Abstract
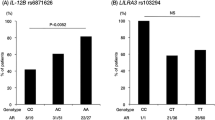
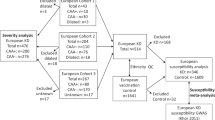
We performed genetic association study for genes encoding angiogenic and angiostatic proteins in patients with Takayasu arteritis (TAK). A total of 96 SNPs involving 60 genes were studied. Genotyping was performed in Fluidigm 96.96 Dynamic Array chip. All statistical analysis for SNP evaluation was performed using PLINK software. Initial analyses revealed five SNPs from three genes [IL-18 (encodes Interleukin-18), FGF2 (encodes Fibroblast Growth Factor-2), and ANGPT1 (encodes Angiopoietin-1)] as significantly different between controls and cases (uncorrected p < 0.05). After permutation-based analysis, two tag SNPs on the promoter region of IL-18 (rs187238 and rs1946518) and one 3’UTR tag SNP (rs1476217) of FGF2 were significantly associated with susceptibility to TAK, with p and OR (95% CI) of 0.0006 and 1.64 (1.25–2.17), 0.03 and 1.28 (1.02–1.64) & 0.016 and 1.33 (1.05–1.67), respectively; while, the two tag SNPs of ANGPT1 gene (rs6469101 and rs16875900) showed a trend (p = 0.055 & p = 0.051, respectively after permutation based correction). There is robust linkage disequilibrium between the two tag SNPs of IL-18 gene as validated by 1000 genome data of South Asian population; the eQTL effects of these tag SNPs of IL-18 and FGF2 genes on adjacent genes further suggest that these tag SNPs act as genetic risks for development of TAK in South Asians, with possible functional implications towards future biomarker development. Genotype phenotype study by genetic model-based analysis also revealed associations between genotype subsets and clinical features like fever, visual loss, left subclavian and coronary artery involvement in our TAK patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Arnaud L, Haroche J, Mathian A, Gorochov G, Amoura Z. Pathogenesis of Takayasu’s arteritis: a 2011 update. Autoimmun Rev. 2011;11:61–7.
Numano F. The story of Takayasu arteritis. Rheumatol Oxf Engl. 2002;41:103–6.
Kaiser M, Younge B, Björnsson J, Goronzy JJ, Weyand CM. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–74.
Weyand CM, Goronzy JJ. Arterial wall injury in giant cell arteritis. Arthritis Rheum. 1999;42:844–53.
Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9:731–40.
Szekanecz Z, Koch AE. Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pr Rheumatol. 2007;3:635–43.
Pulsatelli L, Boiardi L, Assirelli E, Pazzola G, Muratore F, Addimanda O, et al. Imbalance between angiogenic and anti-angiogenic factors in sera from patients with large-vessel vasculitis. Clin Exp Rheumatol. 2020;38:23–30.
Tombetti E, Colombo B, Di Chio MC, Sartorelli S, Papa M, Salerno A, et al. Chromogranin-A production and fragmentation in patients with Takayasu arteritis. Arthritis Res Ther [Internet]. 2016 [cited 2021 Apr 13];18. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4987982/.
Renauer P, Sawalha AH The genetics of Takayasu arteritis. Presse Médicale [Internet]. 2017;26. Available from: http://www.sciencedirect.com/science/article/pii/S0755498217303214.
Charoenwongse P, Kangwanshiratada O, Boonnam R, Hoomsindhu U. The association between the HLA antigens and Takayasu’s arteritis in Thai patients. Int J Cardiol. 1998;66:S117–120.
Kobayashi Y, Numano F. 3. Takayasu arteritis. Intern Med Tokyo Jpn. 2002;41:44–6.
Lee SW, Kwon OJ, Park MC, Oh HB, Park YB, Lee SK. HLA alleles in Korean patients with Takayasu arteritis. Clin Exp Rheumatol. 2007;25:S18–22.
Mehra NK, Jaini R, Balamurugan A, Kanga U, Prabhakaran D, Jain S, et al. Immunogenetic analysis of Takayasu arteritis in Indian patients. Int J Cardiol. 1998;66:S127–132.
Sahin Z, Bıcakcıgil M, Aksu K, Kamali S, Akar S, Onen F, et al. Takayasu’s arteritis is associated with HLA-B*52, but not with HLA-B*51, in Turkey. Arthritis Res Ther. 2012;14:R27.
Kasuya K, Hashimoto Y, Numano F. Left ventricular dysfunction and HLA Bw52 antigen in Takayasu arteritis. Heart Vessels Suppl. 1992;7:116–9.
Ortiz-Fernández L, Saruhan-Direskeneli G, Alibaz-Oner F, Kaymaz-Tahra S, Coit P, Kong X, et al. Identification of susceptibility loci for Takayasu arteritis through a large multi-ancestral genome-wide association study. Am J Hum Genet. 2021;108:84–99.
Terao C, Yoshifuji H, Kimura A, Matsumura T, Ohmura K, Takahashi M, et al. Two Susceptibility Loci to Takayasu Arteritis Reveal a Synergistic Role of the IL12B and HLA-B Regions in a Japanese Population. Am J Hum Genet. 2013;93:289–97.
Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–34.
Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. 1996;54:S155–163.
Goel R, Danda D, Joseph G, Ravindran R, Kumar S, Jayaseelan V, et al. Long-term outcome of 251 patients with Takayasu arteritis on combination immunosuppressant therapy: Single centre experience from a large tertiary care teaching hospital in Southern India. Semin Arthritis Rheum. 2018;47:718–26.
Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–13.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinforma Oxf Engl. 2015;31:3555–7.
Wen D, Zhou XL, Du X, Dong JZ, Ma CS. Association of interleukin-18 gene polymorphisms with Takayasu arteritis in a Chinese Han population. Chin Med J (Engl). 2020;133:2315–20.
Nakanishi K. Unique action of interleukin-18 on T cells and other immune cells. Front Immunol. 2018;9:763.
Kobori T, Hamasaki S, Kitaura A, Yamazaki Y, Nishinaka T, Niwa A, et al. Interleukin-18 amplifies macrophage polarization and morphological alteration, leading to excessive angiogenesis. Front Immunol. 2018;9:334.
Liu W, Tang Q, Jiang H, Ding X, Liu Y, Zhu R, et al. Promoter polymorphism of interleukin-18 in angiographically proven coronary artery disease. Angiology. 2009;60:180–5.
Umare V, Pradhan V, Nath S, Rajadhyaksha A, Ghosh K, Nadkarni A. Impact of functional IL-18 polymorphisms on genetic predisposition and diverse clinical manifestations of the disease in Indian SLE patients. Lupus. 2019;28:545–54.
Correlation Between SNPs at the 3’UTR of the FGF2 Gene and Their Interaction with Environmental Factors in Han Chinese Diabetic Peripheral Neuropathy Patients | SpringerLink [Internet]. [cited 2023 Jan 8]. Available from: https://link.springer.com/article/10.1007/s12031-020-01641-5?utm_source=researcher_app&utm_medium=referral&utm_campaign=RESR_MRKT_Researcher_inbound.
Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82–9.
Cao R, Eriksson A, Kubo H, Alitalo K, Cao Y, Thyberg J. Comparative evaluation of FGF-2–, VEGF-A–, and VEGF-C–induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ Res. 2004;94:664–70.
Fukui S, Kuwahara-Takaki A, Ono N, Sato S, Koga T, Kawashiri SY, et al. Serum levels of fibroblast growth factor-2 distinguish Takayasu arteritis from giant cell arteritis independent of age at diagnosis. Sci Rep. 2019;9:688.
Burgner D, Davila S, Breunis WB, Ng SB, Li Y, Bonnard C, et al. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009;5:e1000319.
Breunis WB, Davila S, Shimizu C, Oharaseki T, Takahashi K, van Houdt M, et al. Disruption of vascular homeostasis in patients with Kawasaki disease: involvement of vascular endothelial growth factor and angiopoietins. Arthritis Rheum. 2012;64:306–15.
Wen X, Chen S, Li P, Li J, Wu Z, Li Y, et al. Single nucleotide polymorphisms of IL12B are associated with Takayasu arteritis in Chinese Han population. Rheumatol Int. 2017;37:547–55.
Danda D, Goel R, Danda S, Mohan H, Joseph G, Kabeerdoss J, et al. Interleukin-17F and interleukin-6 gene polymorphisms in Asian Indian patients with Takayasu arteritis. Hum Immunol. 2017;78:515–20.
Saruhan-Direskeneli G, Biçakçigil M, Yilmaz V, Kamali S, Aksu K, Fresko I, et al. Interleukin (IL)-12, IL-2, and IL-6 gene polymorphisms in Takayasu’s arteritis from Turkey. Hum Immunol. 2006;67:735–40.
Acknowledgements
We thank Ms. Hindumathi Mohan and Babu Raman for collection, storage and processing of blood samples from recruited subjects. Dr. Dolly Daniel, Professor of Transfusion Medicine, Christian Medical College Hospital, Vellore, India for recruitment of healthy donors from Blood Bank.
Funding
This study was funded by the research grant from the Science and Engineering board, Department of Science and Technology to Debashish Danda (SB/SO/HS/170/2013).
Author information
Authors and Affiliations
Contributions
DD for conceptualising the hypothesis, DD, JK, RG and SD were involved in planning and designing of study; DD, RG, JK, AF were involved in data collection and entry; DD, JK, JL, CS, RG, SKN were involved with statistical analysis of genotyping results; and DD, RG, JK, SD and SKN were involved with manuscript writing and critical analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danda, D., Goel, R., Kabeerdoss, J. et al. Angiogenesis related genes in Takayasu Arteritis (TAK): robust association with Tag SNPs of IL-18 and FGF-2 in a South Asian Cohort. J Hum Genet 69, 13–18 (2024). https://doi.org/10.1038/s10038-023-01198-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-023-01198-2