genotypes, with only one patient having the CC genotype. This may have limited the power of this study to identify the association of LILRA3 rs103294 with clinical characteristics of TAK. Another possible explanation is that LILRA3 rs103294 only affects disease susceptibility and is irrelevant to disease progression, thus contributing differently to TAK pathogenesis from IL12B rs6871626. It is unclear why patients with the non-risk allele showed a significantly higher frequency of right subclavian artery lesions (Fig. 2), but this may have been confounded by the systemically advanced vascular lesions of only patients with the CC genotype.
LILRA3 is the only soluble form of LILR and is not detected on the cell surface7. It is speculated that LILRA3 functions as an antagonist or a negative regulator of other LILRs, but its detailed functions and mechanisms are still not known7,8. The risk allele of LILRA3 rs103294 is associated with decreased expression of LILRA35. Therefore, decreased levels of LILRA3 may contribute to the pathogenesis of TAK. To date, associations between LILRA3 and various autoimmune diseases have been reported. LILRA3 deficiency is a risk factor for multiple sclerosis in German and Spanish populations33,34. Homozygous LILRA3 deletion is associated with Sjögren’s syndrome in a German population35, while functional LILRA3 is a susceptibility factor for Sjögren’s syndrome in a Chinese population36. In addition, functional (non-deleted) LILRA3 is a susceptibility factor for systemic lupus erythematosus and rheumatoid arthritis, especially for anti-citrullinated protein antibody-positive cases36,37. Considering these previous reports, it can be speculated that LILRA3 contributes differently to the pathogenesis of different autoimmune diseases. Further study is necessary to elucidate the pathogenic role of LILRA3 in various autoimmune diseases and the function of LILRA3 per se.
This study has some limitations. First, although the number of TAK patients included is relatively large for a single institution, this study is still limited by the sample size. The small sample size of our study made it difficult to draw a firm conclusion especially on the association of LILRA3 rs103294 with vascular damage, because our study included only one patient with CC genotype of LILRA3 rs103294. Further study with a larger sample size is awaited. Second, this study was based on a retrospective review of patients. Further prospective studies are required to confirm the findings of this study. Third, there might be a bias in the assessment and treatment of TAK, because this study was conducted in a single institution. Fourth, we did not assess disease activity measures such as Birmingham Vasculitis Activity Score and Indian Takayasu Clinical Activity Score. Fifth, since all the patients included in this study were Asian, mostly Japanese, our findings may not be applicable to patients with other ethnic backgrounds. Finally, vasculitis-associated damage measures (i.e. TADS and VDI) and angiographic scores (i.e. ASS, ADS, and ACS) were evaluated at different time points in each patient, because a recent whole-body contrast-enhanced angiography was not available in a substantial proportion of patients. This might have led to the discrepancy between the results on vasculitis-associated damage measures and angiographic scores.
In conclusion, our study showed for the first time that IL12B rs6871626 is associated with vascular damage in TAK, whereas LILRA3 rs103294 was not. IL12B rs6871626 cannot be only a susceptibility marker, but also a marker for disease progression in TAK. Better understanding of the pathogenesis with the aid of genetic studies may enable physicians to fine-tune the treatment strategy for TAK based on the genotype of individual patients.
Methods
Patients
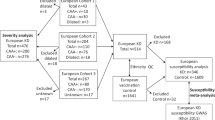
We enrolled 99 TAK patients who were enrolled in our previous genome-wide association study (GWAS)5 and whose genotypes for rs6871626, rs103294, and HLA-B*52 were available. The patients fulfilled either the 1990 American College of Rheumatology classification criteria38 or the diagnostic criteria for the Japan Circulation Society 200839. This was a retrospective cohort study, and patient information in the electronic patient chart was retrospectively reviewed. We obtained approval from the Ethics Committee of Kyoto University Graduate School and the Faculty of Medicine for the study (No. G1006-9). Written informed consent was obtained from all patients. All study procedures were performed in accordance with the principles of the Declaration of Helsinki.
Clinical evaluation
The presence or absence of AR, ischemic heart disease, cerebrovascular events, visual loss, renal replacement therapy, and inflammatory bowel disease were evaluated as organ involvement. The diagnosis of AR was based on transthoracic echocardiography and was used to detect AR. Ischemic heart disease was defined as angina pectoris or myocardial infarction requiring vascular interventions such as coronary artery bypass graft or percutaneous coronary intervention. Cerebrovascular events were included only if they had focal neurological signs such as paresis and weakness. Renal replacement therapy was defined as the condition requiring chronic hemodialysis. Inflammatory bowel disease was diagnosed after scrutiny by gastroenterologists, including endoscopic evaluation and cautious differential diagnosis, to exclude other causes such as drug-induced enterocolitis and infections.
Arterial involvement
Involvement of individual arteries was comprehensively determined using digital subtraction angiography, enhanced computed tomography (CT), magnetic resonance angiography (MRA), ultrasonography, or fluorodeoxyglucose positron emission tomography to differentiate other conditions such as atherosclerosis.
To perform a systemic evaluation of arterial involvement in a qualitative and quantitative manner, we assessed ASS, ADS, and ACS13. These scores were calculated with the last whole-body contrast-enhanced CT angiography or MRA of each patient if available.
Evaluation of vascular damage
Vasculitis-associated damage was evaluated using TADS10 and VDI11. TADS captures TAK-specific vascular damage, whereas VDI is applied to all types of systemic vasculitides9,12. TADS and VDI were measured at the last visit before November 2020.
Treatments
We obtained clinical data regarding the administration of glucocorticoids, immunosuppressants, and biological disease-modifying anti-rheumatic drugs such as infliximab, tocilizumab, and ustekinumab.
Vascular surgery and catheter intervention
The medical history of coronary artery bypass grafting, percutaneous coronary intervention, aortic valve replacement, aortic aneurysm repair, and bypass surgery were retrospectively reviewed.
Genotyping
Illumina Infinium arrays were used for genome scanning, as previously described5. Genotypes of the HLA-B allele were identified by the Luminex microbead method in the NPO HLA laboratory (Kyoto, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation for continuous variables and numbers (%) for categorical variables.
We generally adopted the additive model to analyze the association between genotypes of SNPs and clinical characteristics. To determine the association between genotypes of SNPs and categorical variables, such as the frequency of organ involvement, arterial involvement, and treatment profiles, logistic regression analysis was performed to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) of the risk allele. To determine the correlation between genotypes of SNPs and continuous variables, such as age, TADS, and VDI, linear regression analysis was performed to calculate the effect size (β) of the risk allele.
In addition, we also analyzed the association of IL12B rs6871626 with clinical characteristics using the dominant model. The Mann–Whitney test was used to compare continuous variables, and Fisher’s exact test was used to compare categorical variables. We did not use the dominant model in the analysis of LILRA3 rs103294, because there was only one patient with CC genotype of LILRA3 rs103294.
Statistical analyses were performed using JMP Pro 14.0.0 (SAS Institute, Cary, NC, USA), and p values < 0.05 were considered significant.
Ethics approval and consent to participate
We obtained the approvals of the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine for the study (No. G1006-9). Written informed consent was obtained from all patients. All study procedures were performed in accordance with the principles of the Declaration of Helsinki.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AR:
-
Aortic regurgitation
- CI:
-
Confidence interval
- GWAS:
-
Genome-wide association study
- OR:
-
Odds ratio
- SNP:
-
Single nucleotide polymorphism
- TADS:
-
Takayasu arteritis damage score
- TAK:
-
Takayasu arteritis
- UST:
-
Usutekinumab
- VDI:
-
Vasculitis Damage Index
References
Mason, J. C. Takayasu arteritis—Advances in diagnosis and management. Nat. Rev. Rheumatol. 6(7), 406–415 (2010).
Watanabe, R., Berry, G. J., Liang, D. H., Goronzy, J. J. & Weyand, C. M. Pathogenesis of giant cell arteritis and Takayasu arteritis-similarities and differences. Curr. Rheumatol. Rep. 22(10), 68 (2020).
Terao, C., Yoshifuji, H. & Mimori, T. Recent advances in Takayasu arteritis. Int. J. Rheum. Dis. 17(3), 238–247 (2014).
Terao, C. et al. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. Am. J. Hum. Genet. 93(2), 289–297 (2013).
Terao, C. et al. Genetic determinants and an epistasis of LILRA3 and HLA-B*52 in Takayasu arteritis. Proc. Natl. Acad. Sci. U.S.A. 115(51), 13045–13050 (2018).
Matsumura, T. et al. A novel susceptibility locus for Takayasu arteritis in the IL12B region can be a genetic marker of disease severity. Heart Vessels 31(6), 1016–1019 (2016).
Hirayasu, K. & Arase, H. Functional and genetic diversity of leukocyte immunoglobulin-like receptor and implication for disease associations. J. Hum. Genet. 60(11), 703–708 (2015).
Yoshifuji, H. & Terao, C. Roles of cytotoxic lymphocytes and MIC/LILR families in pathophysiology of Takayasu arteritis. Inflamm. Regen. 40, 9 (2020).
Kaymaz-Tahra, S., Alibaz-Oner, F. & Direskeneli, H. Assessment of damage in Takayasu’s arteritis. Semin. Arthritis Rheum. 50(4), 586–591 (2020).
Goel, R. et al. Childhood-onset Takayasu arteritis—Experience from a tertiary care center in South India. J. Rheumatol. 41(6), 1183–1189 (2014).
Exley, A. R. et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 40(2), 371–380 (1997).
Nakagomi, D. & Jayne, D. Outcome assessment in Takayasu arteritis. Rheumatology (Oxford) 55(7), 1159–1171 (2016).
Tombetti, E. et al. Novel angiographic scores for evaluation of large vessel vasculitis. Sci. Rep. 8(1), 15979 (2018).
Vignali, D. A. & Kuchroo, V. K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 13(8), 722–728 (2012).
Cooper, A. M. & Khader, S. A. IL-12p40: An inherently agonistic cytokine. Trends Immunol. 28(1), 33–38 (2007).
Tripathy, N. K., Chauhan, S. K. & Nityanand, S. Cytokine mRNA repertoire of peripheral blood mononuclear cells in Takayasu’s arteritis. Clin. Exp. Immunol. 138(2), 369–374 (2004).
Verma, D. K., Tripathy, N. K., Verma, N. S. & Tiwari, S. Interleukin 12 in Takayasu’s arteritis: Plasma concentrations and relationship with disease activity. J. Rheumatol. 32(12), 2361–2363 (2005).
Park, M. C., Lee, S. W., Park, Y. B. & Lee, S. K. Serum cytokine profiles and their correlations with disease activity in Takayasu’s arteritis. Rheumatology (Oxford) 45(5), 545–548 (2006).
Tamura, N. et al. Profiles of serum cytokine levels in Takayasu arteritis patients: Potential utility as biomarkers for monitoring disease activity. J. Cardiol. 70(3), 278–285 (2017).
Alibaz-Oner, F., Yentür, S. P., Saruhan-Direskeneli, G. & Direskeneli, H. Serum cytokine profiles in Takayasu’s arteritis: Search for biomarkers. Clin. Exp. Rheumatol. 33(2 Suppl 89), 32–35 (2015).
Saadoun, D. et al. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol. 67(5), 1353–1360 (2015).
Misra, D. P., Chaurasia, S. & Misra, R. Increased circulating Th17 cells, serum IL-17A, and IL-23 in Takayasu arteritis. Autoimmune Dis. 2016, 7841718 (2016).
Nakajima, T. et al. A novel susceptibility locus in the IL12B region is associated with the pathophysiology of Takayasu arteritis through IL-12p40 and IL-12p70 production. Arthritis Res. Ther. 19(1), 197 (2017).
Terao, C. et al. Ustekinumab as a therapeutic option for Takayasu arteritis: From genetic findings to clinical application. Scand. J. Rheumatol. 45(1), 80–82 (2016).
Gon, Y. et al. Long-term outcomes of refractory Takayasu arteritis patients treated with biologics including ustekinumab. Mod. Rheumatol. 31, 1–6 (2020).
Anderson, C. A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43(3), 246–252 (2011).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491(7422), 119–124 (2012).
Cortes, A. et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45(7), 730–738 (2013).
Terao, C. et al. Takayasu arteritis and ulcerative colitis: High rate of co-occurrence and genetic overlap. Arthritis Rheumatol. 67(8), 2226–2232 (2015).
Watanabe, R. et al. Ulcerative colitis is not a rare complication of Takayasu arteritis. Mod. Rheumatol. 24(2), 372–373 (2014).
Sands, B. E. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 381(13), 1201–1214 (2019).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375(20), 1946–1960 (2016).
Koch, S. et al. Association of multiple sclerosis with ILT6 deficiency. Genes Immun. 6(5), 445–447 (2005).
Ordóñez, D. et al. Multiple sclerosis associates with LILRA3 deletion in Spanish patients. Genes Immun. 10(6), 579–585 (2009).
Kabalak, G. et al. Association of immunoglobulin-like transcript 6 deficiency with Sjögren’s syndrome. Arthritis Rheum. 60(10), 2923–2925 (2009).
Du, Y. et al. Impact of the leucocyte immunoglobulin-like receptor A3 (LILRA3) on susceptibility and subphenotypes of systemic lupus erythematosus and Sjögren’s syndrome. Ann. Rheum. Dis. 74(11), 2070–2075 (2015).
Du, Y. et al. Contribution of functional LILRA3, but not nonfunctional LILRA3, to sex bias in susceptibility and severity of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 66(4), 822–830 (2014).
Arend, W. P. et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 33(8), 1129–1134 (1990).
JCS Joint Working Group. Guideline for management of vasculitis syndrome (JCS 2008). Japanese Circulation Society. Circ. J. 75(2), 474–503 (2011).
Funding
This work was supported by JSPS KAKENHI Grant Number 20K17418 and a grant-in-aid of The Cardiovascular Research Fund, Tokyo, Japan to RW.
Author information
Authors and Affiliations
Contributions
K.Kadoba, R.W. and H.Y. designed the study, collected the clinical data, contributed to the data interpretation, and wrote the manuscript. T.I. contributed to the data interpretation, and validated the manuscript. T.N. collected the clinical data, and validated the manuscript. C.T. collected the genomic data, and validated the manuscript. K.Kitagori, S.A., K.M., R.N., M.H., M.T., K.O. and A.M. validated the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The Department of Advanced Medicine for Rheumatic Diseases is supported by Nagahama City, Shiga, Japan, Toyooka City, Hyogo, Japan and five pharmaceutical companies (Mitsubishi Tanabe Pharma Co., Chugai Pharmaceutical Co. Ltd, UCB Japan Co. Ltd, AYUMI Pharmaceutical Co., and Asahi Kasei Pharma Corp.). The above-mentioned pharmaceutical companies were not involved in the study design, data collection and analysis, manuscript writing, and manuscript submission. RW received speaker fees from Mitsubishi Tanabe Pharma, Pfizer, Sanofi, AbbVie, Asahi Kasei, Eisai, Eli Lilly, Bristol-Myers Squibb, and Janssen. KK received research grants from GSK. KMurakami received speaking fees and/or consulting fees from Eisai Co. Ltd, Chugai Pharmaceutical Co. Ltd., Pfizer Inc., Bristol-Myers Squibb, Mitsubishi Tanabe Pharma Corporation, UCB Japan Co. Ltd, Daiichi Sankyo Co. Ltd., and Astellas Pharma Inc. MH received a research grant and/or speaker fees from Bristol-Myers, Eisai, Ely Lilly, Mitsubishi Tanabe Pharma. MT received research grants and/or speaker fees from AbbVie GK, Asahi Kasei Pharma Corporation, Astellas Pharma Inc., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly and Company, Pfizer Inc., UCB Japan Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., and Taisho Pharma Co., Ltd. KO received research grants and/or speaker fees from Abbvie, Actelion, Asahikasei Pharma, Astellas, AYUMI, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, GSK, Janssen, JB, Mitsubishi Tanabe, Nippon Kayaku, Nippon Shinyaku, Novartis, Sanofi, and Takeda. AM received speaking fees and/or research grants from Chugai Pharmaceutical Co. Ltd. HY received lecture fees from Chugai and has been on the advisory board for a clinical trial conducted by Janssen. All other authors have declared no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kadoba, K., Watanabe, R., Iwasaki, T. et al. A susceptibility locus in the IL12B but not LILRA3 region is associated with vascular damage in Takayasu arteritis. Sci Rep 11, 13667 (2021). https://doi.org/10.1038/s41598-021-93213-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93213-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.