Abstract
Biofilm formation by bacterial pathogens is associated with numerous human diseases and can confer resistance to both antibiotics and host defenses. Many strains of Staphylococcus epidermidis are capable of forming biofilms and are important human pathogens. Since S. epidermidis coexists with abundant Cutibacteria acnes on healthy human skin and does not typically form a biofilm in this environment, we hypothesized that C. acnes may influence biofilm formation of S. epidermidis. Culture supernatants from C. acnes and other species of Cutibacteria inhibited S. epidermidis but did not inhibit biofilms by Pseudomonas aeruginosa or Bacillus subtilis, and inhibited biofilms by S. aureus to a lesser extent. Biofilm inhibitory activity exhibited chemical properties of short chain fatty acids known to be produced from C. acnes. The addition of the pure short chain fatty acids propionic, isobutyric or isovaleric acid to S. epidermidis inhibited biofilm formation and, similarly to C. acnes supernatant, reduced polysaccharide synthesis by S. epidermidis. Both short chain fatty acids and C. acnes culture supernatant also increased sensitivity of S. epidermidis to antibiotic killing under biofilm-forming conditions. These observations suggest the presence of C. acnes in a diverse microbial community with S. epidermidis can be beneficial to the host and demonstrates that short chain fatty acids may be useful to limit formation of a biofilm by S. epidermidis.
Similar content being viewed by others
Introduction
As much as 40–80% of bacteria in the terrestrial environment assemble into biofilms1. These biofilms provide mechanical stability and protection from the extracellular environment and can be composed of a matrix with variable polymeric substances such as polysaccharides, proteins, and extracellular DNA2,3. When bacterial biofilms form on foreign implanted devices, or on chronic wounds, this can result in persistent and recalcitrant infection that is more resistant to antibiotic treatment4. At present, limited options are available to inhibit or disrupt biofilms5. Therefore, there is a need to better understand mechanisms to inhibit biofilm formation and thus develop new strategies to limit their deleterious effects to human health.
Although some reports have detected some biofilm formation on healthy human skin, biofilms are not readily apparent on the skin when it is not damaged or diseased6. This is somewhat surprising as healthy human skin is inhabited by several bacterial genera that could potentially form a biofilm, particularly species belonging to Staphylococcus, Corynebacterium, and Cutibacterium7. Coagulase-negative Staphylococci (CoNS) such as Staphylococcus epidermidis and the facultative anaerobic bacterium Cutibacterium acnes, formerly known as Propionibacterium acnes, are particularly abundant on human skin8. Furthermore, CoNS and C. acnes are present at approximately 100 × density in the 5 × 106 follicles present on an average adult7. We hypothesized that the dense bacterial population in the hair follicle would foster development of a biofilm without additional innate mechanisms in place to inhibit or disrupt biofilm formation.
In this paper, we examined if metabolites produced by C. acnes might limit the capacity of S. epidermidis to form a biofilm. Our observations show that culture supernatant from C. acnes can inhibit biofilm formation by S. epidermidis. We further demonstrate that short chain fatty acids (SCFAs), which are known metabolic products of C. acnes9, will recapitulate the action of C. acnes culture supernatant and can enhance susceptibility to antibiotics. These findings reveal how communication in a diverse bacterial environment can benefit the host.
Results
C. acnes inhibits S. epidermidis biofilm formation
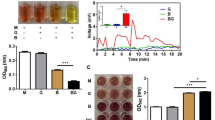
C. acnes and S. epidermidis co-exist on healthy human skin and are each abundant members of the human skin microbiome10. S. epidermidis 1457 is a ST86 strain originally isolated from catheter related bacteremia and can form robust biofilms in culture11,12. To investigate if biofilm formation by S. epidermidis 1457 could be influenced by the presence of C. acnes, we prepared sterile-filtered culture supernatant (CS) from the anaerobic culture of C. acnes ATCC29399 and added this at various concentrations to S. epidermidis 1457. A dose-dependent inhibition of biofilm formation was observed after the addition of C. acnes CS (Fig. 1A). This inhibition of biofilm formation occurred without inhibition of bacterial growth up to a concentration of 25% of CS (Fig. 1B). Biofilm formation by a clinical isolate of S. epidermidis from healthy human skin was also inhibited after exposure to C. acnes CS (Fig. 1C). Inhibition of S. epidermidis 1457 biofilm formation was also observed following the addition of CS from other Cutibacterium species (Fig. 1D).
Cutibacteria inhibited the capacity of S. epidermidis to form a biofilm. (a) Culture supernatant (CS) of C. acnes ATCC29399 inhibited biofilm formation by S. epidermidis s 1457 as seen by crystal violet staining. C. acnes was cultured in reinforced clostridial media (RCM) and fresh RCM was used as control. C. acnes CS or RCM was added to a final concentration of 25% (v/v) during growth of S. epidermidis for 6 or 24 h. (b,c) Dose-dependent inhibition of biofilm formation but not cell growth by C. acnes CS when applied to S. epidermidis 1457 (b) or S. epidermidis clinical isolate (c). (d) CS of several species of Cutibacteria inhibited S. epidermidis 1457 biofilm formation. (e) CS of several strains of C. acnes inhibited S. epidermidis 1457 biofilm formation. Data were expressed as mean ± SEM of a single experiment (n = 6) that was representative of 3 independent experiments. Differences were analyzed using the unpaired Student’s t test (b,c) or one-way ANOVA with Dunnett's test (d,e). Significance was shown as *P < 0.05, **P < 0.01, *** P < 0.001.
C. acnes strains are genetically categorized to several subgroups (IA1, IA2, IB, IC, II, and III). We tested ATCC6919, ATCC29399, 8.CaUCSD, 18.CaUCSD, and 61.CaUCSD (group 1A1); KPA17202 (group 1B); and 35.CaUCSD (group II). Four of those are clinical isolates from acne lesional skin (18.CaUCSD), acne non-lesional skin (8.CaUCSD and 61.CaUCSD), and healthy skin (35CaUCSD). Each of these other strains of C. acnes also inhibited formation of biofilm by S. epidermidis 1457 (Fig. 1E). Importantly, addition of C. acnes CS after the formation of a biofilm by S. epidermidis did not disrupt the pre-existing biofilm (Fig. S1). These observations suggest that a metabolic product or products by C. acnes and related species inhibit biofilm formation by S. epidermidis. On the other hand, biofilm formation by Pseudomonas aeruginosa and Bacillus subtilis were not inhibited by C. acnes CS and biofilm formation by S. aureus was only slightly inhibited (Fig. S2A–S2C).
Chemical properties of the C. acnes metabolites that inhibit S. epidermidis biofilm formation
To identify metabolic products of C. acnes that can inhibit S. epidermidis biofilm formation we examined the chemical properties of CS from C. acnes ATCC29399. To exclude the possibility that the low pH of C. acnes CS was responsible for inhibition of the biofilm, we measured the media pH after the addition of C. acnes CS (Table 1A). 25% C. acnes CS acidified tryptic soy broth (TSB) medium from a pH of 7.2 to a pH of 6.0. However, acidification of TSB medium to a pH of 6.0 by the addition of hydrochloric acid did not inhibit S. epidermidis 1457 biofilm formation or cell growth (Fig. 2A). Thus, media pH reduction by C. acnes was not responsible for inhibition of S. epidermidis biofilm production.
S. epidermidis biofilm formation is not observed at low pH but is increased during fermentation of C. acnes. (a) S. epidermidis 1457 was grown for 6 h in TSB culture medium at the indicated pH range following the addition of hydrogen chloride or sodium hydroxide. Biofilm formation compared to cell growth after the addition of C. acnes ATCC29399 CS or RCM as control were shown. Data are expressed as mean ± SEM of a single experiment (n = 6) that is representative of 3 independent experiments. Differences were analyzed using one-way ANOVA with Dunnett's test. (b) C. acnes ATCC29399 was cultured under anaerobic conditions with the addition of glycerol as a carbon source for fermentation. Sterile media from these cultures was then added at the indicated final concentrations to S. epidermidis 1457 culture. Biofilm formation assayed at 6 h was compared to the results with C. acnes ATCC29399 CS. Data are expressed as mean ± SEM of a single experiment (n = 6) that is representative of 3 independent experiments. Differences were analyzed using the unpaired Student’s t test. Significance was shown as *P < 0.05, **P < 0.01, ***P < 0.01.
Stability analysis of the biofilm inhibitory activity produced by C. acnes further defined the chemical nature of the molecule(s) in the C. acnes CS with activity to inhibit biofilm (Table 1B). The inhibitory activity could not be precipitated from CS by the addition of ammonium sulfate and was resistant to inactivation by digestion with proteinase K or lysozyme. This suggested the bioactive compound(s) were not proteinaceous. Biofilm activity was resistant to heating in a sealed tube at 100 °C for 10 min but was lost when CS was lyophilized. Additionally, the biofilm inhibitory activity was retained after passage through a 500 Da MW filter. These results indicated that the bioactive molecule(s) produced by C. acnes were heat stable and volatile.
C. acnes is a facultative anaerobe that produces short chain fatty acids (SCFAs) when provided a carbon source such as glycerol13. These SCFAs are volatile, heat stable and resistant to proteases and thus matched well with the chemical properties of the biofilm-inhibiting activity in C. acnes CS. To determine if SCFA production by C. acnes correlated with inhibitory activity, we investigated if the addition of glycerol to C. acnes culture media increased the production of SCFAs. Indeed, CS of C. acnes grown in the presence of glycerol has greater potency for biofilm inhibitory activity compared to CS without glycerol supplementation (Fig. 2B). This observation further implied that SCFAs may inhibit S. epidermidis biofilm activity.
SCFAs inhibit biofilm formation by S. epidermidis
SCFAs known to be produced by C. acnes include acetic acid, propionic acid, isobutyric acid, and isovaleric acid13. Therefore, to directly test the hypothesis that SCFAs can inhibit S. epidermidis biofilm, we added these pure SCFAs to S. epidermidis 1457 cultures. Similar to C. acnes CS, SCFAs inhibited biofilm formation at concentrations that did not inhibit cell growth (Fig. 3A). Of note, this inhibition occurred at physiologic concentrations of SCFAs produced by C. acnes on skin13, and was weakest for acetic acid, a SCFA produced by S. epidermidis as a metabolic byproduct. Furthermore, a mixture of SCFAs that mimicked the composition of SCFAs in C. acnes CS (acetic acid, 3.17 mM; propionic acid, 4.59 mM; isobutyric acid, 0.11 mM; isovaleric acid, 2.06 mM) strongly inhibited biofilm formation (Fig. 3B,C). These observations suggested the production of SCFAs by C. acnes inhibits the capacity of S. epidermidis to produce a biofilm.
SCFAs inhibit biofilm formation by S. epidermidis. (a) SCFAs as indicated were added to culture media of S. epidermidis 1457 at concentrations that did not inhibit cell growth. Biofilm formation at 6 h was assayed by crystal violet staining. (b) A mixture of SCFAs simulating the composition measured in C. acnes CS inhibited biofilm formation of S. epidermidis 1457. (c) Representative images of biofilm inhibition by SCFAs were shown. SCFAs or distilled water as a control were added to a final concentration of 25% (v/v) during growth of S. epidermidis for 6 or 24 h. Data were expressed as mean ± SEM of a single experiment (n = 6) that was representative of 3 independent experiments. Differences were analyzed using one-way ANOVA with Dunnett's test. Significance was shown as *P < 0.05, **P < 0.01, *** P < 0.001, **** P < 0.0001.
C. acnes and SCFAs increase capacity of ampicillin and doxycycline to kill S. epidermidis
Since biofilm formation is associated with resistance to killing by antibiotics, we tested whether C. acnes CS would enable antibiotics to kill bacteria grown under conditions that would otherwise lead to formation of a biofilm. S. epidermidis 1457 was cultured with C. acnes CS and with increasing concentrations of ampicillin or doxycycline. After incubation for 6 h, S. epidermidis was killed at lower concentrations of ampicillin or doxycycline when grown with C. acnes CS compared to culture medium that was not conditioned (RCM) as a control (Fig. 4A). The same result was obtained with SCFAs (Fig. 4B). This observation suggested that by inhibiting the biofilm formation, C. acnes or pure SCFAs can increase S. epidermidis susceptibility to antibiotics.
C. acnes and SCFAs increase sensitivity of S. epidermidis to ampicillin and doxycycline in killing (a,b) Minimal inhibitory concentrations of ampicillin and doxycycline were determined in the presence of C. acnes CS (a) or SCFAs (b). A mixture of SCFAs simulated the composition measured in C. acnes CS. S. epidermidis in TSB at 1 × 107 CFU/mL was cultured for 6 h with several concentrations of antibiotics. After the incubation total CFU was counted. Data were expressed as mean ± SEM of a single experiment (n = 3) that was representative of 3 independent experiments. Differences were analyzed using the unpaired Student’s t test. Significance was shown as *P < 0.05, **P < 0.01.
C. acnes and SCFAs inhibit polysaccharide-dependent biofilm formation by S. epidermidis
The process of biofilm formation has at least two distinct phases: initial attachment by surface proteins and biofilm accumulation. The second phase requires cell-to-cell interaction that is mediated by intercellular polysaccharide adhesin (PIA aka PNAG). In the majority of S. epidermidis strains14, including S. epidermidis strain 1457, the production of polysaccharide is important for accumulation of the biofilm.
To determine if C. acnes acts at the phase of attachment or polysaccharide assembly, we stained culture plates during formation of the biofilm with probes to detect total protein, DNA or carbohydrate. At 2 h, protein deposition by S. epidermidis 1457 was unchanged by C. acnes or SCFAs, thus suggesting no effect on initial attachment (Fig. 5A). However, after 6 h, the amount of polysaccharide and DNA was markedly reduced (Fig. 5A). Considering that SYTO 9 stains both intracellular DNA and extracellular DNA, the reduction of SYTO 9 staining can be also interpreted as a reduction of adherent bacteria.
C. acnes CS and SCFAs inhibit assembly of polysaccharide in S. epidermidis biofilms. (a) Protein, DNA, and polysaccharides were detected by fluorescent dyes as indicated. S. epidermidis strain 1457 was cultured on glass plates for indicated time and stained with for each biofilm component and results of fluorometry were summarized. Differences were analyzed one-way ANOVA with Dunnett's test. (b) C. acnes CS was added to a final concentration of 25% (v/v) during growth of S. epidermidis strain 1457 and its Δica mutant strain for 6 or 24 h. The amount of biofilm was detected by crystal violet staining. (c) Dose-dependent inhibition of biofilm formation by C. acnes CS when applied to S. epidermidis 1457 or S. epidermidis 1457 Δica mutant strains. (d) Periodic acid Schiff stain of the biofilm. S. epidermidis strain 1457 and its Δica mutant were cultured for 6 h and then stained. (e) Periodic acid Schiff stain was quantified with absorbance at 550 nm. (f) Gene expressions of aap, icaA, and icaD were assayed by qRT-PCR using the S. epidermidis 1457 biofilm samples treated with C. acnes CS or control for 6 h. Differences were analyzed using the unpaired Student’s t test. Data were expressed as mean ± SEM of a single experiment (n = 6) that was representative of 3 independent experiments. Significance was shown as **P < 0.01, ****P < 0.0001.
In addition, to directly test the effects of C. acnes CS and SCFAs on cell adhesion compared to assembly of the biofilm, we tested the Δica mutant strain of S. epidermidis 1457 which lacks the production of PIA but can still assemble a protein-based biofilm15. As expected, biofilm formation capacity by the S. epidermidis 1457 Δica mutant was less than in the wild-type strain. This biofilm formed by the Δica mutant was not further inhibited by C. acnes CS (Fig. 5B,C), thus demonstrating that the effect of C. acnes does not occur in absence of polysaccharide deposition. To further confirm the inhibition of polysaccharide production, we stained biofilm with periodic acid–Schiff (PAS). PAS is a staining method used to detect polysaccharides such as glycogen, and mucosubstances such as glycoproteins, glycolipids and mucins. As expected, C. acnes CS inhibited the production of PAS-positive substance that was also undetectable in biofilms by S. epidermidis 1457 Δica mutant (Fig. 5D,E). Consistent with this observation, the expression of icaA and icaD, two major genes involved in the synthesis of intercellular adhesin by S. epidermidis16, were significantly decreased in the presence of C. acnes CS. In contrast, the expression of accumulation-associated protein (Aap) was unchanged (Fig. 5F), which is consistent with our observation that the S. epidermidis Δica mutant biofilm is unaffected by C. acnes CS (Fig. 5A,B). Taken together, these results suggest that C. acnes CS may act directly or indirectly to inhibit the synthesis or assembly of polysaccharide in the biofilm, potentially through suppression of icaA and icaD expression.
Discussion
C. acnes is one of the most abundant commensals on human skin7,17. Other commensal skin commensal organisms such as specific strains of CoNS can kill S. aureus18,19 or selectively inhibit the growth of C. acnes20 but limited information has been found to suggest that C. acnes can benefit its host. In contrast, although typically present without deleterious effect, C. acnes can cause infection of implanted medical devices21, and is most frequently thought of due to its involvement in the pathogenesis of acne vulgaris22,23. This study sought to determine if C. acnes could benefit it’s host by influencing the function of S. epidermidis to form a biofilm. We conclude that short chain fatty acids produced by C. acnes will limit biofilm formation by S. epidermidis. This observation may explain in part why highly abundant and dense growth of S. epidermidis in the human hair follicle does not typically result in formation of a biofilm.
To test the capacity of S. epidermidis to form a biofilm, we examined defined laboratory strains of S. epidermidis isolated from infection and clinical isolates obtained from healthy skin. Similarly, we examined multiple strains of C. acnes as well as other related bacterial species relevant to this issue. Our initial reference strain of C. acnes inhibited S. epidermidis isolated from health and disease equally well. Importantly, this occurred at concentrations of C. acnes supernatant that did not inhibit the growth of the opposing species and acted only before the biofilm was formed. Other strains of C. acnes as well as other major members of the Cutibacterium genus also prevented S. epidermidis from forming a biofilm. This suggests the activity produced from C. acnes is likely conserved across the genus. Furthermore, the action against biofilm formation was selective. C. acnes CS strongly inhibited biofilms by S. epidermidis, inhibited biofilms by S. epidermidis to a lesser extent, and did not inhibit biofilms by P. aeruginosa or B. subtilis. Although these findings cannot exclude the potential that some S. epidermidis strains could be resistant, or that some C. acnes may be inactive, our observations support a general conclusion that C. acnes can inhibit S. epidermidis biofilm formation.
To determine the mechanism of biofilm inhibition, we considered the possibility that simple acidification of the environment by the C. acnes CS could be the source of activity. The pH at the surface of the skin is normally acidic, ranging in pH values of 4–624,25. Many bacterial species, including S. epidermidis, also can produce substances that change the pH of the environment26,27, and lower pH has been associated with increased biofilm formation, not a decrease28. Thus, we considered it unlikely that low pH would be the mechanism of inhibition. Analysis of C. acnes culture medium showed a drop in pH from 5.5 to 4.85 after 14 days of anaerobic culture, and 25% mixture of C. acnes CS with TSB had a pH of 6.0. Since acidification of S. epidermidis media from 7.2 to 5.3 did not affect the formation of biofilm in our system, we conclude acidic pH is not a responsible for our observations.
We considered the possibility that C. acnes may produce a specific protein or peptide with the capacity to inhibit biofilm formation. Stability analysis of the biofilm inhibitory activity produced by C. acnes suggested this was likely not the case since the activity was volatile, protease resistant and heat resistant. Since prior reports have shown Cutibacteria can produce SCFAs29 and some SCFAs have similar chemical properties to the observed bioactivity from C. acnes30, we tested if pure SCFAs could have an effect similar to C. acnes CS. These experiments showed direct addition of SCFAs had a similar action to C. acnes CS. Although these results do not rule out the potential that other bioactive C. acnes metabolic products, the totality of our observations strongly support the hypothesis that SCFAs may be at least part of the explanation for how C. acnes acts against S. epidermidis biofilms. Further work to define these as the cause, and understand the mechanism of action against S. epidermidis, is still needed.
Our results indicated that C. acnes CS did not inhibit the growth of S. aureus but prior studies have shown that SCFAs did inhibit S. aureus at concentrations over 250 mM31. The discrepancy with our results may therefore be due to the lower concentrations of pure SCFAs present in C. acnes CS and which we used in these experiments. However, other molecules in the complex C. acnes CS mixture may also influence our observations. For example, some C. acnes phylogroups encode biosynthesis genes for a thiopeptide with possible antimicrobial activity against S. epidermidis, which conversely secretes bacteriocins such as epidermin that kills C. acnes32. The antagonism between S. epidermidis and C. acnes is also noted in acne vulgaris, in which not only SCFAs but many factors like antimicrobial peptides secreted from keratinocytes have an impact33. Considering that S. epidermidis also produces SCFAs, further study of additional, unidentified factors other than SCFAs should be addressed in the future. One example is N-acetylcysteine, which inhibits the growth, adhesion, and biofilm formation of Gram-positive skin bacteria34.
One of the clues to understanding a mechanism of action for C. acnes to inhibit biofilm formation was the observation that pure SCFAs that are produced by C. acnes had a similar effect to C. acnes conditioned medium. SCFAs may have multiple beneficial effects and have been studied in the setting of the intestinal microbiome35 and contribute to the reduction of luminal pH which could inhibit pathogenic microorganisms in gut36. SCFAs also can have direct antimicrobial activity37,38, can increase mucin production39, influence immune responses40 and suppress calcium phosphate-induced itching through activation of IL-6/p-ERK signaling41. In the context of the present study we also observed that higher concentrations of SCFAs can inhibit S. epidermidis survival. Our observations add to this list and suggest that the production of SCFAs may activate host defense, inhibit bacterial survival or act to limit biofilm production. As these effects are dose dependent they will be influenced by the environment since hypoxic conditions within the follicle will favor greater production of SCFAs. Further study is needed to determine if activity observed from C. acnes is solely due to SCFAs, as well as the most relevant functions of SCFA in different specific contexts seen in epithelial biology.
The bacterial biofilm matrix is mainly composed of polysaccharides, proteins, nucleic acids and lipids42. Since S. epidermidis 1457 produces a significant amount of PIA-dependent biofilm, it is considered as an excellent model strain to understand icaADBC transcriptional regulation43. Regulation of biofilm formation may vary depending on the type of biofilm produced as well as the species of organism that produces the biofilm. A previous report suggested C. acnes could induce S. aureus biofilm formation by producing coproporphyrin III44. S. epidermidis was also reported to inhibit S. aureus biofilm formation and nasal colonization45. Our observations did not find lower biofilm formation with S. aureus as we did with S. epidermidis. These vastly different responses from two somewhat similar species of Staphylococci suggest that the mechanisms by which the products of C. acnes act on S. epidermidis are specific. We hypothesize that polysaccharide synthesis or assembly is a primary target for SCFAs and C. acnes CS and we are working to define this mechanism of action. A series of experiments supported this idea. Staining with SYTO 9 showed less staining (intracellular DNA and extracellular DNA) when SCFAs are added, but bacterial growth itself was not inhibited by these concentrations of SCFAs. This suggests that less bacteria were able to adhere and form a biofilm in the presence of SCFA rather than a decrease in DNA synthesis.
While the impact of SCFAs on epithelia is being gradually elucidated, little is known about how SCFAs interact with other microbes on skin. Our data add here a new level of insight and suggest that production of SCFAs by C. acnes is an important mechanism to maintain homeostasis of the microbiome in the cutaneous environment. This may be particularly important in the approximately 5 × 106 follicles present on adult human skin where the density of S. epidermidis is high and hair shafts are present. Such an environment might be expected to foster the frequent development of a biofilm. Despite high density colonization by S. epidermidis, biofilms rarely appear on healthy intact skin. We speculate the observations reported here may be one of the factors that limits biofilm formation and enables homeostasis between S. epidermidis and the host environment. Understanding of mechanisms to maintain the normal balance between humans and commensal microbes may be applicable for development of new strategies to prevent biofilm formation in wounds and medical devices.
Methods
Experimental design
This study was designed to biochemically characterize the activity of C. acnes inhibition of S. epidermis biofilm formation. Pilot experiments were performed to determine the activity. Experimental replicates of at least three (indicated in figure legends) were performed and analyzed to determine statistical significance as defined by P < 0.05. Sample analysis was performed quantitatively in an unblinded manner and confirmed by at least three independent experiments as indicated in the figure legends.
Bacterial culture
Preparation of bacterial cultures was performed as follows. Bacterial stocks frozen at − 80 °C in TSB (Sigma-Aldrich, St. Louis, MO) with 20% glycerol was inoculated into 5 mL of TSB. The culture was aerated by shaking at 120 rpm at 37 °C and grown overnight. Proper concentration of antibiotics was added if bacteria strain contains resistance genes for positive selection.
Crystal violet assay for biofilm formation
S. epidermidis 145712, S. epidermidis clinical isolate, S. aureus USA30046, S. aureus RN422047, P. aeruginosa PAO148, P. aeruginosa P449, and B. subtilis strain ATCC6051 were inoculated into 3% TSB medium, and cultured at 37 °C overnight. Then, the culture was diluted in fresh TSB medium to 1 × 107 CFU/mL by 600-nm optical density. A total of 100 mL of each diluted culture was transferred to flat-bottom 96-well microtiter polystyrene plates (Fisher Scientific, Waltham, MA). The plates were then incubated for 6 h or 24 h at 37 °C without shaking. After 6 h or 24 h of incubation, the supernatants were removed by washing the plates three times using 200 mL of normal saline. Subsequently, 100 mL of 0.01% crystal violet (CV) solution was added to all wells containing completely dry biofilm. After 15 min of dyeing, the excess CV was removed by washing twice with sterile water. Eventually, the fixed CV was released by 33% acetic acid and the absorbance detection at 595 nm was measured50.
Preparation of Cutibacterium culture supernatant
All Cutibacteria species, including all the C. acnes strains, were cultured in RCM media (Sigma-Aldrich, St. Louis, MO), anaerobically for 14 days13. Culture media was then centrifuged for 10 min and this media was then filtered through a 0.22 micron filter (Fisher Scientific, Waltham, MA) to produce culture supernatant (CS). In some experiments ammonium sulfate was added to C. acnes CS, and the solution was centrifuged at 10,000 g for 10 min. At the concentration of 60%, 70%, and 80% (w/v) of ammonium sulfate, precipitate was confirmed. The precipitate was collected and used for further analysis of anti-biofilm activity. C. acnes CS was also tested by lyophilization using SpeedVac Vacuum Concentrators (Thermo Fisher Scientific, Waltham, MA). Volatile portion of a sample was removed by evaporation. For dialysis, C. acnes CS was centrifuged with cellulose membrane (Amicon Ultra Centrifugal Filters; Millipore Sigma, Burlington, MA) to determine the rough molecular weight of the activity. After confirming that the molecular weight was under 3,000 Da, flow-through from the column was set to the dialysis tubes (Float-A-Lyzer Dialysis Devices; Spectrum Chemical Manufacturing, New Brunswick, NJ), and dialyzed in a clean floating water for 24 h. The concentration of SCFAs produced by laboratory strains of C. acnes strain ATCC29399 was measured as previously determined13. Briefly, bacteria were cultured under anaerobic conditions for 14 days. SCFAs concentrations in culture supernatants were measured by gas chromatography–mass spectrometry after ethyl acetate extraction. Concentrations were as follows: acetic acid, 3.17 mM; propionic acid, 4.59 mM; isobutyric acid, 0.11 mM; isovaleric acid, 2.06 mM. All SCFAs were purchased from Sigma-Aldrich (St. Louis, MO).
Colony forming assay
S. epidermidis was inoculated into 3% TSB medium, and cultured at 37 °C overnight. Then, the culture was diluted in fresh TSB with 25% of C. acnes CS or RCM to 1 × 107 CFU/mL by 600-nm optical density. Ampicillin sodium salt (Sigma-Aldrich, St. Louis, MO) or doxycycline hyclate (Sigma-Aldrich, St. Louis, MO) with several final concentrations were also added. A total of 100 μL of each diluted culture were transferred to flat-bottom 96-well microtiter polystyrene plates in which a 5 mm plastic cover slip coupon was put inside. The plates were then incubated for 6 h at 37 °C without shaking. A coverslip was collected from the plates, and we extracted bacteria in biofilm using vortex mixer and sonication51. Colony forming unit was counted on trypticase soy agar plate.
Fluorescent staining of biofilms
Major components of the biofilm (protein, DNA, and polysaccharide) were visualized by fluorescent dyes. Protein was detected with FilmTracer SYPRO Ruby Biofilm Matrix Stain (Thermo Fisher Scientific, Waltham, MA), and observed under microscopy at red channel. DNA was detected with SYTO 9 Green Fluorescent Nucleic Acid Stain (Thermo Fisher Scientific, Waltham, MA), and observed under microscopy at green channel. Note that both intracellular DNA and extracellular DNA are stained with SYTO 9. Polysaccharide was detected with Concanavalin A, Alexa Fluor 350 Conjugate (Thermo Fisher Scientific, Waltham, MA), and observed under microscopy at blue channel. Staining was quantified using a fluorometer. Excitation/emission wavelengths were 450 nm /610 nm for SYPRO Ruby, 480 nm/500 nm for SYTO 9 Green, and 346 nm /442 nm for Concanavalin A, Alexa Fluor 350 Conjugate, respectively.
Periodic acid-Schiff colorimetric assay
Periodic Acid Schiff (PAS) Stain Kit (ab150680; Abcam, Cambridge, MA) was used to detect polysaccharide. The methods to quantify in a microtiter plate format is described elsewhere52. Briefly, after the formation of bacteria, 100 μL of periodic acid was added to the plate and incubated for 30 min. After the washing, 100 μL of Schiff’s reagent was added and incubated for 15 min. Absorbance was measured at 550 nm in a plate reader.
DNA/RNA purification, reverse transcription, and quantitative real-time polymerase chain reaction (qRT-PCR)
Bacterial DNA and RNA were purified using ZymoBIOMICS DNA/RNA Miniprep Kit (Zymo Research, Orange, CA). Total RNA from each sample was reverse-transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Gene expression levels were determined by quantitative real-time reverse transcription PCR using iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA) in triplicates. mRNA levels of target genes were normalized to those of the 16S rRNA gene by the 2−ΔΔCt method. The primer sequences for target genes were as follows: aap, forward 5′-TGATCGGATCTCCATCAACT-3′ and reverse 5′-AAGGTAGCCAAGAGGACGTT-3′; icaA, forward 5′-CTCTTGCAGGAGCAATCAAT-3′ and reverse 5′-AGAGCACGTGGTTCGTACTT-3′; icaD, forward 5′-GAGGCAATATCCAACGGTAA-3′ and reverse 5′-AAATTTCCGTGTTTTCAACATT-3′. The sequences of the universal 16S rRNA primers (V1–V3 region) was as follows: forward 5′-AGTGAAAGACGGTCTTGCTGTC-3′ and reverse 5′-ATTGCGGAAGATTCCCTACTG-3'.
Statistics
Statistical analysis was performed with Prism software (version 6; GraphPad Software). Results are expressed as mean ± SEM. P values less than 0.05 were considered significant.
Data availability
No data sets were generated or analyzed in this study.
References
Flemming, H. C. & Wuertz, S. Bacteria and archaea on earth and their abundance in biofilms. Nat. Rev. Microbiol. 17(4), 247–260 (2019).
Houry, A. et al. Bacterial swimmers that infiltrate and take over the biofilm matrix. Proc. Natl. Acad. Sci. USA 109(32), 13088–13093 (2012).
Schilcher, K. & Horswill, A. R. Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. https://doi.org/10.1128/MMBR.00026-19 (2020).
Lebeaux, D., Ghigo, J. M. & Beloin, C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 78(3), 510–543 (2014).
Bjarnsholt, T., Ciofu, O., Molin, S., Givskov, M. & Høiby, N. Applying insights from biofilm biology to drug development—Can a new approach be developed?. Nat. Rev. Drug Discov. 12(10), 791–808 (2013).
Parsek, M. R. & Singh, P. K. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57, 677–701 (2003).
Gallo, R. L. Human skin is the largest epithelial surface for interaction with microbes. J. Investig. Dermatol. 137(6), 1213–1214 (2017).
Chen, Y. E., Fischbach, M. A. & Belkaid, Y. Skin microbiota–host interactions. Nature 553(7689), 427–436 (2018).
Cogen, A. L., Nizet, V. & Gallo, R. L. Skin microbiota: a source of disease or defence?. Br. J. Dermatol. 158(3), 442–455 (2008).
Sanford, J. A. & Gallo, R. L. Functions of the skin microbiota in health and disease. Semin. Immunol. 25(5), 370–377 (2013).
Mack, D., Siemssen, N. & Laufs, R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 60(5), 2048–2057 (1992).
Galac, M. R. et al. Complete genome sequence of Staphylococcus epidermidis1457. Genome Announc. https://doi.org/10.1128/genomeA.00450-17 (2017).
Sanford, J. A. et al. Inhibition of HDAC8 and HDAC9 by microbial short-chain fatty acids breaks immune tolerance of the epidermis to TLR ligands. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aah4609 (2016).
Dobinsky, S. et al. Glucose-related dissociation between icaADBC transcription and biofilm expression by Staphylococcus epidermidis: evidence for an additional factor required for polysaccharide intercellular adhesin synthesis. J. Bacteriol. 185(9), 2879–2886 (2003).
Schaeffer, C. R. et al. Versatility of biofilm matrix molecules in Staphylococcus epidermidis clinical isolates and importance of polysaccharide intercellular adhesin expression during high shear stress. mSphere https://doi.org/10.1128/msphere.00165-16 (2016).
Arciola, C. R., Baldassarri, L. & Montanaro, L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J. Clin. Microbiol. 39(6), 2151–2156 (2001).
Grice, E. A. et al. Topographical and temporal diversity of the human skin microbiome. Science 324(5931), 1190–1192 (2009).
Nakatsuji, T. et al. Antimicrobials from human skin commensal bacteria protect against. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aah4680 (2017).
Williams, M. R. et al. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aat8329 (2019).
O’Neill, A. M. et al. Identification of a human skin commensal bacterium that selectively kills cutibacterium acnes. J. Investig. Dermatol. https://doi.org/10.1016/j.jid.2019.12.026 (2020).
Gharamti, A. A. & Kanafani, Z. A. Cutibacterium (formerly Propionibacterium) acnes infections associated with implantable devices. Expert Rev. Anti Infect Ther. 15(12), 1083–1094 (2017).
Williams, H. C., Dellavalle, R. P. & Garner, S. Acne vulgaris. Lancet 379(9813), 361–372 (2012).
Ramasamy, S., Barnard, E., Dawson, T. L. & Li, H. The role of the skin microbiota in acne pathophysiology. Br. J. Dermatol. 181(4), 691–699 (2019).
Schmid-Wendtner, M. H. & Korting, H. C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 19(6), 296–302 (2006).
Ali, S. M. & Yosipovitch, G. Skin pH: from basic science to basic skin care. Acta Derm. Venereol. 93(3), 261–267 (2013).
Cotter, P. D. & Hill, C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67(3), 429–453 (2003).
Padan, E., Bibi, E., Ito, M. & Krulwich, T. A. Alkaline pH homeostasis in bacteria: new insights. Biochim. Biophys. Acta 1717(2), 67–88 (2005).
Nostro, A. et al. Effect of alkaline pH on staphylococcal biofilm formation. APMIS 120(9), 733–742 (2012).
Cheung, Y. F., Fung, C. H. & Walsh, C. Stereochemistry of propionyl-coenzyme A and pyruvate carboxylations catalyzed by transcarboxylase. Biochemistry 14(13), 2981–2986 (1975).
Britz, T. J. & Steyn, P. L. Volatile fatty acid production by the dairy and clinical propionibacteria and related coryneforms. Phytophylactica 11(2), 111 (1979).
Jeong, S., Kim, H. Y., Kim, A. R., Yun, C. H. & Han, S. H. Propionate ameliorates Staphylococcus aureus skin infection by attenuating bacterial growth. Front Microbiol. 10, 1363 (2019).
Christensen, G. J. et al. Antagonism between Staphylococcus epidermidis and propionibacterium acnes and its genomic basis. BMC Genom. 17, 152 (2016).
Claudel, J. P. et al. Staphylococcus epidermidis: A potential new player in the physiopathology of acne?. Dermatology 235(4), 287–294 (2019).
Eroshenko, D., Polyudova, T. & Korobov, V. N-acetylcysteine inhibits growth, adhesion and biofilm formation of Gram-positive skin pathogens. Microb. Pathog. 105, 145–152 (2017).
Ríos-Covián, D. et al. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 7, 185 (2016).
Macfarlane, G. T. & Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 95(1), 50–60 (2012).
Selwyn, S. & Ellis, H. Skin bacteria and skin disinfection reconsidered. Br. Med. J. 1(5793), 136–140 (1972).
Huang, C. B., Alimova, Y., Myers, T. M. & Ebersole, J. L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 56(7), 650–654 (2011).
Peng, L., Li, Z. R., Green, R. S., Holzman, I. R. & Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139(9), 1619–1625 (2009).
Donohoe, D. R. et al. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 4(12), 1387–1397 (2014).
Keshari, S. et al. Skin cutibacterium acnes mediates fermentation to suppress the calcium phosphate-induced itching: a butyric acid derivative with potential for uremic pruritus. J. Clin. Med. 9(2), 312 (2020).
Flemming, H. C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8(9), 623–633 (2010).
Mack, D. et al. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178(1), 175–183 (1996).
Wollenberg, M. S. et al. Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio 5(4), e01286-14 (2014).
Iwase, T. et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465(7296), 346–349 (2010).
Diep, B. A. et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367(9512), 731–739 (2006).
Nair, D. et al. Whole-genome sequencing of Staphylococcus aureus strain RN4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J. Bacteriol. 193(9), 2332–2335 (2011).
Stover, C. K. et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406(6799), 959–964 (2000).
Gupta, V., Kumar, G. N. & Buch, A. Colonization by multi-potential Pseudomonas aeruginosa P4 stimulates peanut (Arachis hypogaea L.) growth, defence physiology and root system functioning to benefit the root-rhizobacterial interface. J. Plant Physiol. 248, 153144 (2020).
Rodríguez-Lázaro, D. et al. Characterization of biofilms formed by foodborne methicillin-resistant. Front. Microbiol. 9, 3004 (2018).
Kobayashi, H., Oethinger, M., Tuohy, M. J., Procop, G. W. & Bauer, T. W. Improved detection of biofilm-formative bacteria by vortexing and sonication: a pilot study. Clin. Orthop. Relat. Res. 467(5), 1360–1364 (2009).
Kilcoyne, M., Gerlach, J. Q., Farrell, M. P., Bhavanandan, V. P. & Joshi, L. Periodic acid-Schiff’s reagent assay for carbohydrates in a microtiter plate format. Anal. Biochem. 416(1), 18–26 (2011).
Acknowledgements
RLG and ARH are supported by National Institute of Health Grant R01AI53185. RLG is also supported by R01AR076082, R37AI052453 R01AR069653, R01AR074302, and U01AI52038. K. N. was supported by Grants from Uehara Memorial Foundation, Japan. The authors appreciate support from Gallo lab members including Paul Kotol and Carlos Aguilera for management and assistance.
Author information
Authors and Affiliations
Contributions
Conceptualization: K.N. and R.L.G.; Formal analysis: K.N. Investigation: K.N.; Supervision: A.R.H. and R.L.G.; Writing—original draft: K.N. and R.L.G.; Writing—review and editing: K.N., A.M.O., M.R.W., L.A., T.N., A.R.H. and R.L.G.
Corresponding author
Ethics declarations
Competing interests
R.L.G. is a co-founder, scientific advisor, consultant and has equity in MatriSys Biosciences and is a consultant, receives income and has equity in Sente Inc. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakamura, K., O’Neill, A.M., Williams, M.R. et al. Short chain fatty acids produced by Cutibacterium acnes inhibit biofilm formation by Staphylococcus epidermidis. Sci Rep 10, 21237 (2020). https://doi.org/10.1038/s41598-020-77790-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77790-9
This article is cited by
-
Staphylococcus epidermidis and its dual lifestyle in skin health and infection
Nature Reviews Microbiology (2023)
-
Bacterial Crosstalk via Antimicrobial Peptides on the Human Skin: Therapeutics from a Sustainable Perspective
Journal of Microbiology (2023)
-
Molecular and culture-based assessment of the microbiome in a zebrafish (Danio rerio) housing system during set-up and equilibration
Animal Microbiome (2021)
-
Short-chain fatty acids inhibit the biofilm formation of Streptococcus gordonii through negative regulation of competence-stimulating peptide signaling pathway
Journal of Microbiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.