Abstract
The herb thyme (Thymus vulgaris) has multiple therapeutic uses. In this study, we explored how T. vulgaris leaf extract protects liver cells against sodium nitrite-(NaNO2) induced oxidative stress. Mice were divided into four groups; each group received one of the following treatments orally: saline; T. vulgaris extract alone; NaNO2 alone; or T. vulgaris extract + NaNO2. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), reduced glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), IL-1β, IL-6, TNF-α, and total proteins were measured in serum using standard methods. TNF-α, hemooxygenase-1 (HO-1), thioredoxin, SOD, and GSH synthase, all of which are linked to oxidative stress, were measured using quantitative real-time PCR (qRT-PCR). In mice treated with T. vulgaris extract, the effect of NaNO2 on ALT and AST levels and total proteins was reduced, and its effect on antioxidant levels was reversed. Normally, NaNO2 causes hepatocyte congestion and severe hepatic central vein congestion. Tissues in the mice treated with T. vulgaris were restored to normal conditions. Our results demonstrate that NaNO2-induced hepatic injury is significantly reduced by pretreatment with T. vulgaris extract, which protects against hepatic oxidative stress and its associated genes at the biochemical, molecular, and cellular levels.
Similar content being viewed by others
Introduction
Nitrite is important to organ health. At very low concentrations, and within physiological levels, nitrite has antioxidant properties1,2. When reduced to nitric oxide (NO), which is critical for vascular homeostasis3, it plays a critical role in cell signaling, cellular immunity, and in the activation of certain regulatory proteins3,4. These regulatory proteins are vital to the maintenance of normal human health.
Human beings use nitrites in a wide variety of ways, which makes them hard to avoid. In industry, nitrite and its salts are used in preservatives, colorants, and in manufacturing rubber products and dyes; in food, nitrite is employed for its anti-microbial properties; and in medicine, it is used to treat cyanide poisoning, ischemic heart disease, and as a vasodilator5,6,7. Environmentally, improper waste disposal, over-reliance on nitrite-based fertilizers, and excessive use in food preservatives has led to chronic exposure and the accumulation of high nitrite-nitrate levels in humans.
Overexposure (due either to high concentrations or prolonged low-dose exposure) is toxic and can cause liver, kidney, or other organ failure8, methemoglobinemia9, and eventually death. Overexposure in humans occurs primarily through contaminated food or drinking water. After first affecting the GI tract, it passes into the circulation, where it reaches various other organs10, causing further oxidative stress and toxicity. It has been observed that oxidative stress is a key mediator of nitrite-induced oxidative damage11 and that treatment with antioxidants can ameliorate or even reverse this12. The possible mechanism by which NaNO2 decreases antioxidant enzyme activity is induction of reactive oxygen species (ROS), which mediate the oxidation of enzyme molecules or the binding of NO13.
In the liver, NaNO2 induced hepatic toxicity through inhibition of antioxidant activity and increasing the levels of pro-inflammatory cytokines14. Liver disease is often treated using synthetic chemical drugs, but these can cause serious undesirable side effects, including cirrhosis, cholestatic jaundice, and elevated serum transaminase levels. Consequently, there is a need to explore gentler and lower cost treatments for nitrite toxicity and liver disease. Many herbal compounds contain polyphenols and flavonoids, which have anti-inflammatory and anti-oxidative effects. This has led to increased pharmacological interest in certain herbal medications. Thyme (Thymus vulgaris), an herb commonly used in cooking throughout the world, has antiseptic, antimicrobial, carminative, and anti-oxidative properties15,16 and has been used to treat a range of human illnesses17.
In traditional medicine, T. vulgaris has been used in a variety of ways because of its antiseptic, antispasmodic, anti-asthmatic, bronchodilator, expectorant, antitussive, carminative, anthelmintic, anti-microbial, and antioxidant qualities. It has been used to treat dyspepsia, chronic gastritis, diarrhea, and tonsillitis18. T. vulgaris extracts are believed to contain important anti-inflammatory and immunomodulator factors19 with high free radical scavenging, anti-inflammatory, anti-platelet, anti-obesity, and anti-diabetic actions17,20.
Monoterpenes and their hydrocarbons, as well as phenolics such as rosmarinic acid and flavonoid derivatives21, are found in significant amounts in many species of thyme. The presence of these compounds renders thyme an important herb in research on antioxidants22. The aim of our study was to examine the protective effects of T. vulgaris, at both the cellular and biochemical levels, on NaNO2-induced hepatic oxidative stress.
Materials and methods
Chemicals and kits
Kits for liver biomarkers, aspartate transaminase (AST), and alanine transaminase (ALT) were purchased from Clini Lab (Almanial; Cairo, Egypt). Kits for reduced glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD), and total proteins were purchased from Bio-diagnostic (Giza, Egypt). Chemicals used, including NaNO2 and agarose, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Kits for RNA reverse transcription were purchased from Fermentas (Thermo Fisher Scientific, Waltham, MA, USA). The QIAzol reagents were purchased from QIAGEN (Valencia, CA, USA).
Preparation of T. vulgaris extract
T. vulgaris leaves were collected from Taif markets in April 2020. Plants were identified by Dr. Yassin Alsodani a botanist at the College of Science, Taif University. The protocol and the methods used in this study was complied with relevant Taif University, Saudi Arabia and international guidelines and legislations. The extract was prepared according to the method used by Abdel-Aziem et al.23; the leaves were first air-dried, then powdered. The powder (100 g) was immersed in 500 mL ethanol (70%) and mixed. After 24 h, the mixture was filtered through Whatman paper No.1, then evaporated at 44 ± 1 °C in a vacuum. The solid extract (with a yield of 14.5% per 100 g of original material) was freeze-dried and stored at -20 C°.
Measurement of total polyphenolic and flavonoid compounds
Total polyphenols and flavonoids were estimated, with modifications, using a method previously described by Singleton et al.24, who originally evaluated the total polyphenolic compounds in licorice extract using Foline-Ciocalteu reagent. This method has since been further developed and refined25,26. We combined 200 μL of sample (in triplicate) with 1 mL Folin-Ciocalteu reagent (10% v/v) and 800 μL 7.5% (w/v) Na2CO3, which was then incubated for 30 min at 25 °C. The optical density was measured spectrophotometrically at 760 nm, calibrated using a gallic acid standard curve. Values are presented in terms of mg gallic acid equivalent per 1 g dry thyme extract. Total flavonoid content was determined using an aluminum chloride colorimetric method27, based on a rutin calibration curve (mg rutin equivalent per 1 g dry thyme extract).
Gas chromatography–mass spectrometry (GC–MS)
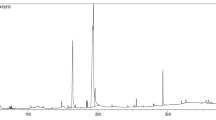
GC–MS analysis was performed using a Focus GC model (Thermo Electron Corporation, Waltham, MA, USA) under specific conditions: (1) DB-5 capillary column (30 m × 0.32 mm, 0.50 mm); (2) column temperature 60 °C (1 min) to 180 °C at 3 °C/min; (3) injector and detector temperature 220 °C; (4) split ratio 1:10; (5) carrier gas He; flow rate: 1.0 mL/min. A sample aliquot of 1 μL was diluted in chloroform at a ratio of 1:10. GC retention indices and ratios were compared on polar columns with specific standard mass spectra28.
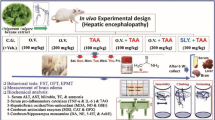
Experimental design and animal handling
Twenty-eight mice (7 weeks old; 35 ± 2 g) were kept at 12 hr /12 hr light dark cycle in the animal quarters at Turabah University College, Taif University, Saudi Arabia. Mice were given free access to food and water and handled daily for 7 days to accustom them to human contact. The Ethical Committee of Turabah University College, Taif University approved all procedures and animal use for this study (project TURSP2020-09). Moreover, the study was carried out in compliance with the ARRIVE guidelines. The animals were split into four groups as follows: Group 1, the negative control group (CNT), received only saline orally; Group 2, the T. vulgaris control group, received 0.5 g/kg bw T. vulgaris extract orally for 15 days23,29; Group 3, the positive NaNO2 intoxication group, received 60 mg/kg bw NaNO2 orally on Day 1430; and Group 4, the protective group, received T. vulgaris extract as for Group 2, and then NaNO2 on Day 14, as for Group 3. Figure 1 shows the details of the experimental protocol. We chose the dose of 60 mg/kg bw of NaNO2 because, while this dose is not lethal, it will nevertheless cause significant toxicity, damaging tissues and cells, which can be measured clearly using biochemical assays13,30,31.
On Day 15, 24 h after the final treatment, the mice were anesthetized and then euthanized by decapitation. Liver samples were taken and preserved in Qiazol for RNA extraction and qRT-PCR; histological samples were prepared using Bouin’s solution; blood samples were collected and the extracted serum stored at -20 °C for later use.
Biochemical assessments
Serum ALT and AST levels were measured using a colorimetric spectrophotometer following the procedures in the user’s manual. MDA levels were assessed using the technique developed by Ohkawa et al.32. SOD was measured using the method of Beutler et al.33, reduced GSH using the method of Nishikimi et al.34, and total protein levels were determined using the method of Lowry et al.35. These colorimetric methods were performed using a Bio-Rad Smart Tech Spectrophotometer according to the procedures in the product inserts provided with each kit. The reduced glutathione (GSH) and oxidized glutathione (GSSG) kits were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). All chemicals used were of the highest purity and purchased from commercial suppliers in Saudi Arabia and Egypt.
Cytokine measurements
Several different kits for measuring parameters in mice, including mouse IL-1β (cat. No. E-EL-M0037), IL-6 (E-EL-M0044), and TNF-α (E-EL-M0049) were purchased from Elabscience (Houston, TX, USA). Serum cytokine levels were determined using Sandwich-ELISA kits in accordance with the product inserts and reference tables available at the Clinical Laboratory Sciences Department, Turabah University College, Taif University.
Quantitative real-time PCR (qRT-PCR) and gene expression
Total liver RNA was extracted and determined to be pure at 260/280 nm. Reverse transcription was performed using the Quanti-Tect reverse transcription kit with 1 µg total RNA, producing single-stranded complementary DNA (cDNA) using a two-step qRT-PCR reaction with a random primer hexamer. Amplification of cDNA was performed using SYBR Green master mix (Thermo Scientific, Waltham, MA, USA). Table 1 lists the primers used in thermal cycle qRT-PCR analysis, using the 2–ΔΔCt method. β-actin was used as a ‘house-keeping’ (internal standard) gene, against which cDNA was normalized. Reactions were run on and analyzed using a 7500 FAST Real-Time PCR Detection System (Applied Biosystems, Foster City, CA, USA). qRT-PCR conditions were: 95 °C for 10 min (1st denaturation stage) and 40 cycles of 95 °C for 15 s (2nd denaturation stage) followed by 60 °C for 1 min (annealing and extension stage).
Changes in intensity and expression of the specific genes listed in Table 1 under Analysis were determined using comparative cycle threshold (CT) values.
Histological examinations
After decapitation, typical tissue specimens from the livers of all animals were harvested according to a standardized necropsy protocol36 and immediately fixed in Bouin's solution for 24 h. The fixed specimens were thoroughly washed under running tap water, dehydrated in an ascending series of ethanol concentrations (70%, 80%, 90%, and 100%; 2 h each), cleared in two changes of xylene (1 h each), impregnated in two changes of soft paraffin (2 h each), embedded in paraffin blocks, cut into Sects. 4 μm thick, and routinely stained with hematoxylin and eosin following standard processing and staining protocols37. The stained sections were examined microscopically and any histopathological alterations observed were recorded. This was followed by multiparametric quantitative lesion scoring for the hepatic parenchyma in all groups following the protocol described by Khalil et al.38 with a few modifications. For each animal, five randomly selected, non-duplicated microscopic profiles (40 × objective) were captured using an AmScope digital camera attached to an Olympus light microscope. These images were analyzed to allow calculation of the ratio of the area fractions of the hepatic central veins, portal blood vessels, sinusoids, necrosis, hemorrhage, fatty change, and leukocytic aggregations to the total areas using the image analysis software ImageJ (version 1.51v; Research Services Branch, NIH, Bethesda, MD, USA). The proportion of hepatic cells that exhibited pyknosis, single-cell necrosis, and vacuolar and hydropic degeneration with respect to the total number of hepatocytes per image was calculated subjectively. The frequency with which other lesions occurred (Kupffer cell hyperplasia, leukocytic infiltration, cholangiocyte hyperplasia and/or necrosis, and cholestasis) was determined by counting the number of lesions per image. Eventually, the results were expressed as percentages (mean ± SEM).
Statistical analysis
Seven mice per group were selected and one-way ANOVA and Dunnett's post hoc descriptive tests were performed using SPSS software version 22 (SPSS, IBM, Chicago, IL, USA) for Windows. The data are presented as mean ± standard error of mean; statistical significance is p < 0.05, indicated by letters with different symbols**.
Ethical Statement
All experimental procedures were carried out under National Institutes of Health Guidelines for the care and use of laboratory animals, and all procedures designed to minimize the suffering of animals were followed.
Results
Analysis and detection of T. vulgaris content
Analysis (using gallic acid as a standard curve) revealed that ethanolic T. vulgaris extract contained rosmarinic acid (38.23 mg/g dry weight thyme extract), luteolin-7-O-glucoside (17.3 mg/g), and caffeic acid (1.96 mg/g). These results are shown in Table 2. Concentrations of phenol and flavonoids were 221.65 and 115 mg/g, respectively, using rutin as a standard calibration curve (Table 1).
GC–MS analysis
Table 3 shows the major compounds contained in T. vulgaris extract. Ten major compounds were detected in T. vulgaris extract following GC–MS analysis; the molecular weight (MW), retention time (RT), and peak area vary among these compounds. The hydroxyl (OH), carbonyl (CO), acetyl (COCH3), and nitro (NO2) functional groups are also shown in Table 3.
Serum hepatic biomarkers
We observed elevated ALT and AST levels and lower total proteins in the NaNO2-intoxicated groups (Groups 3 and 4), which indicated liver damage and dysfunction. In the group pretreated with T.vulgaris extract (Group 4), which received T. vulgaris extract for 14 days and then NaNO2 for 24 h, we observed less pronounced changes in ALT and AST levels and total proteins (Table 4). Pretreatment with T. vulgaris extract normalized and returned ALT and AST levels and total proteins to their relative control levels.
Impact of T. vulgaris extract on oxidative stress biomarkers
Increased levels of MDA (a biomarker of lipid peroxidation), along with lower SOD serum levels, indicated tissue damage in the NaNO2-intoxicated mice (Group 3). Group 2 mice, who received T. vulgaris only, exhibited moderate increases in SOD and GSH. In the NaNO2-intoxicated mice (Group 4) pretreated with T. vulgaris extract, we observed alleviated GSH and SOD levels and a decrease in MDA levels, providing evidence for the ameliorative effect of the T. vulgaris extract against NaNO2-induced hepatic toxicity (Table 5). The effect of T. vulgaris extract on the glutathione system was evaluated by determining the ratio of GSH to oxidized glutathione (GSSG) in serum from NaNO2-intoxicated mice (Table 5). The T. vulgaris extract had a positive effect on the glutathione status in NaNO2-injected mice by causing a concomitant increase in GSH levels and decrease in GSSG levels, which restored the GSH/GSSG ratio to control levels. The profound decrease in the GSH/GSSG ratio induced by NaNO2 was normalized by T. vulgaris treatment for 15 days.
Mitigated impact of T. vulgaris extract on inflammatory cytokines
To examine the destructive impact of NaNo2 on the immune state of mice, we measured the levels of pro-inflammatory cytokines. NaNO2 injection increased serum levels of IL-1β, IL-6, and TNF-α in the NaNO2-intoxicated mice (Groups 3 and 4). Pretreatment of NaNO2-intoxicated mice with T. vulgaris (Group 4) mitigated these effects (Table 6), which eventually returned to normal levels. T. vulgaris extract normalized all altered pro-inflammatory cytokines.
Ameliorative effect of T. vulgaris extract on quantitative expression of liver genes
Nitrite toxicity induced liver damage and hepatic dysfunction as shown by the increases in hemeoxygenase-1 (HO-1), an oxidative stress biomarker, and TNF-α, an inflammatory cytokine (Fig. 2A,B). Gene expression for TNF-α mRNA was downregulated in the group pretreated with T. vulgaris extract (Fig. 2A). Conversely, the HO-1 gene was upregulated in the T. vulgaris-treated group (Fig. 2B). The ameliorative effects of T. vulgaris extract on both TNF-α and HO-1 expression and its potential to restore and recover gene alterations caused by NaNO2 intoxication are shown in Fig. 2A,B.
Ameliorative effect of T. vulgaris extract on mRNA expression of TNF-α (A) and HO-1 (B) in NaNO2-treated mice. Graphic presentation of liver mRNA expression by qRT-PCR analysis of TNF-α (A) and HO-1 (B) in different groups of mice relative to β-actin. *p < 0.05 versus the control group; #p < 0.05 versus the control and T. vulgaris extract groups; and $p < 0.05 versus the NaNO2-treated group.
Impact of T. vulgaris extract on quantitative expression of antioxidant genes in liver
qRT-PCR results showed that NaNO2 downregulated thioredoxin, SOD, and GSH synthase mRNA expression (Fig. 3A–C), leading to oxidative stress in the liver. The T.vulgaris-treated group (Group 2) showed positive correlation in the antioxidant genes examined, as T. vulgaris upregulated thioredoxin, SOD, and GSH synthase mRNA expression, demonstrating that the extract has important antioxidative properties (Fig. 3). In the group pretreated with T. vulgaris extract for 14 days followed by NaNO2 intoxication for 24 h (Group 4), the altered mRNA expression of the examined genes returned to within the normal range as a result of pretreatment.
Ameliorative effect of T. vulgaris extract on mRNA expression of thioredoxin (A), SOD (B), and GSH synthase (C) in NaNO2-treated mice. Graphic presentation of liver mRNA expression by qRT-PCR analysis of thioredoxin, SOD, and GSH synthase in different groups of mice relative to β-actin. *p < 0.05 versus the control group; #p < 0.05 versus the control and T. vulgaris extract groups; and $p < 0.05 versus the NaNO2-treated group.
Histopathological examination
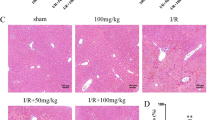
Upon microscopic examination of the specimens taken from the control and T. vulgaris-treated animals, we observed normal hepatic histological architectures: roughly hexagon-shaped hepatic lobules with sinusoids converging from the periphery to the central vein and portal canals present at approximately three of the six angles of the lobule. The hepatic parenchyma between the portal canals consisted of hepatocytes arranged in cell plates (Fig. 4A,B). Exposure to NaNO2 induced a wide variety of hepatopathic histological alterations, including circulatory changes (congestion of the central and portal veins and sinusoids), hepatocyte degeneration and necrosis (pyknosis, cellular swelling associated with vacuolations due to vacuolar and hydropic degenerations or fatty changes, and single cell necrosis), and inflammatory changes (intralobular and/or leukocytic infiltration or aggregations) (Fig. 4C,D). Interestingly, the biliary system did not show any noticeable histological alterations. T. vulgaris showed marked hepatoprotective effects against the NaNO2-induced hepatopathy. Although the hepatic tissue sections from the NaNO2 + T. vulgaris-treated animals were not completely histologically normal, we observed a significant reduction in the severity and frequency of the NaNO2-induced histological changes. The most frequent lesions in this group were vacuolar vascular congestion, sinusoidal dilatation, hepatocyte vacuolation, and tiny mononuclear cell aggregations (Fig. 4E,F). The overall hepatic lesion scoring changes induced by NaNO2 and possible amelioration by thymus extract are shown in Table 7.
Representative photomicrograph of hematoxylin and eosin stained hepatic tissue sections showing the effects on the hepatic histology of exposure to NaNO2 and/or T. vulgaris. (A) and (B), normal histological photomicrographs in the control and T. vulgaris-treated mice, respectively. (C) and (D), livers of the NaNO2-treated mice showing portal congestion (arrows), inflammatory cell infiltrate (ellipses), cellular swelling associated with hydropic degeneration (red arrowheads), and single-cell necrosis (black arrowheads). (E) and (F), livers of NaNO2 + T. vulgaris-treated mice showing focal mononuclear cell aggregates (ellipses), fatty change (black arrowheads), congested blood vessels (black arrows), and sinusoids (blue arrows).
Discussion
Our study confirmed that NaNO2 intoxication increases the levels of ALT and AST in serum, decreases total proteins, affects cytokine levels and gene expression (leading to an imbalance in antioxidant mechanisms), and causes histopathological changes, resulting in severe oxidative stress in liver tissues. However, pretreatment with T. vulgaris extract prevented or reversed these effects, confirming the antioxidant effect of T. vulgaris against NaNO2-induced hepatic dysfunction.
The elevated levels of ALT and AST, decreased total serum proteins, and the histopathological alterations that we observed, are all indicators of toxicity and also well-known quantitative measures of liver activity39. While functional liver damage can be indicated by high serum levels of AST, a more liver-specific indicator is the enzyme ALT. ALT catalyzes the conversion of alanine into pyruvate and glutamate, making serum levels of ALT a more reliable indicator of liver damage40. Our findings demonstrated that the membrane structure and integrity of the liver cells, which would otherwise have been destroyed by NaNO2, remained undamaged and uncongested, providing strong evidence for the protective effect of T. vulgaris extract also described previously17,41.
Sharma et al.42 noted that hepatoprotective plants are characterized by significant levels of polyphenols and flavonoids, major compounds also found in T. vulgaris. Flavonoids promote the expression of enzymes involved in the production of glutamylcysteine synthetase and thioredoxin, leading to increased intracellular GSH levels43,44.The antioxidant assays used in this study, which targeted five different types of ROS molecules (MDA, SOD, GSH, GSSG, and GSH/GSSG), confirmed the strong antioxidant effect of the T. vulgaris extract. Our findings are in agreement with those of previous studies that reported the high phenolic content and strong antioxidative effect of T. vulgaris extract17. Inhibition and decreased function of the antioxidant system causes the accumulation of H2O2 and hepatic cell decomposition45. Treatment with T. vulgaris extract significantly reversed these decreases in antioxidant enzyme activity.
Our study showed that NaNO2 intoxication reduced antioxidant activity and increased both oxidative stress and lipid peroxidation in agreement with previous reports11,30. Antioxidant defenses are affected by the expression of genes such as thioredoxin, SOD, and GSH46, and oxidative stress is regulated by several other downstream genes, including HO-147. Hyun48 showed that many plant extracts play a critical role in increasing antioxidant activity and reducing ROS. Overexpression of a particular hemoxygenase-1 reported here in thymus control group and downregulation in the NaNO2-treated groups, confirmed the potential of T. vulgaris extract. HO-1 is normally involved in the regulation of mitochondrial biogenesis, neurogenesis, and angiogenesis48, causing an increase in protein expression49. As such, HO-1 plays an important role in mechanisms of oxidative stress and inflammation50. By controlling IL-1β activation51, HO-1 regulated the expression of inflammasome-associated genes52. The antioxidant mechanism is regulated by downstream genes that control oxidative stress, such as Nrf2 and HO-147,53. Our findings confirmed that HO-1 expression plays a key role in the regulation of hepatic oxidative stress regulated by T. vulgaris extract. To explore the potential mechanism underlying the hepatoprotective effect of T. vulgaris extract, we examined the role of HO-1 signaling. Most reports have indicated that HO-1 plays a role in the activation and expression of some antioxidant and anti-inflammatory cytokine genes54,55. All were coincided with our reported findings.
Our study confirmed that T. vulgaris extract regulated the expression of pro-inflammatory cytokines, such as IL-1β, thereby ameliorating the NaNO2-induced inflammatory response. The inflammatory response causes increased production of IL-1β, IL-6, and TNF-α cytokines56, which play a leading role in sepsis and fever. NaNO2 intoxication has the same effect57, yet our study found that cytokine levels in mice pretreated with T. vulgaris extract returned to normal. Therefore, T. vulgaris extract prevented NaNO2-induced hepatic toxicity by ameliorating disrupted serum levels of IL-1β, IL-6, and TNF-α.
Reactive oxygen species (ROS) play a vital role in maintaining various human physiological processes58, but at high levels, they can be cytotoxic and cause liver damage59. Therefore, mechanisms exist to maintain appropriate ROS levels in the liver and other organs, thereby preventing oxidative stress and promoting redox homeostasis59. Our study found that while NaNO2 intoxication induced lipid peroxidation, pretreatment with T. vulgaris reversed these effects. In part, this was due to the recovery of depleted GSH, which plays a significant defensive role against oxidative stress60.
Hydroperoxides are converted into alcohols and water in a process catalyzed by thioredoxin, a cytosolic protein61. Thioredoxin has several antioxidant functions, including scavenging for free radicals, removing hydrogen peroxide, protecting against oxidative stress, as well as broader functions in genetic transcription, DNA and protein repair, immunostimulant roles, apoptosis, and cell proliferation. As a thiol-specific antioxidant, thioredoxin shares many of the same functions as glutathione systems and the two are understood to act in tandem in the management of oxidative stress62. In our study, NaNO2 downregulated thioredoxin and GSH synthase expression, but these returned to normal in mice pretreated with T. vulgaris extract.
In addition to biochemical alterations, the hepatotoxic effect of NaNO2 has been further implicated in a wide variety of degenerative, circulatory, and inflammatory alterations. The etiopathogenesis of these hepatopathic morphological alterations are likely to be multifactorial and mediated by NaNO2-induced oxidative damage and inflammation due to the formation of ROS and lipid peroxidation. The latter is associated with lysosomal and mitochondrial membrane rupture and subsequent release of digestive proteases63 and activation of the apoptosis-related Bax and caspase-3 genes64.
Treatment with NaNO2 caused hepatic dysfunction, oxidative stress, and alteration in the levels of inflammatory cytokines. Pretreatment with T. vulgaris extract alleviated these responses to toxicity and restored the levels and mRNA expression of the proteins and genes. The collective summary for the protective impact of T. vulgaris on NaNO2-induced liver dysfunction are clearly shown in Fig. 5.
Schematic representation of the ameliorative effect of T. vulgaris extract on liver dysfunction. NaNO2 intoxication induced hepatic dysfunction, decreased antioxidant activity, and an increase in the levels and expression of inflammatory cytokines. These results of treatment with NaNO2 were normalized by pretreatment with T. vulgaris extract.
Conclusion
T. vulgaris extract exerted a protective effect against oxidative stress in liver tissues caused by NaNO2 intoxication. The effect was observed in different antioxidant systems and through the regulation of pro-inflammatory cytokines. In short, the common herb thyme (T. vulgaris) can be used in the prevention and treatment of hepatic oxidative stress.
Data availability
Data are available upon request.
References
Carlström, M. et al. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 89, 574–585. https://doi.org/10.1093/cvr/cvq366 (2011).
McNally, B., Griffin, J. L. & Roberts, L. D. Dietary inorganic nitrate: from villain to hero in metabolic disease?. Mol. Nutr. Food Res. 60, 67–78. https://doi.org/10.1002/mnfr.201500153 (2016).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167. https://doi.org/10.1038/nrd2466 (2008).
Shiva, S. Nitrite: a physiological store of nitric oxide and modulator of mitochondrial function. Redox Biol. 1, 40–44. https://doi.org/10.1016/j.redox.2012.11.005 (2013).
Butler, A. R. & Feelisch, M. Therapeutic uses of inorganic nitrite and nitrate: from the past to the future. Circulation 117, 2151–2159. https://doi.org/10.1161/circulationaha.107.753814 (2008).
Nossaman, V. E., Nossaman, B. D. & Kadowitz, P. J. Nitrates and nitrites in the treatment of ischemic cardiac disease. Cardiol. Rev. 18, 190–197. https://doi.org/10.1097/CRD.0b013e3181c8e14a (2010).
King, A. M., Glass, K. A., Milkowski, A. L. & Sindelar, J. J. Impact of clean-label antimicrobials and nitrite derived from natural sources on the outgrowth of clostridium perfringens during cooling of deli-style Turkey breast. J. Food Prot. 78, 946–953. https://doi.org/10.4315/0362-028x.Jfp-14-503 (2015).
Al-Rasheed, N. M., Fadda, L. M., Attia, H. A., Ali, H. M. & Al-Rasheed, N. M. Quercetin inhibits sodium nitrite-induced inflammation and apoptosis in different rats organs by suppressing Bax, HIF1-α, TGF-β, Smad-2, and AKT pathways. J. Biochem. Mol. Toxicol. https://doi.org/10.1002/jbt.21883 (2017).
Chui, J. S., Poon, W. T., Chan, K. C., Chan, A. Y. & Buckley, T. A. Nitrite-induced methaemoglobinaemia—aetiology, diagnosis and treatment. Anaesthesia 60, 496–500. https://doi.org/10.1111/j.1365-2044.2004.04076.x (2005).
Ansari, F. A., Ali, S. N., Arif, H., Khan, A. A. & Mahmood, R. Acute oral dose of sodium nitrite induces redox imbalance, DNA damage, metabolic and histological changes in rat intestine. PLoS ONE 12, e0175196. https://doi.org/10.1371/journal.pone.0175196 (2017).
Ansari, F. A. & Mahmood, R. Sodium nitrite enhances generation of reactive oxygen species that decrease antioxidant power and inhibit plasma membrane redox system of human erythrocytes. Cell Biol. Int. 40, 887–894. https://doi.org/10.1002/cbin.10628 (2016).
Sherif, I. O. & Al-Gayyar, M. M. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur. Cytokine Netw. 24, 114–121. https://doi.org/10.1684/ecn.2013.0341 (2013).
Ansari, F. A., Ali, S. N., Khan, A. A. & Mahmood, R. Acute oral dose of sodium nitrite causes redox imbalance and DNA damage in rat kidney. J. Cell. Biochem. 119, 3744–3754. https://doi.org/10.1002/jcb.26611 (2018).
Eissa, M. M. et al. Chlorella vulgaris ameliorates sodium nitrite-induced hepatotoxicity in rats. Environ. Sci. Pollut. Res. Int. https://doi.org/10.1007/s11356-020-11474-9 (2020).
Ulukanli, Z., Cigremis, Y. & Ilcim, A. In vitro antimicrobial and antioxidant activity of acetone and methanol extracts from Thymus leucotrichius (Lamiaceae). Eur. Rev. Med. Pharmacol. Sci. 15, 649–657 (2011).
Sajed, H., Sahebkar, A. & Iranshahi, M. Zataria multiflora Boiss. (Shirazi thyme)—an ancient condiment with modern pharmaceutical uses. J. Ethnopharmacol. 145, 686–698. https://doi.org/10.1016/j.jep.2012.12.018 (2013).
El-Nekeety, A. A. et al. Antioxidant properties of Thymus vulgaris oil against aflatoxin-induce oxidative stress in male rats. Toxicon 57, 984–991. https://doi.org/10.1016/j.toxicon.2011.03.021 (2011).
Pina-Vaz, C. et al. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. JEADV 18, 73–78. https://doi.org/10.1111/j.1468-3083.2004.00886.x (2004).
Ocaña, A. & Reglero, G. Effects of thyme extract oils (from Thymus vulgaris, Thymus zygis, and Thymus hyemalis) on cytokine production and gene expression of oxLDL-stimulated THP-1-macrophages. J. Obes. 2012, 104706. https://doi.org/10.1155/2012/104706 (2012).
Vigo, E., Cepeda, A., Gualillo, O. & Perez-Fernandez, R. In-vitro anti-inflammatory effect of Eucalyptus globulus and Thymus vulgaris: nitric oxide inhibition in J774A.1 murine macrophages. J. Pharm. Pharmacol. 56, 257–263. https://doi.org/10.1211/0022357022665 (2004).
Schött, G. et al. The chemical composition of the pharmacologically active Thymus species, its antibacterial activity against Streptococcus mutans and the antiadherent effects of T. vulgaris on the bacterial colonization of the in situ pellicle. Fitoterapia 121, 118–128. https://doi.org/10.1016/j.fitote.2017.07.005 (2017).
El-Boshy, M. E. et al. The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res. Int. 26, 22736–22746. https://doi.org/10.1007/s11356-019-05562-8 (2019).
Abdel-Aziem, S. H. et al. Ameliorative effects of thyme and calendula extracts alone or in combination against aflatoxins-induced oxidative stress and genotoxicity in rat liver. Cytotechnology 66, 457–470. https://doi.org/10.1007/s10616-013-9598-7 (2014).
Singleton, V. L., Orthofer, R. & Lamuela-Raventós, R. M. Methods in Enzymology Vol. 299, 152–178 (Elsevier, Amsterdam, 1999).
Ordonez, A., Gomez, J. & Vattuone, M. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 97, 452–458 (2006).
Naczk, M. & Shahidi, F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 41, 1523–1542. https://doi.org/10.1016/j.jpba.2006.04.002 (2006).
Rezzoug, M. et al. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 19, 146. https://doi.org/10.1186/s12906-019-2556-y (2019).
Heidari, Z., Salehzadeh, A., Sadat Shandiz, S. A. & Tajdoost, S. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech 8, 177. https://doi.org/10.1007/s13205-018-1199-x (2018).
Abdel-Azeem, A. S., Hegazy, A. M., Zeidan, H. M., Ibrahim, K. S. & El-Sayed, E. M. Potential renoprotective effects of rosemary and thyme against gentamicin toxicity in rats. J. Diet. Suppl. 14, 380–394. https://doi.org/10.1080/19390211.2016.1253632 (2017).
Ansari, F. A., Khan, A. A. & Mahmood, R. Protective effect of carnosine and N-acetylcysteine against sodium nitrite-induced oxidative stress and DNA damage in rat intestine. Environ. Sci. Pollut. Res. Int. 25, 19380–19392. https://doi.org/10.1007/s11356-018-2133-9 (2018).
Ansari, F. A., Khan, A. A. & Mahmood, R. Ameliorative effect of carnosine and N-acetylcysteine against sodium nitrite induced nephrotoxicity in rats. J. Cell. Biochem. https://doi.org/10.1002/jcb.27971 (2018).
Ohkawa, H., Ohishi, N. & Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. https://doi.org/10.1016/0003-2697(79)90738-3 (1979).
Beutler, E., Duron, O. & Kelly, B. M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882–888 (1963).
Nishikimi, M., Appaji, N. & Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46, 849–854. https://doi.org/10.1016/s0006-291x(72)80218-3 (1972).
Lowry, O. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 (1951).
Ruehl-Fehlert, C. et al. Revised guides for organ sampling and trimming in rats and mice–part 1. Exp. Toxicol. Pathol. 55, 91–106 (2003).
Slaoui, M. & Fiette, L. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol. Biol. (Clifton, N.J.) 691, 69–82. https://doi.org/10.1007/978-1-60761-849-2_4 (2011).
Khalil, S. R. et al. Protective effect of Spirulina platensis against physiological, ultrastructural and cell proliferation damage induced by furan in kidney and liver of rat. Ecotoxicol. Environ. Saf. 192, 110256. https://doi.org/10.1016/j.ecoenv.2020.110256 (2020).
Grespan, R. et al. Hepatoprotective effect of pretreatment with Thymus vulgaris essential oil in experimental model of acetaminophen-induced injury. Evid. Based Complement. Altern. Med. eCAM 2014, 954136. https://doi.org/10.1155/2014/954136 (2014).
Sallie, R., Tredger, J. M. & Williams, R. Drugs and the liver. Part 1: testing liver function. Biopharm. Drug Dispos. 12, 251–259. https://doi.org/10.1002/bdd.2510120403 (1991).
Zhang, X. et al. Ameliorative effect of supercritical fluid extract of Chrysanthemum indicum Linnén against D-galactose induced brain and liver injury in senescent mice via suppression of oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 234, 44–56. https://doi.org/10.1016/j.jep.2018.12.050 (2019).
Sharma, A., Sangameswaran, B., Jain, V. & Saluja, M. J. I. C. P. J. Hepatoprotective activity of Adina cordifolia against ethanol induce hepatotoxicity in rats. Int. Curr. Pharm. J. 1, 279–284 (2012).
Myhrstad, M. C., Carlsen, H., Nordström, O., Blomhoff, R. & Moskaug, J. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radical Biol. Med. 32, 386–393. https://doi.org/10.1016/s0891-5849(01)00812-7 (2002).
Moskaug, J., Carlsen, H., Myhrstad, M. C. & Blomhoff, R. Polyphenols and glutathione synthesis regulation. Am. J. Clin. Nutr. 81, 277s–283s. https://doi.org/10.1093/ajcn/81.1.277S (2005).
Halliwell, B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence?. Lancet (London, England) 344, 721–724. https://doi.org/10.1016/s0140-6736(94)92211-x (1994).
Khafaga, A. F. & El-Sayed, Y. S. Spirulina ameliorates methotrexate hepatotoxicity via antioxidant, immune stimulation, and proinflammatory cytokines and apoptotic proteins modulation. Life Sci. 196, 9–17. https://doi.org/10.1016/j.lfs.2018.01.010 (2018).
Ge, M. et al. Brg1-mediated Nrf2/HO-1 pathway activation alleviates hepatic ischemia-reperfusion injury. Cell Death Dis. 8, e2841. https://doi.org/10.1038/cddis.2017.236 (2017).
Hyun, T. K., Kim, H.-C. & Kim, J.-S. Antioxidant and antidiabetic activity of Thymus quinquecostatus Celak. Ind. Crops Prod. 52, 611–616 (2014).
Hull, T. D. et al. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight 1, e85817. https://doi.org/10.1172/jci.insight.85817 (2016).
Abdou, K. H., Moselhy, W. A., Mohamed, H. M., El-Nahass, E. S. & Khalifa, A. G. Moringa oleifera leaves extract protects titanium dioxide nanoparticles-induced nephrotoxicity via Nrf2/HO-1 signaling and amelioration of oxidative stress. Biol. Trace Elem. Res. 187, 181–191. https://doi.org/10.1007/s12011-018-1366-2 (2019).
Abd El-Twab, S. M., Hussein, O. E., Hozayen, W. G., Bin-Jumah, M. & Mahmoud, A. M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-kappaB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. 68, 511–523. https://doi.org/10.1007/s00011-019-01241-z (2019).
Szabo, G. & Csak, T. Inflammasomes in liver diseases. J. Hepatol. 57, 642–654. https://doi.org/10.1016/j.jhep.2012.03.035 (2012).
Li, J. et al. Xiaochaihutang attenuates liver fibrosis by activation of Nrf2 pathway in rats. Biomed. Pharmacother. 96, 847–853. https://doi.org/10.1016/j.biopha.2017.10.065 (2017).
Abd El-Twab, S. M., Hozayen, W. G., Hussein, O. E. & Mahmoud, A. M. 18β-Glycyrrhetinic acid protects against methotrexate-induced kidney injury by up-regulating the Nrf2/ARE/HO-1 pathway and endogenous antioxidants. Renal Fail. 38, 1516–1527. https://doi.org/10.1080/0886022x.2016.1216722 (2016).
Mahmoud, A. M., Hozayen, W. G. & Ramadan, S. M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomed. Pharmacother. 94, 280–291. https://doi.org/10.1016/j.biopha.2017.07.101 (2017).
Bhatia, M. & Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 202, 145–156. https://doi.org/10.1002/path.1491 (2004).
Sherif, I. O. & Al-Gayyar, M. M. Cod liver oil in sodium nitrite induced hepatic injury: does it have a potential protective effect?. Redox Rep. Commun. Free Radical Res. 20, 11–16. https://doi.org/10.1179/1351000214y.0000000097 (2015).
Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 194, 7–15. https://doi.org/10.1083/jcb.201102095 (2011).
Willcox, J. K., Ash, S. L. & Catignani, G. L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 44, 275–295. https://doi.org/10.1080/10408690490468489 (2004).
Gutiérrez, M. B., Miguel, B. S., Villares, C., Gallego, J. G. & Tuñón, M. J. Oxidative stress induced by Cremophor EL is not accompanied by changes in NF-kappaB activation or iNOS expression. Toxicology 222, 125–131. https://doi.org/10.1016/j.tox.2006.02.002 (2006).
Lu, J. & Holmgren, A. The thioredoxin antioxidant system. Free Radical Biol. Med. 66, 75–87. https://doi.org/10.1016/j.freeradbiomed.2013.07.036 (2014).
Telorack, M. et al. A glutathione-Nrf2-thioredoxin cross-talk ensures keratinocyte survival and efficient wound repair. PLoS Genet. 12, e1005800. https://doi.org/10.1371/journal.pgen.1005800 (2016).
Kiani, A., Yousefsani, B. S., Doroudian, P., Seydi, E. & Pourahmad, J. The mechanism of hepatotoxic effects of sodium nitrite on isolated rat hepatocytes. Toxicol. Environ. Health Sci. 9, 244–250. https://doi.org/10.1007/s13530-017-0327-z (2017).
El-Nabarawy, N. A., Gouda, A. S., Khattab, M. A. & Rashed, L. A. Effects of nitrite graded doses on hepatotoxicity and nephrotoxicity, histopathological alterations, and activation of apoptosis in adult rats. Environ. Sci. Pollut. Res. Int. 27, 14019–14032. https://doi.org/10.1007/s11356-020-07901-6 (2020).
Acknowledgements
We appreciate and thank Taif University for the financial support for Taif University Researchers Supporting Project (TURSP-2020/09), Taif University, Taif, Saudi Arabia.
Funding
This study was supported by Taif University Researchers Supporting Project (TURSP-2020/09), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
M.M.S.: designed, supervise experiments, analyzed data and wrote the paper. A.A.: Analyzed data, Real time PCR, Chemistry analysis. M.M.M.M.: Histopathology and Revised paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Soliman, M.M., Aldhahrani, A. & Metwally, M.M.M. Hepatoprotective effect of Thymus vulgaris extract on sodium nitrite-induced changes in oxidative stress, antioxidant and inflammatory marker expression. Sci Rep 11, 5747 (2021). https://doi.org/10.1038/s41598-021-85264-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85264-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.