Abstract
Repurposing existing compounds for new indications may facilitate the discovery of skin prebiotics which have not been well defined. Four compounds that have been registered by the International Nomenclature of Cosmetic Ingredients (INCI) were included to study their abilities to induce the fermentation of Staphylococcus epidermidis (S. epidermidis), a bacterial species abundant in the human skin. Liquid coco-caprylate/caprate (LCC), originally used as an emollient, effectively initiated the fermentation of S. epidermidis ATCC 12228, produced short-chain fatty acids (SCFAs), and provoked robust electricity. Application of LCC plus electrogenic S. epidermidis ATCC 12228 on mouse skin significantly reduced ultraviolet B (UV-B)-induced injuries which were evaluated by the formation of 4-hydroxynonenal (4-HNE), cyclobutane pyrimidine dimers (CPD), and skin lesions. A S. epidermidis S2 isolate with low expressions of genes encoding pyruvate dehydrogenase (pdh), and phosphate acetyltransferase (pta) was found to be poorly electrogenic. The protective action of electrogenic S. epidermidis against UV-B-induced skin injuries was considerably suppressed when mouse skin was applied with LCC in combination with a poorly electrogenic S. epidermidis S2 isolate. Exploring new indication of LCC for promoting S. epidermidis against UV-B provided an example of repurposing INCI-registered compounds as skin prebiotics.
Similar content being viewed by others
Introduction
Drug repurposing or repositioning is an effective approach to rapidly identify novel indications from known compounds1,2. There have been numerous successful cases of repurposed drugs, including sildenafil citrate (Viagra) as a medicine for erectile dysfunction and pulmonary arterial hypertension and raloxifene hydrochloride (Evista) as a treatment for osteoporosis in postmenopausal women3. Repurposing drugs, including Remdesivir4 and Dexamethasone5, to discover potential forms of treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is actively ongoing. Skin diseases affect half of the world's population and novel drugs for treatments of skin diseases are in high demand. The skin is the largest organ in humans and continuously protects the humans from the harmful environment6,7. Over 100 distinct species, which contribute to making up a total of 1 million microbes in the skin microbiome, conquer every square centimeter of human skin6,8. Our previous studies have demonstrated that fermentation of skin probiotic bacteria generated beneficial metabolites, such as short-chain fatty acids (SCFAs), that can attenuate skin disorders9. For example, Staphylococcus epidermidis (S. epidermidis), a common member in the human skin microbiome, can fermentatively metabolize carbon-rich molecules as prebiotics to yield SCFAs against pathogenic Staphylococcus aureus (S. aureus)10.
Prebiotics were originally defined by Gibson and Roberfroid in 1995 as nondigestible food ingredients11. This definition was later revised in12 and13 as “Glucose-based dietary fibers and non-carbohydrate substances including polyunsaturated fatty acid (PUFA) have been used as prebiotics for gut bacteria”. Prebiotics for bacteria in the skin and other human organs are not yet defined. Prebiotics can provide probiotic bacteria as carbon sources to initiate the fermentation and produce SCFAs as acetate and butyrate14. It has been reported that oxidation of acetate or butyrate served as an electron donor to discharge electron to electron acceptors15. The gene-encoding proteins in the extracellular electron transfer (EET) family of homologs are believed to be present in both Gram-negative and Gram-positive bacteria16. Unlike Gram-negative bacteria, Gram-positive bacteria, not having an outer membrane, carry a cell envelope with a textured peptidoglycan layer and teichoic acids that are thought to be poorly electrogenic17. However, a flavin-based EET process has been recently identified in Gram-positive bacteria to produce electricity16 by activation of type II NADH hydrogenase, which can catalyze electron exchange from cytosolic NADH to a quinone derivative such as quinone demethylmenaquinone (DMK)16. Our recent studies demonstrated that S. epidermidis can mediate glycerol as a source to generate electricity and enhanced bacterial resistance to UV-B18.
In this study, we selected four carbon-rich molecules that have been listed on the International Nomenclature of Cosmetic Ingredients (INCI) to examine their prebiotic activities. The liquid coco-caprylate/caprate (LCC) with C8-10 fatty acid connected to C12-C18 fatty alcohols is currently used as an emollient on ultraviolet (UV) filter absorbance19. Isononyl isononanoate (ININ) (C18H36O2) is an ingredient in cosmetics and personal care products as an emollient, texture enhancer, and plasticizer20. Polyethylene glycol (PEG)-150 distearate (PDS) [(C2H4O)n.C36H70O3] is a thickening agent for shampoo products21. PEG-150 pentaerythrityl tetrastearate (PETIS) (C77H148O8) has been used for increasing viscosity of an aqueous agent in cosmetics22. To screen these four carbon-rich molecules for repurposing, we examined their prebiotic activities for induction of fermentation of S. epidermidis. It has been documented that S. epidermidis can mediate fermentation to down-regulate UV-B-induced inflammation in mouse skin9. We thus further explored the mechanism by which repurposing carbon-rich molecules as skin prebiotics influence the skin damage induced by UV-B. UV radiation which provokes free radical formation, making it a primary risk factor for skin cancer23. It has been reported that UV, in particular UV-B, radiation can up-regulate the local neuroendocrine axes, induce the release of hormones to circulation, activate the central hypothalamic–pituitary–adrenal axis, and reset body hemostasis against skin disorders including cancers, aging and autoimmune diseases24. Repurposing may facilitate the discovery of new mechanisms of action for INCI-registered ingredients as skin prebiotics against UV injuries.
Methods
Ethics statement
All animal protocols and experiments have been approved by the National Central University (NCU), Taiwan. Experiments were conducted in accordance with the protocols (NCU-106-016, 19 December 2017) of the Institutional Animal Care and Use Committee (IACUC) of National Central University (NCU). Female ICR mice (8–9 weeks old) were purchased from the National Laboratory Animal Center Taipei, Taiwan. CO2 sedation was used to sacrifice mice in an encased chamber. All human study protocols were approved by Institutional Review Board (IRB) (No. 19-013-B1, 22 May 2019) and Ethics Committee of Landseed International Hospital, Taiwan. The methods followed for skin swab sampling procedure were carried out in accordance with relevant guidelines and regulations of IRB which was approved by Landseed International Hospital, Taiwan. Skin swabs were collected from three healthy subjects and informed consent was obtained from all study participants.
Bacterial fermentation
Staphylococcus epidermidis ATCC 12228 in tryptic soy broth (TSB) (Sigma, St. Louis, MO, USA) was cultured overnight at 37 °C. Bacterial growth was determined at 600 nm wavelength (OD600). The bacterial pellet was collected after centrifugation at 5,000 × g for 10 min, resuspended with 1 × PBS and diluted to 107 CFU/ml before further incubation in rich media [1.5 g/l KH2PO4, 10 g/l yeast extract (Biokar Diagnostics, Beauvais, France), 2.5 g/l K2HPO4, 3 g/l TSB, and 0.002% (w/v) phenol red (Merck, Darmstadt, Germany)] at 37 °C. For fermentation, bacteria in rich media in the presence of 2% LCC, ININ, PDS or PETIS (TNJC corporation, Chiayi, Taiwan) were incubated for 12 h. The color change of phenol red from red to yellow indicated bacterial fermentation, which was quantified by measurement of OD562. Bacteria alone or rich media with or without LCC, ININ, PDS or PETIS served as controls. To examine the effect of LCC on the bacterial growth, S. epidermidis ATCC 12228 [107 colony-forming unit (CFU)/ml)] was incubated with 2% LCC or phosphate buffer saline (PBS) for 12 h at 37 °C. After incubation, bacteria were serially diluted 1: 100–1:105 in a 96 well plate. 10 μl of serially diluted bacteria were dropped on a TSB agar plate for CFU counts.
Electricity detection
Electricity produced by S. epidermidis was detected in vitro using a chamber equipped with cathode and anode. A carbon felt (2.5 cm × 10 cm) and a carbon cloth (10 cm × 10 cm) (Homy Tech, Taoyuan, Taiwan) were utilized to fabricate anode and cathode., respectively. The cathode was wrapped up to a Nafion membrane N117 (6 cm × 6 cm) (Homy Tech), which served as a proton exchange membrane (PEM). Copper wires were used to connect anode and cathode with external resistance (1,000 Ω)18. S. epidermidis in the presence or absence of LCC, ININ, PDS or PETIS was pipetted on the surface of the anode. Electricity was recorded by the changes in voltage (mV) against time (min) using a digital multimeter (Lutron, DM-9962SD, Sydney, Australia). The recorded voltages in every 10 s were used for plotting a graph.
Cyclic voltammetry
The three-electrode autolab potentiostat (PGSTAT 128 N, Metrohm Autolab, Utrecht, Netherland) was used for conducting cyclic voltammetry. The screen-printed carbon electrode (SPCE) (SE-100, Zensor R&D, Taichung, Taiwan) served as a working electrode with a working area of 5 mm. The Ag/AgCl electrode and platinum electrode acted as the reference against applied potential and counter electrode, respectively. All electrodes were purchased from Metrohm Autolab. S. epidermidis (107 CFU/μl) in the presence or absence of 2% of LCC, ININ, PDS or PETIS was drop-coated on the surface of a working electrode. The potential windows were inspected between − 0.8 and 0.2 V at 0.005 V/s. PBS at 7.4 pH was used as an electrolyte. The potentiostat was operated using Autolab Nova 2.0 software (https://metrohm-autolab.com/Products/Echem/Software/Nova.html/; Metrohm Autolab, Utrecht, Netherland).
Extraction of bacterial RNA
RNA was extracted from overnight cultured S. epidermidis ATCC 12228 or a S2 isolate (107 CFU/ml). The cultured bacteria were centrifuged at 5,000 × g for 10 min and the pellet was collected. RNA in bacterial pellet was extracted using a total RNA mini purification kit (Biokit, Miaoli, Taiwan) and quantified by UV spectrophotometry in a Synergy HT multi-mode microplate reader (BioTek Instruments Inc., Highland Park, Winooski, Vermont, USA).
Real-time qPCR (RT-qPCR)
RT q-PCR was used to analyze the expression of genes encoding pyruvate dehydrogenase (pdh), phosphate acetyltransferase (pta) and intracellular adhesion A (ica A) in S. epidermidis ATCC 12228 and S2 isolate. RNA (1 ng) was converted to cDNA using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The cDNA was served as a template in StepOnePlus RT PCR System (Thermo Fisher Scientific, Waltham, MA, USA), which was executed using Power SYBR Green and PCR Master Mix (Thermo Fisher Scientific). The primer-Blast tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi/; Rockville Pike, Bethesda MD, USA) from the National Center for Biotechnology Information (NCBI) was used for designing all primers. Total one step RT-PCR reaction condition was fixed for 40 cycles as follows: 95 °C for 10 min followed by 95 °C for 15 s, 60 °C for 60 s, and 72 °C for 30 s. Gene expression was normalized with the 16S rRNA gene. The cycle threshold (2−ΔΔCt) was implemented to analyze the relative expression of genes. The designed primers for all genes were shown in Table S2.
Gas chromatography-mass spectrometry (GC–MS) analysis
Staphylococcus epidermidis ATCC 12228 (107 CFU/ml) with 2% LCC in rich media was cultured for 24 h and then centrifuged at 5,000 × g for 10 min. Supernatants of bacterial culture were collected and filtered through 0.22 µm filters. The GC–MS protocol for SCFAs detection were obtained following the method25.
UV-B exposure
Mouse hair in the dorsal skin was removed using Nair cream (Church and Dwight, Ewing Township, NJ, USA) one day before experiments started. The dorsal skin of each mouse was exposed to 100 mJ/cm2 UV-B irradiation using a UV lamp (Model EB-280C, Spectronics Corp., Westbury, NY, USA) twice a week, followed by subsequent application of 107 CFU/ml S. epidermidis with or without 2% LCC three times per week for two weeks. The images of mouse skin were captured on day 0, 7 and 14. Skin lysates and sections from dorsal skin (1 cm2) was prepared. Skin sections were stained with hematoxylin and eosin (H&E) and visualized using the Olympus BX63 microscope (Olympus, Tokyo, Japan).
Western blotting
Tissue Protein Extraction Reagent (T-PER) (Thermo Fisher Scientific) was used for preparing the skin lysates. Protein concentrations of skin lysates were measured by a bicinchoninic acid (BCA) assay (Bio-Rad). Skin lysates (30 μg) were loaded to a 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a PVDF membrane (Millipore Sigma, Burlington, MA, USA). The membrane was blocked with 5% (w/v) non-fat milk, and incubated with primary antibodies to cyclobutane pyrimidine dimer (CPD) (1:1,000; Cosmo Bio, Tokyo, Japan), 4-hydroxynonenal (4-HNE) (1:2,000; Abcam, Cambridge, MA, USA), or β-actin (1:5,000; ACE Biolabs, Taoyuan, Taiwan) overnight at 4 °C. The membrane was subsequently incubated with secondary antibodies goat anti-rabbit or anti-mouse IgG (H + L) horseradish peroxidase (HRP) (1:5000; ACE Biolabs) for 1 h. Protein bands in membranes were developed using chemiluminescent detection reagent (Thermo Fisher Scientific) and visualized by an Omega Lum C Imaging System (Gel Co., San Francisco, CA, USA). ImageJ software (https://imagej.nih.gov/ij/index.html/; National Institutes of Health, Bethesda, MD, USA) was employed to quantify the intensities of protein bands.
Statistical analysis
GraphPad Prism 8 (https://www.graphpad.com/; GraphPad Software, La Jolla, CA, USA) software was employed for data analysis by unpaired t-test. The significant difference was considered by P-values observation as follows: P-values of < 0.05 (*), < 0.01 (**), and < 0.001 (***). The mean ± standard deviation (SD) was obtained from at least three separate experiments.
Results
INCI-registered compounds function as skin probiotics for induction of SCFA production and bacterial electricity
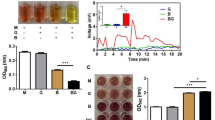
Our previous studies have demonstrated the fermentation and electrogenic activities of S. epidermidis in the presence of glycerol as a carbon source18. To examine if INCI-registered compounds can act as skin prebiotics that provides carbon sources to induce fermentation of skin bacteria, we cultured S. epidermidis ATCC 12228 (107 CFU/ml), a non-biofilm forming skin bacterium, in rich media containing phenol red with 2% each individual INCI-registered compound including LCC, ININ, PDS, and PETIS for 12 h. Media with bacteria alone served as controls. A change in color of phenol red from red to yellow and a significant reduction of the optical density of 562 nm (OD562) due to low pH values26 in the culture media of the S. epidermidis in the presence of 2% each individual INCI-registered compound served as indications of bacterial fermentation. As shown in Fig. 1A, 2% LCC and ININ, compared to PDS and PETIS, induced significant fermentation of S. epidermidis by detection of yellowish media and OD562 reduction. The reduction of OD562 induced by LCC was greater than that by ININ. To validate the occurrence of fermentation, six SCFAs including acetate, butyrate, hexanoate, isobutyrate, isovalerate, and propionate in the media of LCC fermentation were detected by GC–MS analysis (Fig. 1B). A high amount of acetate (> 15 mM) was produced by LCC fermentation of S. epidermidis. SCFAs, especially acetate and butyrate, have been proved as potent electron donors during the EET process in bacteria27,28,29. We thus investigated the electrogenic activity of S. epidermidis in the presence of INCI-registered compounds. Changes in voltages in an in vitro chamber and current values detected by cyclic voltammetry30 were used to monitor bacterial electricity. Compared to media with S. epidermidis alone, media with S. epidermidis plus ININ, PDS or PETIS did not elicit high voltage changes and currents. By contrast, a robust increase in voltage changes with a peak voltage of approximately 6 mV and currents was detected in the media with S. epidermidis plus LCC (Fig. 2). The result provided evidence for repurposing INCI-registered LCC as a prebiotic to provoke the fermentation and electricity production of skin S. epidermidis bacteria.
LCC fermentation of S. epidermidis. (A) S. epidermidis ATCC 12228 (107 CFU/ml; B) was incubated for 12 h in rich media in the presence or absence of 2% LCC, ININ, PDS or PETIS. The prevalence of fermentation was indicated by colour change of phenol red from red to yellow and quantified by OD562. (B) S. epidermidis ATCC 12228 (107 CFU/ml) in the presence of 2% LCC in rich media was cultured for 24 h. The levels (mM) of six SCFAs (acetate, butyrate, hexanoate, isobutyrate, isovalerate and propionate) in fermentation media were quantified. Data are the mean ± SD from three separate experiments. ***P < 0.001 (two-tailed t-test).
Electricity production by LCC fermentation of S. epidermidis. (A) The electricity measured by voltage changes (mV) was recorded for 20 min after pipetting S. epidermidis alone or with LCC (B/LCC), ININ (B/ININ), PDS (B/PDS) or PETIS (B/PETIS) on the surface of an anode. (B) Cyclic voltammetry was employed to measure the current (µA) generated by various experimental conditions above. Data presented the mean ± SD from three separate experiments. ***P < 0.001 (two-tailed t-test).
Topical application of S. epidermidis plus LCC reduced UV-B-induced the formation of 4-HNE and CPD
It has been documented that UV-B-induced free radicals can cause skin hyperplasia31, lipid peroxidation32 and the CPD formation33. We next assessed the influence of fermentation and electricity produced by S. epidermidis plus LCC on the UV-B-induced skin injuries. The recurrent exposure of UV-B significantly fostered the formation of 4-HNE and CPD on mouse skin topically applied with LCC or S. epidermidis alone. In addition, UV-B-induced epidermal hyperplasia, as characterized by an increase in epidermal thickness (Figure S1) and lesions (Fig. 3C), can be detected on mouse skin topically applied with LCC or S. epidermidis alone. However, the UV-B-induced the formation of 4-HNE, CPD, epidermal hyperplasia and lesions were considerably attenuated when mouse skin was topically applied with S. epidermidis plus LCC (Fig. 3, Figure S1). Topical application of PBS or LCC alone without S. epidermidis on mouse skin before UV-B irradiation exhibited the same levels of 4-HNE and CPD as well as skin lesions (Figure S2), indicating that LCC itself was insufficient to impede the UV-B-induced skin injuries. Data above defined a novel function for the repurposed LCC in conjunction with S. epidermidis for suppressing UV-B-induced skin injuries.
Effect of S. epidermidis in the presence of LCC on the UV-B-induced formation of 4-HNE, CPD and lesions. The dorsal skin of ICR mice topically applied with LCC alone, S. epidermidis ATCC 12228 alone (B), or S. epidermidis plus LCC (B/LCC) was irradiated with (+ UV) or without (− UV) UV-B. The images of protein bands of (A) 4-HNE or (B) CPD analyzed by western blot were displayed. The ratio of intensities of protein bands of 4-HNE or CPD normalized to β-actin was shown. (C) Morphologies of mouse skin irradiated with (+ UV) or without (− UV) 100 mJ/cm2 UV-B 0, 7, and 14 days after irradiation were shown. Skin lesions were indicated by white arrows. Data are the mean ± SD from three independent experiments. ***P < 0.001 (two-tailed t-test). ns = non-significant.
The S. epidermidis S2 isolate with low expression of pdh and pta genes was poorly electrogenic
Since LCC effectively induced S. epidermidis to undergo fermentation and yield electricity, we next determined the expression of genes related to fermentation, acetate production, and biofilm formation in S. epidermidis ATCC 12228 and a S. epidermidis S2 strain isolated from human skin. The pdh gene encodes for pyruvate dehydrogenase, which catalyzes pyruvate to acetyl coenzyme A (acetyl-CoA) at the upstream site of the fermentation pathway34. The pta gene encoding for phosphate acetyltransferase is involved in the conversion of acetyl-CoA to acetate35. The icaA gene encoding for intracellular adhesion A has been well characterized in the engagement of the biofilm formation in S. epidermidis36,37. The 16 s rRNA sequence of S. epidermidis S2 isolate shared 99.8% identity to that of S. epidermidis ATCC 12228 (Table S1). However, the expressions of pdh and pta genes in S. epidermidis S2 isolate were much lower than those in S. epidermidis ATCC 12228 (Fig. 4A). Furthermore, the activity of LCC fermentation monitored by OD562 reduction of phenol red-containing rich media for S. epidermidis S2 isolate was relatively low compared to that in S. epidermidis ATCC 12228 (Fig. 4B). The high expression of icaA gene (Fig. 4A) and obvious biofilms (Fig. 4D) were detected in S. epidermidis S2 isolate, but not in the non-biofilm forming bacterial strain, S. epidermidis ATCC 12228. Interestingly, unlike S. epidermidis ATCC 12228, the S. epidermidis S2 isolate that expressed low levels of pdh and pta genes was poorly electrogenic. As shown in Fig. 4C, consistent with Fig. 2, a peak voltage of approximately 6 mV was detected in media with S. epidermidis ATCC 12228 plus LCC whereas little or no voltage change was measured in S. epidermidis S2 isolate plus LCC. Collectively, the pdh and pta genes may participate in fermentation and electricity production of S. epidermidis triggered by LCC.
The gene (pdh, pta, and icaA) expression, electricity and biofilm formation in S. epidermidis ATCC 12228 and S2 isolate. (A) Relative expression of pdh, pta, and icaA genes normalized to 16S rRNA was analyzed by the RT-qPCR. (B) LCC fermentation for 12 h in rich media was quantified by measurement of OD562. (C) Electricity production of bacteria in the presence of 2% LCC was analyzed by voltage changes (mV) in an in vitro chamber. (D) Bacterial biofilms were stained by crystal violet after culture of bacteria in TSB on a 24-well plate for 48 h. B/LCC: S. epidermidis ATCC 12228 plus 2% LCC; S2/LCC: S. epidermidis S2 isolate plus 2% LCC. Scale bars = 1 cm. Data are the mean ± SD from three separate experiments. **P < 0.01; ***P < 0.001 (two-tailed t-test).
LCC plus S. epidermidis S2 isolate did not confer protection against UV-B-induced skin injuries
To confirm the critical roles of pdh and pta genes in LCC fermentation of S. epidermidis against UV-B in vivo, dorsal skin of ICR mice was topically applied with S. epidermidis ATCC 12228 or S2 isolate in the presence of LCC before UV-B irradiation. The levels of 4-HNE and CPD in mouse skin were measured by western blot. In agreement with data in Fig. 3, after UV-B irradiation, the formation of 4-HNE, CPD and skin lesions were detected at low levels and moderate on mouse skin topically applied with S. epidermidis plus LCC. However, when mouse skin was topically applied with S. epidermidis S2 isolate plus LCC (Fig. 5A–C), UV-B induced significantly increasing levels of 4-HNE and CPD as well as skin lesions. Since the expressions of pdh and pta genes in S. epidermidis S2 isolate were much lower than those in S. epidermidis ATCC 12228 (Fig. 4), the expressions of pdh and pta genes may mediate the LCC-triggered promotion of S. epidermidis against skin injuries caused by UV-B.
The UV-B-induced formation of 4-HNE, CPD and lesions in mouse skin applied with LCC in combination with S. epidermidis S2 isolate. The dorsal skin of ICR mice was topically applied with S. epidermidis ATCC 12228 (B/LCC) or S. epidermidis S2 (S2/LCC) in the presence of 2% LCC. The levels of (A) 4-HNE, (B) CPD related to β-actin in western blot analysis and (C) lesions (at 0, 7, and 14 days post-irradiation) on mouse skin irradiated with 100 mJ/cm2 UV-B (+ UV) were shown. Skin lesions were indicated by white arrows. Data are the mean ± SD from three separate experiments. **P < 0.01; ***P < 0.001 (two-tailed t-test).
Discussion
LCC is an ester obtained from the reaction of the coconut alcohol-derived fatty acids with a mixture of caprylic acid and capric acid38. Both caprylic acid and capric acid, as medium-chain fatty acids, can be extracted from coconut oil and have been used as ingredients for skincare formulation to protect against UV radiation39,40. It has been reported that addition of caprylic acid into the fermentation process enhanced acetate production41. Coconut oil has been used as a fuel for electricity production42. Although many medium-chain fatty acids exhibited potent bactericidal activities43, our result in Figure S3 demonstrated that LCC did not change the growth of S. epidermidis. Compared to other INCI-registered compounds (ININ, PDS and PETIS), LCC displayed higher activity in promoting fermentation and electricity production of S. epidermidis (Fig. 1). Biofilms are electroactive and can promote the electricity production of bacteria44. By using S. epidermidis ATCC 12228, a non-biofilm forming strain, we demonstrated that skin bacteria can produce electricity without biofilm formation on electrodes. Data in our recent publication has revealed that addition of glycerol into the S. epidermidis culture can induce fermentation and instantly produce detectable electricity, highlighting a possible mechanism that skin bacteria underwent fermentation to accumulate SCFAs as electron donors to intensify electricity18.
Results in Fig. 1 demonstrated that the electricity measured by changes in voltage and currents was considerably produced when the anode was pipetted with S. epidermidis ATCC 12228 plus 2% LCC. However, the electricity produced by bacteria plus LCC was largely reduced in the S. epidermidis S2 isolate which expressed lower levels of pdh and pta genes (Fig. 3). The data indicated that proteins corresponding to pdh and pta genes may play a role in electricity production of S. epidermidis in the presence of LCC. Electrons are derived by the reduction reaction of NAD+ to NADH in the metabolic pathway of bacterial fermentation45. The conversion of NAD+ to NADH is involved in pyruvate dehydrogenase (pdh), which initiates the process of electron transport chain45,46. Previous studies have shown that electricity production of Shewanella oneidensis MR-1, a representative electrochemically active bacterium (EAB) extensively studied in the laboratory, was mediated by activation of NAD+-linked PDH47. Phosphate acetyltransferase (pta) can catalyze the conversion of acetyl-CoA to acetate, a known electron donor. A pta knockout strain of Shewanella oneidensis strain has been used to study the effect of electricity on bacterial fermentation48. The protective effect of bacterial fermentation on the suppression of the UV-B-induced formation of 4-HNE and CPD was remarkably diminished when mouse skin was topically applied with LCC and S. epidermidis S2 isolate which yielded low electricity and expressed lower levels of pdh and pta genes (Fig. 5). The data suggested that SCFAs and electricity induced by LCC fermentation of S. epidermidis may synergistically provide mice protection from UV-B injuries. Future studies will include the construction of a pdh knockout S. epidermidis strain and investigation of the essential role of pdh gene in S. epidermidis for production of electricity against UV-B injuries.
4-HNE is known to be genotoxic and damages DNA by producing bulky 1,N2-propano-2′-deoxyguanosine adducts49. Here, we showed that 4-HNE and CPD were formed when mouse skin was constantly bombarded with UV-B. Results in our previous studies have demonstrated that butyrate produced by glycerol fermentation of S. epidermidis can down-regulate UV-B-induced pro-inflammatory interleukin (IL)-6 cytokines through SCFA receptor 2 (FFAR2)50. Thus, skin bacteria may take advantage of endogenous glycerol as a carbon source to provoke fermentation and simultaneously produced SCFAs and electrons. Besides being electron donors, SCFAs may regulate FFAR2 and/or histone deacetylases (HDAC)51 to mitigate the inflammation induced by UV irradiation. Electrons may play a key role in neutralizing free radicals generated by UV irradiation. It has been shown that UV light can mediate nitrate, a constituent of sweat in skin, to generate free radicals52, which can subsequently induce lipid peroxidation to produce 4-HNE53. However, commensal bacteria can utilize nitrate as an electron acceptor for energy transduction54. For example, under anoxic or oxygen-depleted conduction, Pseudomonas species can mediate denitrification by using nitrate as an electron acceptor to convert nitrate to nitrogenous gases55. Thus, a possible mechanism behind the protective effect of S. epidermidis plus LCC on UV-B skin injuries is that LCC triggers bacteria to produce electrons, activating bacterial denitrification to reduce UV-B-induced free radicals.
In summary, prebiotics for skin probiotic bacteria have been not defined by the Food and Drug Administration (FAD) even though they may become novel therapeutics for treatments of skin disorders via induction of fermentation of skin bacteria. Our study here demonstrated an approach by repurposing INCI-registered compounds as skin prebiotics and revealed their capabilities of generating electricity from skin bacteria to combat UV-B-induced skin damages.
References
Sleigh, S. H. & Barton, C. L. Repurposing strategies for therapeutics. Pharm. Med. 24, 151–159. https://doi.org/10.1007/BF03256811 (2010).
Ashburn, T. T. & Thor, K. B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discovery 3, 673–683. https://doi.org/10.1038/nrd1468 (2004).
Tartaglia, L. A. Complementary new approaches enable repositioning of failed drug candidates. Expert Opin. Investig. Drugs 15, 1295–1298. https://doi.org/10.1517/13543784.15.11.1295 (2006).
Beigel, J. H. et al. Remdesivir for the Treatment of Covid-19—Preliminary Report. https://doi.org/10.1056/NEJMoa2007764 (2020).
Tomazini, B. M. et al. COVID-19-associated ARDS treated with DEXamethasone (CoDEX): study design and rationale for a randomized trial. Rev. Bras. Terapia Intens. https://doi.org/10.1101/2020.06.24.20139303 (2020).
Zeeuwen, P. L., Kleerebezem, M., Timmerman, H. M. & Schalkwijk, J. Microbiome and skin diseases. Curr. Opin. Allergy Clin. Immunol. 13, 514–520 (2013).
Edmonds-Wilson, S. L., Nurinova, N. I., Zapka, C. A., Fierer, N. & Wilson, M. Review of human hand microbiome research. J. Dermatol. Sci. 80, 3–12. https://doi.org/10.1016/j.jdermsci.2015.07.006 (2015).
Grice, E. A. et al. A diversity profile of the human skin microbiota. Genome Res. 18, 1043–1050. https://doi.org/10.1101/gr.075549.107 (2008).
Keshari, S. et al. Butyric acid from probiotic staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int. J. Mol. Sci. 20, 4477. https://doi.org/10.3390/ijms20184477 (2019).
Kao, M.-S. et al. Microbiome precision editing: using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. https://doi.org/10.1002/biot.201600399 (2016).
Gibson, G. R. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412. https://doi.org/10.1093/jn/125.6.1401 (1995).
Gibson, G. R., Probert, H. M., Loo, J. V., Rastall, R. A. & Roberfroid, M. B. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17, 259–275. https://doi.org/10.1079/nrr200479 (2004).
Gibson, G. R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502. https://doi.org/10.1038/nrgastro.2017.75 (2017).
Wang, Y. et al. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of propionibacterium acnes: implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 98, 411–424. https://doi.org/10.1007/s00253-013-5394-8 (2014).
Shi, L. et al. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 14, 651–662. https://doi.org/10.1038/nrmicro.2016.93 (2016).
Light, S. H. et al. A flavin-based extracellular electron transfer mechanism in diverse gram-positive bacteria. Nature 562, 140–144 (2018).
Matsuda, S., Liu, H., Kato, S., Hashimoto, K. & Nakanishi, S. Negative faradaic resistance in extracellular electron transfer by anode-respiring Geobacter sulfurreducens cells. Environ. Sci. Technol. 45, 10163–10169. https://doi.org/10.1021/es200834b (2011).
Balasubramaniam, A. et al. Skin bacteria mediate glycerol fermentation to produce electricity and resist UV-B. Microorganisms 8, 1092 (2020).
Sohn, M. et al. Effect of emollients on UV filter absorbance and sunscreen efficiency. J. Photochem. Photobiol. B Biol. 205, 111818 (2020).
Subongkot, T., Sirirak, T. J. C. & Biointerfaces, S. B. Development and skin penetration pathway evaluation of microemulsions for enhancing the dermal delivery of celecoxib. 111103 (2020).
Penfield, K. in AIP Conference Proceedings. 899–901 (American Institute of Physics).
Johnson, W. Jr. et al. Safety assessment of PEG-150 pentaerythrityl tetrastearate as used in cosmetics. Int. J. Toxicol. 37, 5s–9s. https://doi.org/10.1177/1091581818794457 (2018).
Jurkiewicz, B. A. & Buettner, G. R. Ultraviolet light-induced free radical formation in skin: an electron paramagnetic resonance study. Photochem. Photobiol. 59, 1–4. https://doi.org/10.1111/j.1751-1097.1994.tb04993.x (1994).
Slominski, A. T., Zmijewski, M. A., Plonka, P. M., Szaflarski, J. P. & Paus, R. How UV light touches the brain and endocrine system through skin, and why. Endocrinology 159, 1992–2007. https://doi.org/10.1210/en.2017-03230%JEndocrinology (2018).
Kao, M.-S. et al. Microbiome precision editing: using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol. J. https://doi.org/10.1002/biot.201600399 (2017).
Kao, M. S. et al. Microbiome precision editing: using PEG as a selective fermentation initiator against methicillin-resistant Staphylococcus aureus. Biotechnol J. https://doi.org/10.1002/biot.201600399 (2017).
Finke, N., Vandieken, V. & Jorgensen, B. B. Acetate, lactate, propionate, and isobutyrate as electron donors for iron and sulfate reduction in Arctic marine sediments, Svalbard. FEMS Microbiol. Ecol. 59, 10–22. https://doi.org/10.1111/j.1574-6941.2006.00214.x (2007).
Chen, C., Shen, Y., An, D. & Voordouw, G. Use of acetate, propionate, and butyrate for reduction of nitrate and sulfate and methanogenesis in microcosms and bioreactors simulating an oil reservoir. Appl Environ Microbiol. https://doi.org/10.1128/aem.02983-16 (2017).
Sorokin, D., Detkova, E. & Muyzer, G. Propionate butyrate dependent bacterial sulfate reduction at extremely haloalkaline conditions description of Desulfobotulus alkaliphilus sp. nov. extremophiles. Extremophiles Life Under Extreme Conditions 14, 71–77. https://doi.org/10.1007/s00792-009-0288-5 (2009).
Kumar, S. et al. Improved levulinic acid production from agri-residue biomass in biphasic solvent system through synergistic catalytic effect of acid and products. Bioresour. Technol. 251, 143–150. https://doi.org/10.1016/j.biortech.2017.12.033 (2018).
Tyrrell, R. M. Ultraviolet radiation and free radical damage to skin. Biochem. Soc. Symp. 61, 47–53. https://doi.org/10.1042/bss0610047%JBiochemicalSocietySymposia (1995).
Halliday, G. M. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat. Res. 571, 107–120. https://doi.org/10.1016/j.mrfmmm.2004.09.013 (2005).
Bowden, G. T. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer 4, 23–35. https://doi.org/10.1038/nrc1253 (2004).
Zheng, P., Wereath, K., Sun, J., van den Heuvel, J. & Zeng, A.-P. Overexpression of genes of the dha regulon and its effects on cell growth, glycerol fermentation to 1, 3-propanediol and plasmid stability in Klebsiella pneumoniae. Process Biochem. 41, 2160–2169 (2006).
Wolin, M. J. Interactions between H2-producing and methane-producing species. Microbial formation and utilization of gases, 141–150 (1976).
Namvar, A. E., Asghari, B., Ezzatifar, F., Azizi, G. & Lari, A. R. Detection of the intercellular adhesion gene cluster (ICA) in clinical Staphylococcus aureus isolates. GMS Hyg. Infect. Control 8, Doc03–Doc03. https://doi.org/10.3205/dgkh000203 (2013).
Kıvanç, S. A., Arık, G., Akova-Budak, B. & Kıvanç, M. Biofilm forming capacity and antibiotic susceptibility of Staphylococcus spp. with the icaA/icaD/bap genotype isolated from ocular surface of patients with diabetes. Malawi Med J 30, 243–249. https://doi.org/10.4314/mmj.v30i4.6 (2018).
Fiume, M. M. et al. Safety assessment of alkyl esters as used in cosmetics. Int. J. Toxicol. 34, 5S-69S (2015).
Fonseca, B. L. et al. Neuroprotective effects of a new skin care formulation following ultraviolet exposure. Cell Prolif. 45, 48–52. https://doi.org/10.1111/j.1365-2184.2011.00795.x (2012).
Bacqueville, D. et al. Efficacy of a dermocosmetic serum combining bakuchiol and vanilla tahitensis extract to prevent skin photoaging in vitro and to improve clinical outcomes for naturally aged skin. Clin. Cosmet. Investig. Dermatol. 13, 359–370. https://doi.org/10.2147/CCID.S235880 (2020).
Abel, H., Immig, I. & Harman, E. Effect of adding caprylic and capric acid to grass on fermentation characteristics during ensiling and in the artificial rumen system RUSITEC. Anim. Feed Sci. Technol. 99, 65–72. https://doi.org/10.1016/S0377-8401(02)00084-6 (2002).
Włodarczyk, P., Włodarczyk, B. & Kalinichenko, A. Direct electricity production from coconut oil - the electrooxidation of coconut oil in an acid electrolyte. E3S Web Conf. 45, 00103. https://doi.org/10.1051/e3sconf/20184500103 (2018).
Liu, S.-T., Sugimoto, T., Azakami, H. & Kato, A. Lipophilization of lysozyme by short and middle chain fatty acids. J. Agric. Food Chem. https://doi.org/10.1021/jf9904822 (2000).
Reguera, G. Microbial nanowires and electroactive biofilms. FEMS Microbiol. Ecol. https://doi.org/10.1093/femsec/fiy086 (2018).
Cahoon, L. A. & Freitag, N. E. The electrifying energy of gut microbes. Nature 562, 43–44. https://doi.org/10.1038/d41586-018-06180-z (2018).
Serwańska-Leja, K., Czaczyk, K. & Myszka, K. Biotechnological synthesis of 1,3-propanediol using Clostridium ssp. Afr. J. Biotech. 10, 11093–11101. https://doi.org/10.5897/AJB11.873 (2011).
Madsen, C. S. & TerAvest, M. A. NADH dehydrogenases Nuo and Nqr1 contribute to extracellular electron transfer by Shewanella oneidensis MR-1 in bioelectrochemical systems. Sci. Rep. 9, 14959. https://doi.org/10.1038/s41598-019-51452-x (2019).
Flynn, J. M., Ross, D. E., Hunt, K. A., Bond, D. R. & Gralnick, J. A. Enabling unbalanced fermentations by using engineered electrode-interfaced bacteria. mBio https://doi.org/10.1128/mBio.00190-10 (2010).
Choudhury, S., Pan, J., Amin, S., Chung, F.-L. & Roy, R. Repair kinetics of trans-4-hydroxynonenal-induced cyclic 1, N2-propanodeoxyguanine DNA adducts by human cell nuclear extracts. Biochemistry 43, 7514–7521. https://doi.org/10.1021/bi049877r (2004).
Keshari, S. et al. Butyric acid from probiotic Staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20184477 (2019).
Vinolo, M. A. R., Rodrigues, H. G., Nachbar, R. T. & Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 3, 858–876. https://doi.org/10.3390/nu3100858 (2011).
Suzuki, T. & Inukai, M. Effects of nitrite and nitrate on DNA damage induced by ultraviolet light. Chem. Res. Toxicol. 19, 457–462. https://doi.org/10.1021/tx050347l (2006).
Yang, Y., Sharma, R., Sharma, A., Awasthi, S. & Awasthi, Y. C. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim. Pol. 50, 319–336 (2003).
Wilson, L. P. & Bouwer, E. J. Biodegradation of aromatic compounds under mixed oxygen/denitrifying conditions: a review. J. Ind. Microbiol. Biotechnol. 18, 116–130. https://doi.org/10.1038/sj.jim.2900288 (1997).
Borrero-de Acuña, J. M., Timmis, K. N., Jahn, M. & Jahn, D. Protein complex formation during denitrification by Pseudomonas aeruginosa. Microbial. Biotechnol. 10, 1523–1534. https://doi.org/10.1111/1751-7915.12851 (2017).
Acknowledgements
The study was supported by the Ministry of Science and Technology (MOST) Grants (108-2622-B-008-001-CC1; 108-2314-B-008-003-MY3, and 107-2923-B-008-001-MY3) and 106/107/108-Landseed Hospital-NCU joint Grants. We thank Supitchaya Traisaeng at National Central University for assistance at biofilm staining.
Author information
Authors and Affiliations
Contributions
A.B.: Methodology; A.B., and P.A.: Software; A.B., P.A., D.T.T.M., S.K., and R.S.: Data curation; A.B.: Writing- Original draft preparation; C.-M.H. and C.-L.C.: Ethical evaluation; C.-M.H.: Conceptualization, Visualization, Investigation, Methodology, Supervision, Writing-Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Balasubramaniam, A., Adi, P., Tra My, D.T. et al. Repurposing INCI-registered compounds as skin prebiotics for probiotic Staphylococcus epidermidis against UV-B. Sci Rep 10, 21585 (2020). https://doi.org/10.1038/s41598-020-78132-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78132-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.