Abstract
Staphylococcus epidermidis (S. epidermidis) ATCC 12228 was incubated with 2% polyethylene glycol (PEG)-8 Laurate to yield electricity which was measured by a voltage difference between electrodes. Production of electron was validated by a Ferrozine assay. The anti-Cutibacterium acnes (C. acnes) activity of electrogenic S. epidermidis was assessed in vitro and in vivo. The voltage change (~ 4.4 mV) reached a peak 60 min after pipetting S. epidermidis plus 2% PEG-8 Laurate onto anodes. The electricity produced by S. epidermidis caused significant growth attenuation and cell lysis of C. acnes. Intradermal injection of C. acnes and S. epidermidis plus PEG-8 Laurate into the mouse ear considerably suppressed the growth of C. acnes. This suppressive effect was noticeably reversed when cyclophilin A of S. epidermidis was inhibited, indicating the essential role of cyclophilin A in electricity production of S. epidermidis against C. acnes. In summary, we demonstrate for the first time that skin S. epidermidis, in the presence of PEG-8 Laurate, can mediate cyclophilin A to elicit an electrical current that has anti-C. acnes effects. Electricity generated by S. epidermidis may confer immediate innate immunity in acne lesions to rein in the overgrowth of C. acnes at the onset of acne vulgaris.
Similar content being viewed by others
Introduction
Microbes in the human skin microbiome play a vital role in modulating both the cutaneous innate immunity and adaptive immunity, while maintaining the skin homeostasis. Dysbiosis of this system may actively influence cutaneous immunity1,2. Inflammatory acne vulgaris is a canonical example of a dysbiotic microbiome, where patients exhibit a striking alteration in bacterial abundance in acne lesions3,4,5. Although it has been reported that the relative abundances of Cutibacterium acnes (C. acnes), formally named as Propionibacterium acnes, were similar in the pilosebaceous units of healthy individuals and acne patients6, overgrowth of certain phylotypes of C. acnes correlates with the development of inflammatory acne vulgaris, which affects a 94–95% of the pubertal population, 20–40% of adults and < 25% of women worldwide7. Our previous studies have demonstrated that Staphylococcus epidermidis (S. epidermidis) can fermentatively metabolize molecules with multiple carbon atoms such as sucrose or polyethylene glycol (PEG)-based polyether to produce short-chain fatty acids (SCFAs)8. Several SCFAs including acetic, butyric, lactic, and succinic acids exert robust antimicrobial activities against the growth of C. acnes9. Carbon-rich carbohydrates and SCFAs, especially acetic acid and butyric acid, have been recognized as redox mediators for electricity production of bacteria10,11,12. Here we investigate whether S. epidermidis can metabolize carbon-rich molecules to yield electricity.
Electricity-producing bacteria in the environment, such as Gram-negative Shewanella, Geobacter and Pseudomonas species, can mediate the formation of biofilms to generate electrons and engage in the process of extracellular electron transfer (EET) to transport electrons from the bacterial cytosol to the extracellular space. This facilitates the cycling of minerals and biomaterials in nature13,14. Several genes encoding multiheme cytochrome C are expressed by Gram-negative bacteria to mediate electricity production15. Gram-positive bacteria express a single protein, called peptide pheromone encoding lipoprotein A (PplA), which contains two flavin molecules enabling electrons to exit the membrane to reach the cell’s exterior13,14,16. The lactic acid bacteria are Gram-positive fermenting bacteria that have electrogenic properties due to the expression quinol oxidase or quinone reductase in the EET17. During the process of flavin-based EET, the type II NADH dehydrogenase (Ndh2) transfers electrons from adenine dinucleotide (NAD) to demethylmenaquinone (DMK) then to flavin mononucleotide (FMN) groups on PplA or free flavin shuttle molecules17. Cytochrome C in Gram-negative bacteria acts as an electron donor or acceptor to shuttle electrons between its reductase and oxidase18. It has been documented that cytochrome C can be sequestered by cyclophilin A, a ubiquitously distributed protein with peptidyl prolyl cis–trans isomerase (PPIase) activity19. The binding of cyclophilin A to peroxiredoxin proteins supports its peroxidase activity as an immediate electron donor20. Both electrogenic Shewanella and Geobacter can produce sufficient amounts of cytochrome C to mediate their electron transfer21. However, electron transfer mediators in Gram-positive bacteria, which do not synthesize soluble cytochrome C, have not been characterized. Cyclophilin A is critical for bacterial growth and biofilm formation22 and may play a role in mediating electron transfer in Gram-positive bacteria.
Although the physiological function of electrons produced by bacteria in humans remains largely unknown, recent studies revealed that human gut bacteria including Listeria monocytogenes, Enterococcus faecalis, Klebsiella pneumonia, Escherichia coli, and many probiotic Lactobacillus species, express the genes encoding proteins responsible for growth promoting EET23,24,25. Previous studies have shown that an electric field generated by insulated electrodes can effectively inhibit the growth of Staphylococcus aureus and Pseudomonas aeruginosa26. This low-energy electron irradiation has been used to inactivate Escherichia coli and viruses due to damage to their nucleic acids27,28 and cell membranes27,29. By using S. epidermidis ATCC 12228, a non-biofilm forming strain, we found that S. epidermidis can utilize a PDG ester of lauric acid containing 50 carbon atoms to yield electricity, demonstrating non-biofilm-mediated electricity production from human bacteria30,31. Both S. epidermidis and C. acnes can be isolated from a single skin lesion in patients with acne vulgaris32. Previously, a genomic basis of antagonism between S. epidermidis and C. acnes has been proposed32. Here we investigate the interference of electrogenic S. epidermidis with C. acnes in vitro and in vivo. Our results demonstrate a novel mechanism by which S. epidermidis inhibits the growth of C. acnes through the production of a weak electrical current, thus identifying a previously unknown process underlying bacterial homeostasis in the skin microbiome.
Methods
Ethics statement
This research was carried out in strict accordance with an approved Institutional Animal Care and Use Committee (IACUC) protocol at National Central University (NCU), Taiwan (NCU-106-016) and in compliance with the Arrive guidelines (https://arriveguidelines.org/).
Bacterial culture
S. epidermidis (ATCC 12,228) was cultured in tryptic soy broth (TSB) (Sigma, St. Louis, MO, USA). C. acnes (ATCC 6919) was cultured on Reinforced Clostridium Medium (RCM, Oxford, Hampshire, England) under anaerobic conditions using a Gas-Pak (BD Biosciences, San Jose, CA, USA). Bacteria were cultured at 37 °C until the logarithmic growth phase. Bacterial pellets were harvested by centrifugation at 5000 × g for 10 min, washed in phosphate-buffered saline (PBS), and then suspended in PBS or TSB for further experiments.
Fermentation of bacteria
S. epidermidis [107 colony-forming unit (CFU)/mL] was incubated in 10 ml rich media (10 g/L yeast extract (Biokar Diagnostics, Beauvais, France), 3 g/L TSB, 2.5 g/L K2HPO4, and 1.5 g/L KH2PO4) with and without 2% (20 g/L) of polyoxyethylene glycol 400 monolaurate designated as PEG-8 Laurate (C28H56O10) by The International Nomenclature of Cosmetic Ingredients (INCI) (Taiwan NJC Corporation, Ltd, Chiayi, Taiwan). In some experiments, S. epidermidis was pretreated with 1 μM TMN 355 (UNI-ONWARD, New Taipei, Taiwan)33, under aerobic conditions at 37 °C with shaking at 200 rpm for 12 h before adding into rich media. The 0.002% (w/v) phenol red (Sigma) in rich media acted as a fermentation indicator. A color change from red–orange to yellow indicated the occurrence of bacterial fermentation which was detected by optical density (OD)560 nm. To determine if PEG-8 Laurate affects the bacterial growth, bacteria (107 CFU) was incubated with 2% PEG-8 Laurate in TSB media for 24 h. Bacteria were diluted 1: 100 −1: 105 into PBS and 10 μl from each dilution was spotted onto a TSB agar plate to count CFU. Plates were incubated at 37 °C for 12 h for S. epidermidis and 72 h for C. acnes to count the colony numbers.
Detection of bacterial electricity
The voltage difference between the electrodes (cathode and anode) was used to detect the bacterial electricity in vitro. A carbon felt (2.5 cm × 10 cm) and a carbon cloth (10 cm × 10 cm) (Homy Tech, Taoyuan, Taiwan) were used as an anode and a cathode, respectively. The carbon cloth was wrapped up in a nafion membrane N117 (6 cm × 6 cm) (Homy Tech), a proton exchange membrane (PEM), and placed in a 10 cm diameter petri dish. Anode and cathode were connected by copper wires, which in turn were bridged to external resistance of 200 Ω. Bacteria (S. epidermidis or C. acnes) cultured overnight to 107 CFU in rich media with/without 2% PEG-8 Laurate (200 μL) were pipetted onto the surface of the anode. In some experiments, S. epidermidis was pretreated with 1 μM TMN 355 for 12 h before pipetting onto the anode. The voltage difference (mV) against time (min) was monitored by a digital multimeter (Lutron, DM-9962SD, Sydney, Australia). The voltage was recorded every 10 s to plot a graph of voltage against time34.
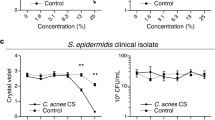
The interference of S. epidermidis with the growth of C. acnes in vitro
C. acnes ATCC 6919 (107 CFU) was incubated with 0.22 µm-filtered supernatants obtained from the culture of S. epidermidis (107 CFU) in the presence or absence of 2% PEG-8 Laurate for 60 or 300 min. After incubation for 1 h, C. acnes was serially diluted and spotted onto a C. acnes selective agar plate to count CFU. In other experiment, rich media containing C. acnes ATCC 6919 (107 CFU) was added into a 10 cm diameter petri dish where a carbon felt, a carbon cloth and PEM were placed. S. epidermidis (107 CFU) pretreated with/without 1 µM TMN 355 in rich media supplemented with/without 2% PEG-8 Laurate was pipetted on the surface of the anode. After that, C. acnes was collected from the petri dish, serially diluted, and spotted onto a selective agar plate containing rich media plus furazolidone (10 µg/mL) to count CFU. Results in our previous publication9 have demonstrated that furazolidone (10 µg/mL) in a selective agar plate completely inhibits the growth of S. epidermidis, but not C. acnes. To measure the membrane permeability35, C. acnes was suspended in 0.5 mM PBS (pH 7.4) containing 10 µg/mL crystal violet (Sigma) followed by centrifugation at 9300 × g for 10 min. After further incubation at 37 °C for 10 min, the suspension was centrifuged at 13,400 × g for 15 min. The OD590 of the supernatant was measured using untreated C. acnes as a blank. The OD590 value of 10 µg/mL crystal violet solution was considered as 100%. The percentage of crystal violet uptake was calculated as follows: (OD590 value of sample/ OD590 value of crystal violet solution) × 100 = Percentage uptake of crystal violet.
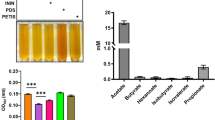
High-performance liquid chromatography (HPLC) analysis
Cultured media of S. epidermidis (107 CFU) with/without 2% PEG-8 Laurate after 60 or 300 min of incubation were centrifuged at 5000 rpm for 10 min. The supernatants were filtered through a 0.22 μm microfiltration membrane and butyric acid in supernatants was detected according to the previous protocol36. The concentrations of butyric acid were quantified based on a calibration curve of a butyric acid analytical standard.
Ferrozine assays
Pellets from S. epidermidis (107 CFU) pretreated with/without 1 μM TMN 355 were suspended in TSB media supplemented with/without 2% PEG-8 Laurate and 4 mM ferrozine (Alfa Aesar Chemicals, Tewsbury, MA, USA). An equal volume of 50 mM ferric ammonium citrate (Sigma) with 100 μL of bacteria in a 96-well plate was incubated for 1 h at 37 °C. Media with S. epidermidis alone or PEG-8 Laurate alone in the presence of ferrozine and ferric ammonium citrate were used as negative controls. The color change of media containing ferrozine and ferric ammonium citrate was measured at OD562 and quantified by a calibration curve16.
S. epidermidis against C. acnes in vivo
Institute of Cancer Research (ICR) female mice (8–9 weeks old) purchased from the National Laboratory Animal Center, Taipei, Taiwan were anesthetized by isoflurane (Sigma). Five mice per group were used in each experiment. The ears of ICR mice were injected intradermally with C. acnes (ATCC 6919) (107 CFU) and S. epidermidis (ATCC 12228) (107 CFU) with/without 2% PEG-8 Laurate using an insulin syringe with 29 G × 1/2 inches (BD Biosciences, San Jose, CA, USA). The mouse ears co-injected with C. acnes and S. epidermidis pretreated in the presence and absence of 1 μM TMN 355 for 12 h were included as controls. After 24 h, ears were excised and homogenized for bacterial counts. Ear homogenates were made by a tissue grinder in 200 μL of sterile PBS. CFUs of C. acnes in ear homogenates were enumerated by plating serial dilutions (1:100–1:105) of homogenates on C. acnes selective agar plates containing rich media and 10 μg/mL of furazolidone (Sigma)37. Plates were incubated for 72 h at 37 °C under anaerobic conditions using a Gas-Pak.
Statistical analysis
Experiments were repeated at least three times to ensure reproducibility. Data are presented as mean values ± standard deviation (SD). Statistical significance was determined using Student’s unpaired two-tailed t-test, as indicated in the legend (*P < 0.05, **P < 0.01, ***P < 0.001 and ns = non-significant).
Results
PEG-8 Laurate induces electricity production by S. epidermidis
Bacteria develop biofilms on microbial fuel cell (MFC) electrodes, enhancing electricity production during the EET process38. The biofilm matrix produced by the bacteria is conductive, which allows electrons to move efficiently to the electrodes. Addition of organic acids such as acetic acid generates electrons by oxidation of organic acids by bacteria. Here we investigate the electrogenicity of S. epidermidis ATCC 12228, a non-biofilm forming skin bacterium, in the presence of 2% PEG-8 Laurate, a carbon-rich PEG ester of lauric acid. S. epidermidis 107 CFU and 2% PEG-8 Laurate in rich media were added onto the surface of the anode. Addition of the same volume of S. epidermidis (107 CFU) alone or 2% PEG-8 Laurate alone acted as controls. As shown in Fig. 1, little or no voltage change was detected in media containing S. epidermidis or PEG-8 Laurate alone throughout the monitoring period of 360 min. However, a marked increase in voltage to 4.4 mV was detected when 2% PEG-8 Laurate was added to media containing S. epidermidis. Voltage production peaked at 60 min, and remained steady for 180 min before declining to a baseline voltage of 0.2 mV at 300 min. This result clearly illustrates the biofilm-independent electrogenic properties of skin S. epidermidis in the presence of PEG-8 Laurate.
PEG-8 Laurate triggered S. epidermidis to produce electricity measured by voltage changes. (a) The surface of anode (a carbon felt) was spotted with S. epidermidis ATCC 12228 (S. epi) (107 CFU) or C. acnes ATCC 6919 (107 CFU). PEM (a nafion membrane) was used to separate the anode and cathode (a carbon cloth) which were connected via copper wires to a conductive resister. The mV was detected by a digital multimeter. (b) Schematic of diagram represented in vitro detection of bacterial electricity. Electrons produced by S. epidermidis in the presence of PEG-8 Laurate on anode were transferred to cathode. (c) The voltage difference (mV) between anode and cathode in the presence of S. epidermidis or C. acnes with or without PEG-8 Laurate was monitored for 360 min. Illustration (a, b) is from own resources.
Electrogenic S. epidermidis impedes the growth of C. acnes
Since both S. epidermidis and C. acnes are bacterial members in an acne microbiome39, we sought to assess if electricity generated by S. epidermidis in the presence of PEG-8 Laurate can alter the growth of C. acnes. The culture media of S. epidermidis plus PEG-8 Laurate which elicited the high and low voltage peaks at 60 and 300 min, respectively (Fig. 1), were added into a culture of C. acnes for 60 min. The incubation of C. acnes with media of S. epidermidis alone was included as a control. As shown in Fig. 2a,b, the number (7.0 ± 0.5 × 107 CFU) of C. acnes incubated with media collected from 60 min after the culture of S. epidermidis plus PEG-8 Laurate was significantly lower than that (11.6 ± 0.8 × 107 CFU) incubated with media collected from in the culture of S. epidermidis alone. Higher C. acnes counts were detected when C. acnes incubated with media collected from 300 min after culture of S. epidermidis in the presence (11.0 ± 0.5 × 107 CFU) or absence (11.3 ± 0.8 × 107 CFU) of PEG-8 Laurate. This result suggests that high electricity in media collected from 60 min after culture of S. epidermidis plus PEG-8 Laurate hindered the growth of C. acnes.
High production of electricity, not butyric acid, contributed to anti-C. acnes effect of S. epidermidis plus PEG-8 Laurate. (a) C. acnes ATCC 6919 (107 CFU) was incubated with media collected from the culture of S. epidermidis (S. epi) in the presence or absence of PEG-8 Laurate for 60 min or 300 min. C. acnes was serially diluted (1: 100 −1: 105) and spotted on a TSB plate for CFU counts. (b) The graph represents the CFU/mL of C. acnes from three independent experiments. (c) Representative chromatography of HPLC analysis of butyric acid in media collected from the culture of S. epidermidis in the presence or absence of PEG-8 Laurate for 60 min or 300 min. (d) The concentration (mM) of butyric acid was quantified based on the heights [milli-absorbance unit (mAU)] of standard (STD) peaks with concentrations of butyric acid from 0–20 mM. Data are represented as mean ± SD). *P < 0.05 (two-tailed t-tests). ns = non-significant.
Our previous studies have demonstrated that S. epidermidis can mediate fermentation to produce SCFAs including butyric acid to suppress the growth of C. acnes40. We next measured the production of butyric acid in the culture media of S. epidermidis with/without PEG-8 Laurate for 60 and 300 min by HPLC. As shown in Fig. 2c,d, the levels of butyric acid in media of S. epidermidis plus PEG-8 Laurate for both 60 and 300 min cultures are higher than those in media of S. epidermidis alone. However, there is no difference in the level (approximately 0.08 mM) of butyric acid in media of the culture of S. epidermidis plus PEG-8 Laurate for either 60 or 300 min. Furthermore, PEG-8 Laurate itself did not affect the growth of both S. epidermidis and C. acnes (Fig. S1). These results suggested that high electricity produced from 60 min-culture of S. epidermidis plus PEG-8 Laurate, neither butyric acid nor PEG-8 Laurate itself, contributed to the suppressive effect of S. epidermidis against C. acnes.
The blockade of electricity production attenuates the suppressive effect of S. epidermidis against C. acnes in vitro
To examine the essential role of electricity produced by S. epidermidis in the growth inhibition of C. acnes, S. epidermidis was pretreated with 1 µM TMN 355, a potent inhibitor of cyclophilin A, for 12 h. To confirm that this treatment prevents electricity generation, S. epidermidis pretreated with/without TMN 355 in the presence or absence of PEG-8 Laurate in rich media was pipetted on the surface of an anode. Electron produced by S. epidermidis plus PEG-8 Laurate can instantly interact with C. acnes which was placed in rich media in a 10 cm diameter petri dish containing anode, cathode and PEM. As shown in Fig. 3a, a high peak of voltage at approximately 1.3 mV was detected when S. epidermidis plus PEG-8 Laurate, not S. epidermidis alone, was pipetted on anode. Blocking cyclophilin A of S. epidermidis significantly reduced the voltage (~ 0.1 mV) induced by PEG-8 Laurate. Furthermore, inhibition of S. epidermidis cyclophilin A by TMN 355 did not influence the activity of PEG-8 Laurate fermentation of S. epidermidis (Fig. S2). This result demonstrates that cyclophilin A is an essential mediator of electricity production in S. epidermidis.
Electrons produced by S. epidermidis plus PEG-8 Laurate caused lysis of C. acnes. (a) The voltage differences (mV) which were measured in conditions of anodes pipetted with media containing S. epidermidis (S. epi) pretreated with/without TMN 355 in the presence or absence of PEG-8 Laurate, and C. acnes were placed in a petri dish containing anode, cathode and PEM. (b) Electrons produced by S. epidermidis plus PEG-8 Laurate were detected using a ferrozine assay. S. epidermidis pretreated with/without TMN 355 was added into TSB media supplemented with ferrozine and ferric ammonium citrate in the presence or absence of PEG-8 Laurate. Addition of PEG-8 Laurate alone into TSB media supplemented with ferrozine and ferric ammonium citrate served as a control. The formation (dark brown) of ferrozine-chelatable irons (mM) was quantified by OD562 measurements. (c) C. acnes in anodes was collected and serially diluted (1 : 100–1 : 105) before spotting on a selective agar plate consisting of rich media plus furazolidone (10 µg/mL). The graph represents the CFU/mL of C. acnes from three separate experiments. (d) The percentage (%) of lysed C. acnes assayed by the uptake of crystal violet was displayed after response of C. acnes in cathodes to anodes pipetted with S. epidermidis or TMN 355-pretreated S. epidermidis in the presence or absence of PEG-8 Laurate. Data are represented as mean ± SD (n = 3 each group). ***P < 0.001, (two-tailed t-tests).
Production of electrons by S. epidermidis is detected by ferrozine assays and induces the lysis of C. acnes
In the presence of electrons, ferric (Fe3+) ammonium citrate is converted to ferrozine-cheltable irons (dark brown) which can be quantified by measurement of OD562. As shown in Fig. 3b, S. epidermidis plus PEG-8 Laurate induced a higher amount of ferrozine-cheltable irons than S. epidermidis alone. Inhibition of cyclophilin A by TMN 355 considerably lowered the amount of ferrozine-cheltable irons induced by S. epidermidis plus PEG-8 Laurate. These data confirmed the production of electrons by S. epidermidis in the presence of PEG-8 Laurate. After pipetting S. epidermidis with/without PEG-8 Laurate on an anode for 360 min, C. acnes in a petri dish containing an anode and a cathode were collected and spotted on a furazolidone-supplemented selective agar plate to determine if electrons influence the growth of C. acnes. Results in Fig. 3c demonstrate that a two log10 reduction in the number (1.1 ± 0.1 × 107 CFU) of C. acnes was detected when an anode was coated with S. epidermidis plus PEG-8 Laurate compared to that (1.2 ± 0.04 × 105 CFU) obtained from an anode coated with S. epidermidis alone. To examine if membranes of C. acnes are compromised by electrons generated by S. epidermidis plus PEG-8 Laurate, C. acnes were collected following the treatment in Fig. 3c and were stained with crystal violet to visualized lysed cells. As shown in Fig. 3d, treatment with S. epidermidis plus PEG-8 Laurate, not S. epidermidis alone, resulted in a noticeable increase in the percentage of lysed C. acnes. These results demonstrate that electrons produced by S. epidermidis plus PEG-8 Laurate can suppress the growth of C. acnes by disruption of membrane integrity. Inhibition of S. epidermidis cyclophilin A by TMN 355 significantly weakened this effect (Fig. 3c,d), illustrating a cyclophilin A-mediated pathway of electricity production in S. epidermidis against C. acnes.
Electron production mediated by cyclophilin A in S. epidermidis attenuates viability of C. acnes in vivo
Integrating all in vitro data above supports the model that electrogenic S. epidermidis suppresses the growth of C. acnes, thus we employed a mouse ear model to investigate the counteraction of S. epidermidis to C. acnes in vivo. Ears of ICR mice were intradermally injected with S. epidermidis and C. acnes in the presence or absence PEG-8 Laurate for 24 h. As shown in Fig. 4a,b, the number (5.7 ± 0.3 × 107 CFU) of C. acnes from mouse ears injected with S. epidermidis and C. acnes in the presence of PEG-8 Laurate was significantly lower than that (55.7 ± 0.9 × 107 CFU) injected with S. epidermidis and C. acnes in the absence of PEG-8 Laurate. This result suggests that PEG-8 Laurate provokes electricity production in S. epidermidis against C. acnes. An extremely low voltage difference (< 0.5 mV) was detected when C. acnes and PEG-8 Laurate were pipetted on an anode (Fig. 1 and Fig. S3). To verify the cyclophilin A-mediated pathway of electricity production in S. epidermidis against C. acnes in vivo, S. epidermidis was pretreated with or without TMN 355 before intradermally injecting into mouse ear with C. acnes and PEG-8 Laurate. The effect of lowering the number of C. acnes by S. epidermidis plus PEG-8 Laurate was significantly reversed when TMN 355-pretreated S. epidermidis was injected into mouse ear (Fig. 4c,d). These data suggest that PEG-8 Laurate-induced electricity mediated by cyclophilin A in S. epidermidis contributes to bacterial interference in vivo.
Cyclophilin A is an essential component for the interference of electrogenic S. epidermidis with C. acnes in vivo. (a) The number of C. acnes in ears of ICR mice after co-injection of C. acnes (107 CFU) and S. epidermidis (S. epi) (107 CFU) in the presence or absence of 2% PEG-8 Laurate was quantified after 72 h. (b) The number of C. acnes in ears of ICR mice after co-injection of C. acnes and S. epidermidis pretreated with/without TMN 355 in the presence of 2% PEG-8 Laurate was calculated after 72 h. The CFUs of C. acnes in mouse ears were enumerated by plating serial dilutions (1 : 100 −1 : 105) of the ear homogenate on furazolidone-supplemented agar plates. Data are represented as mean ± SD, in triplicate from five mice per group. ***P < 0.001 (two-tailed t-tests).
Discussion
Antibiotics without bacterial selectivity for the treatment of acne vulgaris carry a risk of developing antibiotic resistant C. acnes and may result in dysbiosis in the skin human microbiome. Transferring electron from the cytosol to the exterior of the cell via the EET process represents an alternative strategy that may selectively target pathogenic bacteria. Media containing a high content of electricity (> 4 mV) collected from the culture of S. epidermidis plus PEG-8 Laurate for 60 min exerted a marked anti-C. acnes activity (Fig. 2a,b). Although a lower electricity (~ 1.2 mV) was detected when both C. acnes and S. epidermidis plus PEG-8 Laurate were simultaneously present in media (Fig. 3a,c), the anti-C. acnes activity of electrogenic S. epidermidis still remained. Although low electricity of C. acnes in the presence of PEG-8 Laurate was detectable (Fig. 1), electron acceptors on the cell wall of C. acnes may interrupt the intensity of electricity produced by S. epidermidis. It has been reported that electrogenesis can be influenced by different bacteria when they are co-cultured 41. Addition of C. acnes in the presence of PEG-8 Laurate onto the surface of anode created an extremely low voltage difference (< 0.5 mV) which did not affect the growth of S. epidermidis (Fig. S3), supporting that S. epidermidis, not C. acnes, exerted the electrogenicity and antibacterial activity when both bacteria were co-cultured in the presence of PEG-8 Laurate.
It has been reported that membrane-bound electron transport protein entrapped inside an electrochemically active biofilm facilitates electron transfer42. For example, electron transfer in Shewanella oneidensis (S. oneidensis) or many lactic acid bacteria in the human gut occurs directly by the formation of biofilm, as shown to occur on the surface of electrodes through extensions in the form of nano-wires43,44. Interestingly, an indirect electron transfer was also detected in S. oneidensis that involves excreting redox-active mediators such as flavin molecules that act as small diffusible shuttle molecules to transfer electron between electrodes44. However, the formation of biofilm is associated with the production of exopolysaccharides (EPS), quorum sensing signaling molecules and other stress factors such as heavy metal stress45, salinity46, pH47, nutrient starvation48, and pathogen invasion. Moreover, the products from microbial fermentation, such as hydrogen, formate, or acetate as redox mediators, prove an advantage over the electron transfer by the biofilm formation process49,50. A decrease in electron transport observed in S. oneidensis by inhibiting fermentation supports the model that fermentation is likely to be associated with enhancing electron transport51. As shown in Fig. S2, S. epidermidis can utilize PEG-8 Laurate to undergo fermentation which may generate SCFAs as redox mediators for electricity production. We, here, identify S. epidermidis ATCC 12,228 as a non-biofilm producing skin commensal bacteria which may generate electricity through PEG-8 Laurate as an electron donor31,52. The electricity produced by S. epidermidis plus PEG-8 Laurate can be enhanced by addition of FMN (Fig. S4), supporting flavin-based EET in Gram-positive bacteria53.
The addition of S. epidermidis plus PEG-8 Laurate induced a higher voltage (4.4 mV) within 60 min, which remained elevated for ~ 120 min, before declining to baseline by 300 min (Fig. 1). This indicates high electron availability at 60 min, with little remaining 300 min after addition of PEG-8 Laurate into bacterial media. Incubation of C. acnes with media collected at 60 min induced a significantly greater reduction in C. acnes viability relative to the incubation of C. acnes with media collected at 300 min (Fig. 2). There was no statistically significant difference in the content of butyric acid present in media of S. epidermidis plus PEG-8 Laurate at 60 min versus 300 min after culture (Fig. 2c,d). A butyric acid concentration less than 0.1 mM was produced within a 300 min culture of S. epidermidis plus PEG-8 Laurate (Fig. 2c,d). Data from our previous study demonstrated that the minimum bactericidal concentration (MBC) of butyric acid for C. acnes was 10 Mm54, indicating that the amount of butyric acid produced within a 300 min culture of S. epidermidis plus PEG-8 Laurate was not sufficient to kill C. acnes. Results in Fig. 2 indicated that electrons, not butyric acid, in culture media exerted the anti-C. acnes property in vitro. Furthermore, direct delivery of a voltage at 4.4 mV to C. acnes in vitro in the absence of S. epidermidis plus PEG-8 Laurate reduced the growth of C. acnes (Fig. S5). Although the reduction (< one log10) of C. acnes growth by 4.4 mV voltage was less than that (> two log10) by S. epidermidis plus PEG-8 Laureate (Fig. 3c), the results in Fig. S5 clearly demonstrated the anti-a. acnes property of electricity. However, we cannot rule out the possibility that SCFAs and other fermentation metabolites potentiated the effect of the electrons to fully eradicate C. acnes in vivo.
Genome-based antagonism between S. epidermidis and C. acnes highlighted the expression of antimicrobial substances in S. epidermidis against C. acnes55. Succinate in the metabolites of glycerol fermentation of S. epidermidis effectively inhibited the growth of C. acnes in vivo56. The C. acnes phylotype IA1, a high predominance of phylotype in acne lesions, can trigger inflammatory responses including activation of Toll-like receptors (TLRs), secretion of pro-inflammatory cytokines and infiltration of immune cells57,58. Although we do not know whether electricity generated by S. epidermidis can directly activate immune cells to eliminate C. acnes in vivo, it has been reported that electric fields increased the phagocytosis in macrophages59. Inhibition of cyclophilin A in S. epidermidis by 1 µM TMN 355 for 12 h completely abolished the electricity production (Fig. 3a). Intradermal co-injection of C. acnes with TMN 355 pretreated S. epidermidis in the presence of PEG-8 Laurate only partially reduced the suppressive effect of S. epidermidis plus PEG-8 Laurate on the growth of C. acnes in vivo (Fig. 4c,d). Several microbes have been identified in subepidermal compartments of normal skin60. It is worth investigating how S. epidermidis pretreated with or without TMN 355 works together with other skin microbes to fully eliminate C. acnes in vivo. The PEG-8 Laurate fermentation activity of S. epidermidis still remained after pretreatment of S. epidermidis with TMN 355 (Fig. S2), indicating that SCFAs can be produced by TMN 355-preteated S. epidermidis. Both electrons produced by S. epidermidis and SCFA-activated skin immunity54 may be required for complete eradication of C. acnes in vivo. Electron production by PEG-8 Laurate fermentation of S. epidermidis may provide an immediate innate immunity to lyse C. acnes at an early stage of C. acnes overgrowth. We further show that cyclophilin A is a key mediator of electron production of S. epidermidis. Cyclophilin is highly conserved across genera in both prokaryotes and eukaryotes. TMN 355, a potent cyclophilin A inhibitor, can down-regulate the gene expression of cyclophilin A and Ndh2 in S. epidermidis (data not shown). This is not due to toxicity since the inhibition of cyclophilin A by TMN 355 does not influence bacterial fermentation or survival (Figs S1 and S2). This is in line with a previous study that identified a flavin-based EET mechanism in Gram-positive bacteria that utilizes membrane-anchored Ndh2 for electricity production61. Our results demonstrate for the first time that cytoplasmic cyclophilin A in S. epidermidis is an essential component for electricity production.
Low-intensity direct currents or low-frequency alternating electric fields use conductive electrodes to produce free radicals, modify pH or alter exopolysaccharide matrix in bacterial biofilm. The interaction of the electromagnetic field with charged particles present in that matrix causes electron-mediated bacterial cell lysis26. In eukaryotic cells, including cancer cells, cell death through an electric field is associated with plasma membrane permeabilization, cytochrome C release into the cytoplasm, disorientation of the spindle microtubules or internucleosomal DNA fragmentation, resulting in necrotic cell transformation62,63. The increase in lysis of C. acnes (Fig. 3d) is likely to be due to electrolysis activity generated by S. epidermidis in the presence of PEG-8 Laurate. This has been reported to result from several processes including cell wall degradation64, or inactivation or breakdown of membrane proteins involved in cellular respiration glycolysis or cell division such as glycerol-3-phosphate dehydrogenase (GPDH)65 or filamenting temperature-sensitive mutant Z (FtsZ)66,67. These proteins share the similar dipole moments with tubulin in eukaryotes68, which is known to undergo disruption by low electric field. In addition, exposure of bacteria to a high pulsed electric field induces plasma membrane permeabilization and disrupts cell wall integrity, leading to loss of viability64. In the case of Gram-positive bacteria, a high electric pulse can lead to a change in the zeta potential of the surface charge especially at the phosphoryl groups of teichoic acids, causing cell wall degradation69.
In summary, deciphering the electrogenic characteristics of each bacterium leads to a better understanding of complex interplay involved in the human microbiome. Here, we present a novel modality to inhibit the growth of C. acnes using cyclophilin A-mediated electricity generated by skin S. epidermidis in the presence of PEG-8 Laurate and illustrate an electric pathway for drug targeting in acne vulgaris.
References
Grice, E. A. & Segre, J. A. J. N. r. m. The skin microbiome. 9, 244–253 (2011).
Levy, M., Kolodziejczyk, A. A., Thaiss, C. A. & Elinav, E. Dysbiosis and the immune system. Nat Rev Immunol 17, 219–232. https://doi.org/10.1038/nri.2017.7 (2017).
Shi, B. et al. The skin microbiome is different in pediatric versus adult atopic dermatitis. J Allergy Clin Immunol 138, 1233–1236. https://doi.org/10.1016/j.jaci.2016.04.053 (2016).
Gao, Z., Tseng, C. H., Strober, B. E., Pei, Z. & Blaser, M. J. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS ONE 3, https://doi.org/10.1371/journal.pone.0002719 (2008).
Kong, H. H. et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22, 850–859. https://doi.org/10.1101/gr.131029.111 (2012).
Fitz-Gibbon, S. et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. 133, 2152–2160 (2013).
Kanwar, I. et al. Models for acne: A comprehensive study. Drug Discoveries & Therapeutics 12, 329–340. https://doi.org/10.5582/ddt.2018.01079 (2018).
Kao, M. S. et al. Microbiome precision editing: Using PEG as a selective fermentation initiator against methicillin‐resistant Staphylococcus aureus. Biotechnology journal 12 (2017).
Wang, Y. et al. A precision microbiome approach using sucrose for selective augmentation of Staphylococcus epidermidis fermentation against Propionibacterium acnes. Int. J. Mol. Sci. 17, 1870 (2016).
Finke, N., Vandieken, V. & Jørgensen, B. B. Acetate, lactate, propionate, and isobutyrate as electron donors for iron and sulfate reduction in Arctic marine sediments. Svalbard. FEMS Microbiology Ecology 59, 10–22 (2007).
Chen, C., Shen, Y., An, D. & Voordouw, G. Use of acetate, propionate, and butyrate for reduction of nitrate and sulfate and methanogenesis in microcosms and bioreactors simulating an oil reservoir. Appl. Environ. Microbiol. 83, e02983-e12916 (2017).
Sorokin, D. Y., Detkova, E. & Muyzer, G. Propionate and butyrate dependent bacterial sulfate reduction at extremely haloalkaline conditions and description of Desulfobotulus alkaliphilus sp. nov. Extremophiles 14, 71–77 (2010).
Pankratova, G., Hederstedt, L. & Gorton, L. Extracellular electron transfer features of Gram-positive bacteria. Anal. Chim. Acta 1076, 32–47 (2019).
Xayarath, B., Alonzo III, F. & Freitag, N. E. Identification of a peptide-pheromone that enhances Listeria monocytogenes escape from host cell vacuoles. PLoS pathogens 11 (2015).
Matsuda, S., Liu, H., Kato, S., Hashimoto, K. & Nakanishi, S. Negative faradaic resistance in extracellular electron transfer by anode-respiring Geobacter sulfurreducens cells. Environ. Sci. Technol. 45, 10163–10169 (2011).
Pankratova, G., Leech, D. n., Gorton, L. & Hederstedt, L. Extracellular electron transfer by the Gram-positive bacterium Enterococcus faecalis. Biochemistry 57, 4597–4603 (2018).
Light, S. H. et al. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562, 140 (2018).
Carpenter, J. M. et al. Structure and redox properties of the diheme electron carrier cytochrome c4 from Pseudomonas aeruginosa. Journal of inorganic biochemistry 203, 110889 (2020).
Bonfils, C. et al. Cyclophilin A as negative regulator of apoptosis by sequestering cytochrome c. Biochem. Biophys. Res. Commun. 393, 325–330 (2010).
Lee, S. P. et al. Cyclophilin a binds to peroxiredoxins and activates its peroxidase activity. J. Biol. Chem. 276, 29826–29832 (2001).
Lovley, D. R. Electromicrobiology. Annu. Rev. Microbiol. 66, 391–409 (2012).
Roset, M. S., Fernández, L. G., DelVecchio, V. G. & Briones, G. Intracellularly induced cyclophilins play an important role in stress adaptation and virulence of Brucella abortus. Infect. Immun. 81, 521–530 (2013).
Liu, X. et al. Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 103, 1535–1544 (2019).
Wang, W. et al. Bacterial Extracellular Electron Transfer Occurs in Mammalian Gut. Anal. Chem. 91, 12138–12141 (2019).
Kim, M. Y. et al. Metabolic shift of Klebsiella pneumoniae L17 by electrode-based electron transfer using glycerol in a microbial fuel cell. Bioelectrochemistry 125, 1–7 (2019).
Giladi, M. et al. Microbial growth inhibition by alternating electric fields. Antimicrob. Agents Chemother. 52, 3517–3522 (2008).
Fertey, J. et al. Pathogens inactivated by low-energy-electron irradiation maintain antigenic properties and induce protective immune responses. Viruses 8, 319 (2016).
Bayer, L., Fertey, J., Ulbert, S. & Grunwald, T. Immunization with an adjuvanted low-energy electron irradiation inactivated respiratory syncytial virus vaccine shows immunoprotective activity in mice. Vaccine 36, 1561–1569 (2018).
Valle, A. et al. Effects of low electric current (LEC) treatment on pure bacterial cultures. J. Appl. Microbiol. 103, 1376–1385 (2007).
Angelaalincy, M. J. et al. Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. 6, 63 (2018).
Balasubramaniam, A. et al. Repurposing INCI-registered compounds as skin prebiotics for probiotic Staphylococcus epidermidis against UV-B. 10, 1–10 (2020).
Christensen, G. J. et al. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genomics 17, 152 (2016).
Ni, S. et al. Discovering potent small molecule inhibitors of cyclophilin A using de novo drug design approach. J. Med. Chem. 52, 5295–5298 (2009).
Balasubramaniam, A. et al. Skin Bacteria Mediate Glycerol Fermentation to Produce Electricity and Resist UV-B. 8, 1092 (2020).
Halder, S. et al. Alteration of Zeta potential and membrane permeability in bacteria: a study with cationic agents. Springerplus 4, 1–14 (2015).
Keshari, S. et al. Butyric Acid from Probiotic Staphylococcus epidermidis in the Skin Microbiome Down-Regulates the Ultraviolet-Induced Pro-Inflammatory IL-6 Cytokine via Short-Chain Fatty Acid Receptor. Int J Mol Sci 20, https://doi.org/10.3390/ijms20184477 (2019).
Marito, S., Keshari, S. & Huang, C.-M. J. I. J. o. M. S. PEG-8 Laurate Fermentation of Staphylococcus epidermidis Reduces the Required Dose of Clindamycin Against Cutibacterium acnes. 21, 5103 (2020).
Read, S. T., Dutta, P., Bond, P. L., Keller, J. & Rabaey, K. Initial development and structure of biofilms on microbial fuel cell anodes. BMC Microbiol. 10, 98 (2010).
Goodarzi, A., Mozafarpoor, S., Bodaghabadi, M. & Mohamadi, M. The potential of probiotics for treating acne vulgaris: A review of literature on acne and microbiota. Dermatologic Therapy (2020).
Wang, Y. et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 98, 411–424 (2014).
Modestra, J. A. & Mohan, S. V. Bio-electrocatalyzed electron efflux in Gram positive and Gram negative bacteria: an insight into disparity in electron transfer kinetics. RSC Adv. 4, 34045–34055 (2014).
Di Domenico, E. G. et al. Development of electroactive and anaerobic ammonium-oxidizing (anammox) biofilms from digestate in microbial fuel cells. BioMed research international 2015 (2015).
Liu, R., Zhao, Y., Lu, S. & Huang, Q. Electricity generation from lactate using microbial fuel cell and the distribution characteristics of anode microbial community. Wei sheng wu xue bao= Acta microbiologica Sinica 52, 744–752 (2012).
Marsili, E. et al. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105, 3968–3973. https://doi.org/10.1073/pnas.0710525105 (2008).
Chen, B. et al. Role of extracellular polymeric substances from Chlorella vulgaris in the removal of ammonium and orthophosphate under the stress of cadmium. Biores. Technol. 190, 299–306 (2015).
Hong, B. et al. Influence of salinity variations on exocellular polysaccharide production, biofilm formation and flocculation in halotolerant bacteria. J. Environ. Biol. 38, 657 (2017).
Christenson, L. Algal biofilm production and harvesting system for wastewater treatment with biofuels by-products. (2011).
Angelaalincy, M. et al. Enhanced extracellular polysaccharide production and self-sustainable electricity generation for PAMFCs by Scenedesmus sp. SB1. ACS omega 2, 3754–3765 (2017).
Choi, O. & Sang, B.-I. Extracellular electron transfer from cathode to microbes: application for biofuel production. Biotechnol. Biofuels 9, 11. https://doi.org/10.1186/s13068-016-0426-0 (2016).
Rabaey, K. & Rozendal, R. A. Microbial electrosynthesis - revisiting the electrical route for microbial production. Nat Rev Microbiol 8, 706–716. https://doi.org/10.1038/nrmicro2422 (2010).
Pinchuk, G. E. et al. Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation, and fumarate respiration conditions. Appl. Environ. Microbiol. 77, 8234–8240 (2011).
Angelaalincy, M. J. et al. Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. Frontiers in Energy Research 6, 63 (2018).
Pankratova, G., Hederstedt, L. & Gorton, L. J. A. c. a. Extracellular electron transfer features of Gram-positive bacteria. 1076, 32–47 (2019).
Schwarz, A., Bruhs, A. & Schwarz, T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J. Investig. Dermatol. 137, 855–864 (2017).
Christensen, G. J. et al. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. 17, 1–14 (2016).
Wang, Y. et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. 98, 411–424 (2014).
Claudel, J.-P. et al. Staphylococcus epidermidis: a potential new player in the physiopathology of acne? 235, 287–294 (2019).
Kim, J. J. D. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. 211, 193–198 (2005).
Hoare, J. I., Rajnicek, A. M., McCaig, C. D., Barker, R. N. & Wilson, H. M. J. J. o. l. b. Electric fields are novel determinants of human macrophage functions. 99, 1141–1151 (2016).
Nakatsuji, T. et al. The microbiome extends to subepidermal compartments of normal skin. 4, 1–8 (2013).
Pouillot, A., Polla, A. & S Polla, B. Iron and iron chelators: a review on potential effects on skin aging. Current aging science 6, 225–231 (2013).
Pakhomova, O. N., Gregory, B. W., Semenov, I. & Pakhomov, A. G. Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS ONE 8, https://doi.org/10.1371/journal.pone.0070278 (2013).
Kirson, E. D. et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res 64, 3288–3295. https://doi.org/10.1158/0008-5472.can-04-0083 (2004).
Pillet, F., Formosa-Dague, C., Baaziz, H., Dague, E. & Rols, M.-P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 6, 19778. https://doi.org/10.1038/srep19778 (2016).
Yeh, J. I., Chinte, U. & Du, S. Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism. Proc Natl Acad Sci U S A 105, 3280–3285. https://doi.org/10.1073/pnas.0712331105 (2008).
Massey, T. H., Mercogliano, C. P., Yates, J., Sherratt, D. J. & Lowe, J. Double-stranded DNA translocation: structure and mechanism of hexameric FtsK. Mol Cell 23, 457–469. https://doi.org/10.1016/j.molcel.2006.06.019 (2006).
Carballido-Lopez, R. & Errington, J. A dynamic bacterial cytoskeleton. Trends Cell Biol 13, 577–583. https://doi.org/10.1016/j.tcb.2003.09.005 (2003).
Felder, C. E., Prilusky, J., Silman, I. & Sussman, J. L. A server and database for dipole moments of proteins. Nucleic Acids Res 35, W512-521. https://doi.org/10.1093/nar/gkm307 (2007).
Ahimou, F., Paquot, M., Jacques, P., Thonart, P. & Rouxhet, P. G. Influence of electrical properties on the evaluation of the surface hydrophobicity of Bacillus subtilis. J Microbiol Methods 45, 119–126. https://doi.org/10.1016/s0167-7012(01)00240-8 (2001).
Acknowledgements
The authors thank to Li Huiying [Chun Yuan Christian University (CYCU)] for substantial teaching of HPLC and Dr. Hsin Chen (National Tsing Hua University) for establishment of a power supply using ELITE. This work was supported by 106/107/108-Landseed Hospital-NCU joint grants and Ministry of Science and Technology (MOST) Grants 108-2622-B-008-001-CC1; 108-2314-B-008-003-MY3, and 107-2923-B-008-001-MY3.
Author information
Authors and Affiliations
Contributions
S.M., S.K., S.T., DTT.M., A.B., and P.A. conducted experiments and analyzed data. S.K. and CM.H. wrote the manuscript. MF.H. assisted experimental design. CM. H. and D.R.H. review and editing. CM.H. designed experiments and is the guarantor of this work. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marito, S., Keshari, S., Traisaeng, S. et al. Electricity-producing Staphylococcus epidermidis counteracts Cutibacterium acnes. Sci Rep 11, 12001 (2021). https://doi.org/10.1038/s41598-021-91398-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-91398-7
This article is cited by
-
The updates and implications of cutaneous microbiota in acne
Cell & Bioscience (2023)
-
Recent advances in single-cell engineered live biotherapeutic products research for skin repair and disease treatment
npj Biofilms and Microbiomes (2023)
-
Extracellular electrons transferred from honey probiotic Bacillus circulans inhibits inflammatory acne vulgaris
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.