Abstract
6-Gingerol, the main bioactive compound of ginger, has antioxidant, anti-inflammatory, anti-cancer and neuroprotective effects. However, it is unclear whether 6-Gingerol has protective effects against hepatic ischemia/reperfusion (I/R) injury. In this study, the mouse liver I/R injury model and the mouse AML12 cell hypoxia/reoxygenation (H/R) model were established by pretreatment with 6-Gingerol at different concentrations to explore the potential effects of 6-Gingerol. Serum transaminase levels, liver necrotic area, cell viability, inflammatory response, and cell apoptosis were used to assess the effect of 6-Gingerol on hepatic I/R or cell H/R injury. Quantitative polymerase chain reaction (qPCR) and Western blotting were used to detect the mRNA and protein expression. The results show that 6-Gingerol decreased serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels, liver necrosis, inflammatory cytokines IL-1β, IL-6, MCP-1, TNF-α expression, Ly6g+ inflammatory cell infiltration, protein phosphorylation of NF-κB signaling pathway, Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) positive cells, cell apoptosis rate, the protein expression of pro-apoptotic protein BAX and C-Caspase3, increased cell viability, and expression of anti-apoptotic protein BCL-2. Moreover, 6-Gingerol could increase the mRNA and protein expression of mitogen activated protein kinase phosphatase 5 (MKP5) and inhibit the activation of P38/JNK signaling pathway. In MKP5 knockout (KO) mice, the protective effect of 6-gingerol and the inhibition of P38/JNK pathway were significantly weakened. Therefore, our results suggest that 6-Gingerol exerts anti-inflammatory and anti-apoptotic effects to attenuate hepatic I/R injury by regulating the MKP5-mediated P38/JNK signaling pathway.
Similar content being viewed by others
Introduction
Hepatic I/R injury refers to the pathological process of ischemic injury in the liver caused by various factors that are aggravated by the restoration of blood supply1. Hepatic I/R injury is a critical cause of postoperative liver function decline, failure, or non-functioning of transplanted liver. In liver transplantation, hepatic I/R injury leads to 10% early organ failure and a high rate of acute and chronic rejection2. Thus, finding new therapeutic methods to reduce hepatic I/R injury is of great theoretical significance and clinical value.
In recent years, various approaches have been explored to mitigate hepatic I/R injury, such as ischemic preconditioning, gene targeting techniques and pharmaceutical interventions, with a growing emphasis on pharmacological interventions3,4,5. 6-Gingerol is the main bioactive compound of ginger, which has been extensively studied and shown to possess antioxidant, anti-inflammatory, anti-tumor, immunomodulatory, antibacterial, and other biological properties6. Research has also demonstrated that 6-Gingerol can effectively ameliorate cognitive impairment, oxidative stress, neuroplasticity, amyloidosis, and inflammation in a rat model of neuroinflammation induced by lipopolysaccharide (LPS)7. Furthermore, 6-Gingerol has been found to inhibit pyroptosis and mitigate sepsis-induced liver injury through activation of the Nrf2 signaling pathway8. In addition, 6-Gingerol attenuated I/R injury in heart, brain and small intestine9,10,11,12, but the role of 6-Gingerol in hepatic I/R injury has not yet been reported.
The activation of the mitogen-activated protein kinase (MAPK) pathway is closely associated with hepatic I/R injury, and inhibition of MAPK signaling pathway activation can significantly reduce hepatic I/R injury13. The mitogen activated protein kinase phosphatase (MKP) family acts as a negative regulator of the MAPK signalling pathway by dephosphorylating phosphoserine/threonine and phosphotyrosine residues, which is closely related to many human diseases14. MKP5, a member of the MKP family, can inhibit the activation of P38 and JNK to reduce lipotoxicity-induced pancreatic β-cell injury15. Previous studies have shown that 6-Gingerol can reduce hypoxia-induced apoptosis in PC-12 cells by inhibiting the P38/JNK signaling pathway16. Additionally, in prostate cells, 6-Gingerol has been found to exert anti-inflammatory effects by promoting the expression of MKP517. Based on these findings, we hypothesized that 6-Gingerol could play a protective role in hepatic I/R injury by promoting MKP5 expression and thus inhibiting the P38/JNK signaling pathway.
In this study, we investigated the protective effects of 6-Gingerol on hepatic I/R injury using mouse liver I/R and AML12 cell H/R models. Our results showed that 6-Gingerol could effectively reduce serum ALT and AST levels, attenuate tissue necrosis, decrease hepatocyte apoptosis and inflammatory response after hepatic I/R, and inhibit the phosphorylation of p38/JNK protein. Furthermore, the protective effect of 6-Gingerol was associated with the promotion of MKP5 expression. Therefore, 6-Gingerol has the potential to become a promising drug for the treatment of hepatic I/R injury.
Materials and methods
Animals and hepatic I/R injury model
Male C57BL/6 mice (6–8 weeks, 20–25 g) were provided by Zhengzhou University Experimental Animal Center. The mice were housed under pathogen-free conditions with a 12 h of light/darkness cycle, and given ad libitum access to food and water. MKP5 knockout mice were provided by Professor Liang Yinming of Xinxiang Medical University. Information on the construction of MKP5 knockout mice can be found in the supplementary materials. All animal experimental procedures followed the ethical codes of ethics established by the International Animal Committee and the ARRIVE guidelines. The experiment was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (ethics approval number: 2018-KY-78). 6-Gingerol was dissolved in dimethyl sulfoxide (Solarbio, Beijing, China) and then diluted with normal saline. 6-Gingerol (50 mg/kg and 100 mg/kg) group mice and MKP5 KO groups mice were administered by gavage once a day for five days, and the control group was given equal amounts of saline8.
A mouse hepatic I/R injury model was established following the methods reported in the literature18,19. Briefly, mice were anesthetized by intraperitoneal injection of 60 mg/kg sodium pentobarbital, a 2 cm incision was made at the mid-abdomen with surgical scissors. The middle and left lobes of the hepatic were separated, and non-invasive arterial clamps were used to clamp the blood vessels of both lobes to simulate ischemia. After 90 min, the clamps were released to restore liver perfusion, and the mid-abdominal incision was closed using silk suture. Mice in the sham-operated group did not undergo clamping and reperfusion operations, and the rest of the operations were the same. Blood and tissue specimens were collected after 6 h of reperfusion for subsequent experimental testing.
Measurement of serum transaminase levels
After reperfusion, blood samples were collected and centrifuged at 3000 g/min for 5 min, and the supernatant was collected for assay. Serum aspertate aminotransferase (AST) and alanine aminotransferase (ALT) were detected according to the kit instructions (JianChen Bioengineering Institute, Nanjing, China).
H&E
After reperfusion, liver tissue was obtained, fixed in 10% formalin solution, embedded in paraffin, and cut into 5-μm thick sections. The sections were treated and stained according to the instructions of hematoxylin and eosin reagent (servicebio, Wuhan, China). Briefly, the sections were baked at 60 °C for 2 h, then dewaxed with xylene and soaked in gradient ethanol. The sections were stained with hematoxylin for 5 min, differentiated with hydrochloric ethanol for 30 s, rinsed with water for 15 min, restained with eosin for 2 min, washed with water. The sections were conventionally dehydrated with gradient ethanol, transparent with xylene, and sealed with neutral gum. Photographs were collected under the microscope (Olympus, Tokyo, Japan) and analyzed for histopathological changes in the liver. Statistical analysis of the necrotic area of the liver was performed using Image Pro Plus software (version 6.0), percentage of necrotic area = necrotic area/total area of the field of view.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining
Paraffin sections were dewaxed, followed by incubation with proteinase K at 37 °C for 20 min. The sections were then washed with PBS and incubated with TUNEL detection solution (servicebio, Wuhan, China) at 37 °C for 10 min. After incubation, the sections were washed three times with PBS for 5 min each, stained with DAPI (servicebio, Wuhan, China), and incubated at room temperature for 10 min. The sections were washed with PBS and sealed with anti-fluorescence quenching solution (servicebio, Wuhan, China) before being photographed under a fluorescent microscope (Olympus, Tokyo, Japan).
Western blot
Hepatic tissue and cellular proteins were extracted according to the RIPA instructions (Solarbio, Beijing, China), and the proteins concentration were determined using the BCA kit (Solarbio, Beijing, China). 10% or 12% SDS-PAGE were used to separated proteins, then transferred to PVDF membrane, blocked with 5% skim milk powder for 1 h. Primary antibody was added and incubated overnight at 4 °C, followed by incubation with goat anti-rabbit or goat anti-mouse secondary antibody (Proteintech, Wuhan, China) at room temperature for 1 h. The gel imaging system (Cytiva, America) was used for color development after the addition of ECL luminescent solution (NCM Biotech, Suzhou, China). The primary antibodies used for western blot are shown in Table 1. (Original protein bands are provided in the Supplementary file).
Real-time polymerase chain reaction (PCR) analyses
Total RNA was extracted from liver tissues and cells using a Trizol regent (Solarbio, Beijing, China), and the concentration of total RNA was determined using a micro UV spectrophotometer. Total RNA (1 μg) was reverse transcribed to cDNA using a reverse transcription kit (vazyme,Nanjing, China). cDNA was amplified using SYBR Green qPCR Mix reagent (vazyme,Nanjing, China) on a qPCR instrument. GAPDH was used as an internal reference. 2−△△CT method was used for analysis. The primer sequences used for amplification are shown in Table 2.
Immunohistochemical staining
Paraffin sections were baked at 60 °C for 2 h, dewaxed by xylene immersion, dehydrated in gradient ethanol, and sections were subjected to thermal antigen repair with 0.01 mol/L citrate buffer (pH 6.0) and incubated with 3% H2O2 at room temperature for 10 min to inactivate endogenous enzymes. The sections were incubated with 10% goat serum for 1 h at room temperature, and incubated with Ly6g primary antibody (1:200, servicebio, Wuhan, China) overnight at 4 °C. On the second day, secondary antibody (1:500, servicebio, Wuhan, China) was added and incubated at room temperature for 30 min. The sections were washed with PBS three times before and after the secondary antibody incubation. DAB chromogenic solution was added dropwise, followed by restaining with hematoxylin and sealing with neutral gum. Microscopic examination was performed and images were collected.
Cell culture and H/R model
AML12 mouse hepatocytes were purchased from Procell Biotechnology Co., Ltd. Cells were cultured in DMEM/F12 medium (Procell, Wuhan, China) containing 10% fetal bovine serum (Gibco, Carlsbad,CA, USA), 1 × 105 U/L penicillin and 100 mg/L streptomycin (Solarbio, Beijing, China) in a constant temperature incubator at 37 °C with 5% CO2. The H/R model of AML12 cells was established according to reference20. When the cell density reached 70%-80%, the medium was discarded, and the cells were washed twice with PBS, sugar-free and serum-free medium (Procell, Wuhan, China) was added, and the cells were placed in a triple-vapor incubator (5% CO2, 94% N2 and 1% O2) for hypoxia. After 12 h, the medium was changed to normal medium, and the cells were placed in a normoxic incubator for another 6 h to complete reoxygenation.
Cell counting kit-8 assay
AML12 cells were inoculated in 96-well plates (100 μL/well) at a density of 0.5 × 104 cells/mL and incubated in an incubator at 37 °C with 5% CO2. When the cell density reached 70–80%, H/R was performed according to the experimental groups. After reoxygenation, 10 μL of CCK-8 reagent (Beyotime, Shanghai, China) was added to each well, and the cells were incubated in the incubator for 1h, and the OD value at 450 nm was measured using an enzyme marker (Thermo Fisher Scientific, Inc.), and the cell activity was calculated according to the absorbance value.
Flow cytometry analysis
After reoxygenation, cells were collected by trypsin digestion, centrifuged at 200 g/min for 5 min, washed twice with PBS, and resuspended in 195 μL of 1× Annexin-binding buffer. The cells were then incubated with 5 μL of Annexin V-FITC and 10 μL of PI for 15 min in the dark. Cell apoptosis rates were analyzed using flow cytometry (BD Biosciences, USA).
Statistical analysis
The experimental data were analyzed using SPSS 2 2.0 statistical software. The measurement data were expressed as mean ± standard deviation. The t-test was used for comparisons between two groups. One-way ANOVA was used for comparisons between multiple groups. The difference was statistically significant at P < 0.0 5.
Results
6-Gingerol alleviates hepatic damage during liver I/R injury
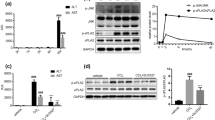
Serum ALT and AST levels were significantly higher in the I/R group compared to the sham-operated group (Fig. 1A,B). However, the I/R+6-Gingerol (50 mg/kg, 100 mg/kg) group showed significantly lower levels compared to the I/R group (Fig. 1A,B). H&E staining revealed that the hepatocytes in the I/R group exhibited focal or large areas of degenerative necrosis, with disrupted liver lobule structure (Fig. 1C). In contrast, the area of cell necrosis in the I/R+6-Gingerol (50 mg/kg, 100 mg/kg) group was significantly reduced compared to the I/R group, and the liver tissue structure was largely intact (Fig. 1C,D). These results suggest that 6-Gingerol plays a protective role against I/R injury in mouse liver.
6-Gingerol alleviates apoptosis in hepatic I/R injury
The number of TUNEL-positive cells and the protein expression of BAX, C- caspase3 were significantly increased, while protein expression of BCL-2 was significantly decreased in hepatic tissues of mice after I/R injury (Fig. 2A–F). In contrast, 6-Gingerol pretreatment significantly decreased the protein of BAX, C-caspase3 as well as the number of TUNEL-positive cells in liver tissue after hepatic I/R, and increased the protein expression of BCL-2 (Fig. 2A–F). These results suggest that 6-Gingerol pretreatment restrained the activation of apoptotic pathways in mouse hepatic after I/R injury.
6-Gingerol alleviates apoptosis in hepatic I/R injury. (A) TUNEL staining (red fluorescence indicates TUNEL positives cells) and (B) statistical analysis of mouse liver tissue, (C) detection of apoptotic proteins and (D–F) statistical analysis in mouse liver tissue. **P < 0.01 versus sham group; ##P < 0.01 versus I/R group.
6-Gingerol alleviates inflammation in hepatic I/R injury
The mRNA expression of inflammatory factors Il-1β, Il-6, Tnf-α and MCP-1 was significantly increased in mouse liver tissues after I/R (Fig. 3A–D), while 6-Gingerol pretreatment significantly decreased the mRNA expression of these inflammatory factors in liver tissues after I/R (Fig. 3A–D). Moreover, immunohistochemical results showed that the number of Ly6g-positive cells was significantly increased in liver tissues after I/R, while the number of Ly6g-positive cells was significantly decreased in the IR+6-Gingerol (50 mg/kg, 100 mg/kg) group (Fig. 3E,F). Additionally, western blotting results showed that 6-Gingerol pretreatment inhibited the activation of the NF-κB signaling pathway after hepatic I/R, which was manifested as reduced phosphorylation of p65, IKKβ and IκBα proteins (Fig. 3G,H). These results suggest that 6-Gingerol reduces the tissue inflammatory response after I/R injury in mice.
6-Gingerol alleviates inflammation in hepatic I/R injury. (A–D) mRNA expression of inflammatory cytokines Il-1β, Il-6, Tnf-α and MCP-1, (E) Ly6g immunohistochemical staining (brown stained cells indicate Ly6g positive) and (F) statistical analysis in mouse liver tissues, (G) Western blotting was used to detect NF-κB signaling pathway proteins and (H) statistical analysis **P < 0.01 versus sham group; #P < 0.05 and ##P < 0.01 versus I/R group.
6-Gingerol alleviates cell injury induced by H/R
To determine the concentration of 6-gingerol used in cellular experiments, the CCK-8 assay was used to detect cell activity after 24 h pretreatment with different concentrations (5, 10, 25, 50, 75, 100 and 200 µM) of 6-gingerol. The results showed that 6-gingerol produced cytotoxic effects when the drug concentration exceeded 50 µM (Fig. 4A), thus the concentration of 6-gingerol was kept below 50 μM in cell H/R experiments. We then examined the protective effect of different concentrations of 6-gingerol on H/R-induced cell damage in AML12 cells. Cell activity was significantly reduced after H/R compared to the control group, but pretreatment with 6-gingerol (5, 10, 25, and 50 µM) increased cell activity compared to the H/R group, and there was no difference in the increase of cell activity between 25 and 50 µM 6-gingerol (Fig. 4B), so the final experimental concentration of 6-gingerol was 25 µM. H/R injury significantly increased the apoptosis rates (Fig. 4C,D) and the expressions of pro-apoptotic proteins BAX and C-Caspase3 (Fig. 4E–H), while inhibited the expression of anti-apoptotic protein BCL-2 (Fig. 4E–H). 6-Gingerol pretreatment inhibited H/R-induced apoptosis, reversed the alteration of apoptosis-related proteins (Fig. 4E–H). Moreover, 6-gingerol pretreatment significantly decreased the mRNA expression of inflammatory factors Il-1β, Il-6, Tnfα and MCP-1 after H/R in AML12 cells (Fig. 4I–L). These results suggest that 6-Gingerol alleviates H/R-induced cell damage.
6-Gingerol alleviates cell injury induced by H/R. (A) Effect different concentrations of 6-Gingerol on the activity of AML12 cells under normal oxygen conditions, (B) effects of different concentrations of 6-Gingerol on cell viability after H/R. (C, D) Cell apoptosis was determined by flow cytometry. (E) Western blotting was used to detect apoptosis-related proteins and (F–H) statistical analysis, (I–L) mRNA expression of inflammatory cytokines Il-1β, Il-6, Tnf-α and MCP-1. **P < 0.01 versus control group; #P < 0.05 and ##P < 0.01 versus H/R group.
6-Gingerol inhibits P38/JNK signaling during hepatic I/R injury in vivo and in vitro
The P38/JNK signaling is involved in the regulation of hepatic I/R injury. To detect changes in the P38/JNK signaling pathway, we examined the changes in P38/JNK protein phosphorylation in mice and cells. Compared with the control group, protein phosphorylation of P38 and JNK was significantly increased in liver tissue after I/R injury (Fig. 5A–C) and AML12 cells subjected to H/R injury (Fig. 5D–F). And 6-Gingerol pretreatment significantly reduced the protein phosphorylation of P38 and JNK after I/R and H/R injury (Fig. 5A–F). These results suggest that 6-Gingerol pretreatment inhibits the activation of P38/JNK signaling pathway after hepatic I/R in mice.
6-Gingerol inhibits P38/JNK signaling during hepatic I/R injury in vivo and in vitro. (A) Proteins detection and (B, C) statistical analysis in mouse liver tissue, (D) proteins detection and (E, F) statistical analysis in AML12 cells. **P < 0.01 versus sham group; ##P < 0.01 versus I/R group; $$P < 0.01 versus control group; &&P < 0.01 versus H/R group.
6-Gingerol promoted the expression of MKP5 in vivo and in vitro
Previous study has also shown that 6-Gingerol could promote MKP5 expression in prostate cells17, and our previous studies have shown that MKP5 is involved in the regulation of hepatic I/R injury by inhibiting the P38/JNK signaling pathway21. We verified by molecular docking that 6-gingerol was able to bind to the MKP5 protein (Results can be found in the supplementary materials). Then, we examined the effect of 6-Gingerol on MKP5 mRNA and protein expression after hepatic I/R injury. As shown in Fig. 6A,B, MKP5 mRNA expression was significantly decreased after I/R and H/R injury, and 6-Gingerol could promote MKP5 mRNA expression after I/R and H/R injury. We further examined the changes in MKP5 protein expression, and the results showed that MKP5 protein expression was significantly increased after 6-Gingerol pretreatment compared with sham group mice and control cells, and also increased the I/R and H/R-induced decrease in MKP5 protein expression (Fig. 6C–F). These results suggest that 6-Gingerol can promote MKP5 expression.
6-Gingerol promoted the expression of MKP5 in vivo and in vitro. (A) mRNA expression of MKP5 in liver tissues, (B) mRNA expression of MKP5 in AML12 cells, (C) protein detection and (D) statistical analysis of MKP5 in liver tissues, (E) protein detection and (F) statistical analysis of MKP5 in liver AML12 cells. **P < 0.01 versus sham group; ##P < 0.01 versus I/R group; $$P < 0.01 versus control group; &&P < 0.01 versus H/R group.
Knockout of MKP5 attenuated the hepatoprotective effect of 6-Gingerol
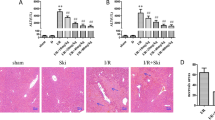
To further investigate whether 6-Gingerol protects against hepatic I/R injury depends on MKP5, we performed a mechanistic investigation using MKP5 KO mouse. Compared with WT mice, the ALT and AST levels (Fig. 7A,B), liver necrosis area (Fig. 7C,D), inflammatory response (Fig. 7E–H), and hepatocyte apoptosis (Fig. 7I,J) were significantly increased, and P38/JNK protein phosphorylation was more pronounced after MKP5 KO (Fig. 7K–N). The protective effects and inhibition of P38/JNK protein phosphorylation by 6-Gingerol were significantly diminished in MKP5 KO mice compared with WT mice (Fig. 7A–N), and these results suggest that MKP5 KO attenuated the protective effects of 6-Gingerol and that the hepatoprotective effects of 6-Gingerol were associated with modulation of MKP5 expression.
Knockout of MKP5 attenuated the hepatoprotective effect of 6-Gingerol. (A, B) Serum ALT and AST level, (C) H&E staining and (D) necrotic area statistics, (E–H) Il-1β, Il-6, Tnf-α and Mcp1 mRNA expression, (I) TUNEL staining (red fluorescence indicates TUNEL positives cells) and (J) statistical analysis, (K–N) MKP5 protein and P38/JNK pathway protein detection. **P < 0.01 versus sham group; ##P < 0.01 versus I/R group; $$P < 0.01 versus I/R group; ##P < 0.01 versus I/R +100 mg/kg group.
Discussion
Apoptosis and inflammatory response are key causes of liver I/R injury and important targets to mitigate hepatic I/R injury22. In this study, we established a mouse hepatic I/R injury model and AML12 cells H/R injury model to investigate the potential effects of 6-Gingerol on hepatic injury. The results showed that 6-Gingerol inhibited liver injury, apoptosis and inflammation, increased cellular activity, and inhibited JNK/P38 protein phosphorylation after liver I/R or cellular H/R injury. The protective effect of 6-Gingerol was associated with the promotion of MKP5 expression, and the protective effects and inhibition of p38/JNK pathway of 6-Gingerol was significantly diminished after MKP5 KO. Thus, our results suggest that 6-Gingerol exerts anti-inflammatory and anti-apoptotic effects to attenuate hepatic I/R injury by regulating MKP5-mediated P38/JNK signaling pathway.
Studies have shown that hepatic I/R injury manifests as an increase in inflammatory factors and apoptosis, and that protection against I/R-induced hepatic injury may be achieved by inhibiting inflammatory or apoptosis-related signaling pathways. Han et al. reported that 6-Gingerol attenuated cardiotoxicity caused by arsenic trioxide by inhibiting apoptotic and inflammatory responses23. Luo et al. showed that 6-Gingerol exerted a protective effect against cerebral I/R injury by inhibiting NLRP3 inflammatory vesicles and apoptosis10. These studies suggest that 6-Gingerol has potent anti-inflammatory and anti-apoptotic effects. In the present study, we examined the mRNA expression of inflammatory factors Il-1β, Il-6, Tnf-α, MCP-1, and demonstrated that 6-Gingerol pretreatment significantly reduced the expression of these inflammatory factors. Furthermore, immunohistochemical staining showed that 6-Gingerol pretreatment significantly reduced the infiltration of Ly6g-positive cells. The detection of NF-κB pathway protein also proved the anti-inflammatory effect of 6-Gingerol after hepatic I/R injury. And TUNEL staining showed that 6-Gingerol pretreatment significantly reduced apoptosis. Apoptosis-associated protein further verified the anti-apoptotic effect of 6-Gingerol. The results of in vitro AML12 cells H/R test were consistent with those of in vivo I/R test. Thus our results suggest that 6-Gingerol can attenuate hepatic I/R injury by inhibiting the inflammatory response and apoptosis.
The MAPK signaling pathway is an important pathway for the transduction of membrane receptor signals into cells and is involved in the regulation of cell growth, differentiation, migration, and inflammation24. MAPK family includes the extracellular signal-regulated kinase (ERK) pathway, the c-Jun N-terminal kinase (JNK) pathway, and the P38 MAPK pathway25. The MAPK family plays a critical role in mediating hepatic I/R injury, regulating hepatocyte death and survival by mediating signaling pathways related to apoptosis and inflammatory responses. For example, Regulator of G-protein signaling 14 attenuates hepatic I/R injury by inhibiting the TAK1-mediated JNK/p38 pathways signaling pathway26. Ring finger protein 5 protects against hepatic I/R injury by inhibiting the activity of ASK1-JNK/p38 pathway27. 6-Gingerol has been reported to inhibit the secretion of pro-inflammatory cytokines by targeting the MAPK signaling pathway, thereby comprehensively inhibiting the development of sepsis28. Similarly, in our study, 6-Gingerol inhibited the JNK/p38 pathway in mouse hepatic I/R injury and hepatocyte H/R injury, indicating that 6-Gingerol may protect against hepatic I/R injury by regulating the P38/JNK signaling pathway.
MKP5 belongs to the MKP family, which inactivates target kinase activity by dephosphorylating phosphoserine/threonine and phosphotyrosine residues, acting as a negative regulatory element that regulates the MAPK signaling pathway, which is closely associated with a variety of human diseases29,30. Studies have shown that MKP5 can inhibit the activation of P38/JNK signaling pathway to attenuate lipotoxicity-induced pancreatic β-cell injury15. Conversely, MKP5 KO in mice aggravates LPS-induced lung injury by enhancing the activation of the P38/JNK signaling pathway31. Research has also shown that 6-Gingerol could promote MKP5 expression in prostate cells, exerting anti-inflammatory effects17. Our studies shown that 6-Gingerol could promote mRNA and protein expression of MKP5 in the hepatic and AML12 cells. To explore whether the hepatoprotective effect of 6-Gingerol is related to the promotion of MKP5 expression, we verified it using MKP5 KO mice. Consistent with our previous research, the activation of P38/JNK signaling pathway was more pronounced and liver damage was more severe after MKP5 KO21, and the protective effect of 6-Gingerol was significantly diminished in MKP5 KO mice. These results suggest that the hepatoprotective effect of 6-Gingerol is dependent on MKP5-mediated P38/JNK signaling pathway.
To summarize, the results of this study suggest that 6-Gingerol has potential as a therapeutic agent for attenuating hepatic I/R injury by inhibiting inflammation and apoptosis. The protective mechanism may involve the promotion of MKP5 expression, which inhibits the activation of the JNK/P38 signaling pathway. However, this study also has some limitations. the present study focused on investigating the effects of 6-Gingerol on apoptosis and inflammatory responses via the P38/JNK signaling pathway in hepatic I/R injury, while whether 6-Gingerol can ameliorate hepatic I/R injury by regulating other signaling pathways remains to be further investigated, and the regulatory relationship between 6-gingerol and MKP5 needs further study. Moreover, we used MKP5 global knockout mice to investigate the mechanism of 6-Gingerol on hepatic I/R injury, and the results would be more convincing if liver-specific knockout mice were used.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Abbreviations
- I/R:
-
Ischemia reperfusion
- H/R:
-
Hypoxia/reoxygenation
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- MKP5:
-
Mitogen activated protein kinase phosphatase 5
- MAPK:
-
Mitogen-activated protein kinase
- JNK:
-
c-Jun N-terminal kinase
- KO:
-
Knockout
- ERK:
-
Extracellular signal-regulated kinase
- TUNEL:
-
Terminal-deoxynucleoitidyl transferase mediated nick end labeling
- DHE:
-
Dihydroethidium
- Bax:
-
Bcl2-associated x protein
- Bcl2:
-
B-cell leukemia/lymphoma
- PVDF:
-
Polyvinylidene fluoride
- BCA:
-
Bicinchoninic acid
- CCK-8:
-
Cell counting kit-8
- PBS:
-
Phosphate-buffered saline
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- ANOVA:
-
Analysis of variance
References
Saidi, R. & Kenari, S. Liver ischemia/reperfusion injury: an overview. J. Investig. Surg. 27, 366–379. https://doi.org/10.3109/08941939.2014.932473 (2014).
Liu, H. & Man, K. New insights in mechanisms and therapeutics for short- and long-term impacts of hepatic ischemia reperfusion injury post liver transplantation. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22158210 (2021).
Kong, E. et al. Protective efficiency comparison of direct and remote ischemic preconditioning on ischemia reperfusion injury of the liver in patients undergoing partial hepatectomy. Biomed. Res. Int. 2023, 2763320. https://doi.org/10.1155/2023/2763320 (2023).
Dossi, C., Vargas, R., Valenzuela, R. & Videla, L. Beneficial effects of natural compounds on experimental liver ischemia-reperfusion injury. Food Funct. 12, 3787–3798. https://doi.org/10.1039/d1fo00289a (2021).
Marinho, H., Marcelino, P., Soares, H. & Corvo, M. Gene silencing using siRNA for preventing liver ischaemia-reperfusion injury. Curr. Pharm. Des. 24, 2692–2700. https://doi.org/10.2174/1381612824666180807124356 (2018).
Adetuyi, B. & Farombi, E. 6-Gingerol, an active constituent of ginger, attenuates lipopolysaccharide-induced oxidation, inflammation, cognitive deficits, neuroplasticity, and amyloidogenesis in rat. J. Food Biochem. 45, e13660. https://doi.org/10.1111/jfbc.13660 (2021).
Zhang, F. et al. 6-Gingerol attenuates LPS-induced neuroinflammation and cognitive impairment partially via suppressing astrocyte overactivation. Biomed. Pharmacother. 107, 1523–1529. https://doi.org/10.1016/j.biopha.2018.08.136 (2018).
Hong, M. et al. 6-Gingerol ameliorates sepsis-induced liver injury through the Nrf2 pathway. Int. Immunopharmacol. 80, 106196. https://doi.org/10.1016/j.intimp.2020.106196 (2020).
Lv, X., Wang, M., Qin, Q., Lu, P. & Qin, G. 6-Gingerol relieves myocardial ischaemia/reperfusion injury by regulating lncRNA H19/miR-143/ATG7 signaling axis-mediated autophagy. Lab. Investig. 101, 865–877. https://doi.org/10.1038/s41374-021-00575-9 (2021).
Luo, J. et al. 6-Gingerol protects against cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and apoptosis via TRPV1/FAF1 complex dissociation-mediated autophagy. Int. Immunopharmacol. 100, 108146. https://doi.org/10.1016/j.intimp.2021.108146 (2021).
Lv, X. et al. 6-Gingerol activates PI3K/Akt and inhibits apoptosis to attenuate myocardial ischemia/reperfusion injury. Evid-Based Compl. Altern. 2018, 9024034. https://doi.org/10.1155/2018/9024034 (2018).
Li, Y. et al. 6-Gingerol protects intestinal barrier from ischemia/reperfusion-induced damage via inhibition of p38 MAPK to NF-κB signalling. Pharmacol. Res. 119, 137–148. https://doi.org/10.1016/j.phrs.2017.01.026 (2017).
Yu, B. et al. MAPK signaling pathways in hepatic ischemia/reperfusion injury. J. Inflamm. Res. 16, 1405–1418. https://doi.org/10.2147/jir.S396604 (2023).
Shillingford, S. & Bennett, A. Mitogen-activated protein kinase phosphatases: No longer undruggable?. Annu. Rev. Pharmacol. 63, 617–636. https://doi.org/10.1146/annurev-pharmtox-051921-121923 (2023).
Zhao, T. et al. MKP-5 relieves lipotoxicity-induced Islet β-cell dysfunction and apoptosis via regulation of autophagy. Int. J. Mol. Sci. 21, 2. https://doi.org/10.3390/ijms21197161 (2020).
Kang, C. et al. 6-Gingerols (6G) reduces hypoxia-induced PC-12 cells apoptosis and autophagy through regulation of miR-103/BNIP3. Artif. Cell. Nanomed. B. 47, 1653–1661. https://doi.org/10.1080/21691401.2019.1606010 (2019).
Nonn, L., Duong, D. & Peehl, D. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis 28, 1188–1196. https://doi.org/10.1093/carcin/bgl241 (2007).
Zhang, J., Zhang, M., Zhang, J. & Xia, Q. A novel mouse model of liver ischemic/reperfusion injury and its differences to the existing model. J. Investig. Surg. 28, 283–291. https://doi.org/10.3109/08941939.2014.983621 (2015).
Zheng, J. et al. MSCs ameliorate hepatocellular apoptosis mediated by PINK1-dependent mitophagy in liver ischemia/reperfusion injury through AMPKα activation. Cell. Death Dis. 11, 256. https://doi.org/10.1038/s41419-020-2424-1 (2020).
Yu, Q. et al. Veratric acid alleviates liver ischemia/reperfusion injury by activating the Nrf2 signaling pathway. Int. Immunopharmacol. 101, 108294. https://doi.org/10.1016/j.intimp.2021.108294 (2021).
Yu, Q. et al. Mitogen activated protein kinase phosphatase 5 alleviates liver ischemia-reperfusion injury by inhibiting TAK1/JNK/p38 pathway. Sci. Rep. 13, 11110. https://doi.org/10.1038/s41598-023-37768-9 (2023).
Jiménez-Castro, M., Cornide-Petronio, M., Gracia-Sancho, J. & Peralta, C. Inflammasome-mediated inflammation in liver ischemia-reperfusion injury. Cells https://doi.org/10.3390/cells8101131 (2019).
Han, X. et al. Protective effects of 6-Gingerol on cardiotoxicity induced by arsenic trioxide through AMPK/SIRT1/PGC-1α signaling pathway. Front. Pharmacol. 13, 868393. https://doi.org/10.3389/fphar.2022.868393 (2022).
Kim, E. & Choi, E. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 396–405, 2010. https://doi.org/10.1016/j.bbadis.2009.12.009 (1802).
Liang, Y. & Yang, W. Kinesins in MAPK cascade: How kinesin motors are involved in the MAPK pathway?. Gene 684, 1–9. https://doi.org/10.1016/j.gene.2018.10.042 (2019).
Zhang, J. et al. Regulator of G-protein signaling 14 protects the liver from ischemia-reperfusion injury by suppressing TGF-β-activated kinase 1 activation. Hepatology 75, 338–352. https://doi.org/10.1002/hep.32133 (2022).
Ding, M. et al. E3 ubiquitin ligase ring finger protein 5 protects against hepatic ischemia reperfusion injury by mediating phosphoglycerate mutase family member 5 ubiquitination. Hepatology 76, 94–111. https://doi.org/10.1002/hep.32226 (2022).
Zhang, F. et al. 6-Gingerol attenuates macrophages pyroptosis via the inhibition of MAPK signaling pathways and predicts a good prognosis in sepsis. Cytokine 125, 154854. https://doi.org/10.1016/j.cyto.2019.154854 (2020).
Theodosiou, A., Smith, A., Gillieron, C., Arkinstall, S. & Ashworth, A. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene 18, 6981–6988. https://doi.org/10.1038/sj.onc.1203185 (1999).
Jiménez-Martínez, M., Stamatakis, K. & Fresno, M. The dual-specificity phosphatase 10 (DUSP10): its role in cancer, inflammation, and immunity. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20071626 (2019).
Qian, F. et al. Map kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am. J. Physiol Lung Cell. Mol. Physiol. 302, L866-874. https://doi.org/10.1152/ajplung.00277.2011 (2012).
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 82300688, 82201963). We thank Professor Yinming Liang from Xinxiang Medical College for providing us with MKP5 knockout mice.
Author information
Authors and Affiliations
Contributions
Q.Y.: Conceptualization, Investigation, Data curation, Writing—original draft. J.L.: Conceptualization, Investigation. M.C.: Investigation, Data curation. C.M.: Investigation, Data curation. Q.H.: Investigation, Resources. X.D.: Supervision, Validation, Writing—review and editing, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Q., Li, J., Cui, M. et al. 6-Gingerol attenuates hepatic ischemia/reperfusion injury through regulating MKP5-mediated P38/JNK pathway. Sci Rep 14, 7747 (2024). https://doi.org/10.1038/s41598-024-58392-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58392-1
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.