Abstract
In the FUGA-BT trial (JCOG1113), gemcitabine plus S-1 (GS) showed non-inferiority to gemcitabine plus cisplatin (GC) in overall survival (OS) with good tolerance for patients with advanced biliary tract cancer (BTC). We performed a subgroup analysis focused on the elderly cohort of this trial. All 354 enrolled patients in JCOG1113 were classify into two groups; < 75 (non-elderly) and ≥ 75 years (elderly) group. We investigated the influence of age on the safety analysis, including the incidence of chemotherapeutic adverse events and the efficacy analysis, including OS. There were no remarkable differences in OS between the elderly (n = 60) and the non-elderly groups (n = 294). In the elderly group, median OS was 12.7 and 17.7 months for those who received GC (n = 20) and GS (n = 40), respectively. The prevalence of all-grade adverse events was similar between the elderly and the non-elderly groups. However, among the elderly group, Grade ≥ 3 hematological adverse events were more frequently observed in the GC arm than in the GS arm. The clinical outcomes of combination chemotherapy in elderly patients with advanced BTC were comparable to non-elderly patients. GS may be the more favorable treatment for elderly patients with advanced BTC.
Similar content being viewed by others
Introduction
Biliary tract cancer (BTC) is a malignant tumor arising from the biliary tract, which includes the intrahepatic bile duct, extrahepatic bile duct, gallbladder (GB), and ampulla of Vater. The number of patients with BTC has been increasing in Japan, and 63.9% of patients are over 75 years1,2. Most patients have been diagnosed with locally advanced, metastatic, or recurrent disease, therefore palliative chemotherapy is important for improving the prognosis of patients with BTC. However, the 5-year overall survival remains dismal at 10–20%3,4.
With an aging global population, there is a growing need to evaluate treatment outcomes in elderly patients with cancer. Several studies have reported the clinical outcomes of palliative chemotherapy in elderly patients with BTC5,6. Although gemcitabine plus cisplatin combination therapy (GC) is currently the standard regimen following the ABC-02 trial7, physicians in general clinical practice may be hesitant to administer GC for elderly patients due to perceptions of the potential for increased toxicity in a population with a high proportion of comorbidity.
Recently, Hepatobiliary and Pancreatic Oncology Group of Japan Clinical Oncology Group (JCOG) reported the results of the randomized phase III trial (FUGA-BT/JCOG1113) that compared GC and gemcitabine plus S-1 (GS), an oral fluoropyrimidine combination consisting of tegafur (a prodrug of 5-fluorouracil [5-FU]) and the 5-FU modulators gimeracil and oteracil, combination therapy as a first-line treatment for patients with advanced BTC8. In JCOG1113, GS showed non-inferiority to GC in overall survival (OS) with good tolerance (median OS: 13.4 months with GC and 15.1 months with GS, hazard ratio 0.945; 90% confidence interval 0.78–1.15).
For elderly patients, GS may also show good tolerance, however, there is less data regarding clinical outcomes of combination chemotherapy in elderly patients with advanced BTC. Therefore, we planned the present subgroup analysis focused on the elderly cohort of JCOG1113.
Methods
Study design and patients
JCOG1113 was a randomized phase III trial that enrolled patients from 33 institutions in Japan8. Main eligibility criteria were: histologically proven adenocarcinoma or adenosquamous carcinoma of the biliary tract; unresectable or recurrent disease; no previous chemotherapy; Eastern Cooperative Oncology Group performance status of 0 or 1; preserved major organ function; and written informed consent to participate. The study protocol was approved by the institutional review board of each participating institution (Cancer Institute Hospital of Japanese Foundation for Cancer Research, National Cancer Center Hospital, Kanagawa Cancer Center, National Cancer Center Hospital East, Kyorin University Faculty of Medicine, Yokohama City University Medical Center, Shizuoka Cancer Center, Saitama Cancer Center, Aichi Cancer Center Hospital, Kansai Medical University Hospital, Teikyo University School of Medicine, Kobe University Graduate School of Medicine, Teine Keijinkai Hospital, Osaka National Hospital, Jichi Medical University, National Hospital Organization Shikoku Cancer Center, Tochigi Cancer Center, National Center for Global Health and Medicine, Kyushu University, Hokkaido University Hospital, National Hospital Organization Kyushu Cancer Center, Kitasato University School of Medicine, Chiba Cancer Center, Sapporo Kousei General Hospital, Kanazawa University, University of Toyama, Osaka International Cancer Institute, Chiba University, Niigata Cancer Center Hospital, Tokyo Women's Medical University, Tokai university School of Medicine, Kindai University Faculty of Medicine). The study was conducted in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
Between May 8, 2013 and March 4, 2016, a total of 354 patients were enrolled to JCOG1113; 175 were assigned to the GC arm and 179 to the GS arm. For this subgroup analysis to investigate the influence of age, all registered patients of JCOG1113 were stratified by age; < 75 (non-elderly group) and ≥ 75 years (elderly group). Due to the growing of global aging society and most patients with BTC are over 75 years in Japan, the age 75 years was selected that it was an acceptable cut-off value to define the ‘elderly’ population. Additionally, the efficacy and the safety data were compared by regimen (GC vs. GS) in each group.
Outcomes
The primary endpoint of this subgroup analysis was OS. The secondary endpoints were progression-free survival (PFS), response rate (RR), adverse events (AEs), serious AEs, clinically significant AEs, and percent planned dose administered. Clinically significant AEs were predefined as those of Grade ≥ 2, including fatigue, anorexia, nausea, vomiting, oral mucositis, and diarrhea, occurring at least once during the monitoring period, which was the duration from the start of treatment until completion of eight cycles of treatment, or 24 weeks, whichever was longer. OS was calculated from the date of randomization to the date of death, or censored on the date of last contact for surviving patients. PFS was counted from the date on which disease progression or death was detected, or was censored on the last date when progression-free status was confirmed. Tumor response was assessed every six weeks according to the Response Evaluation Criteria in Solid Tumors version 1.1. RR was calculated without confirmation.
Treatment
Patients were randomly assigned to either the GC or GS treatment arm. Patients assigned to GC arm received gemcitabine (1000 mg/m2) and cisplatin (25 mg/m2) via infusion on days 1 and 8; this regimen was repeated every 3 weeks. Cisplatin was administered up to 16 times (total 400 mg/m2) unless patients met the termination criteria. After cisplatin termination, patients received gemcitabine (1000 mg/m2) via infusion on days 1, 8, and 15, repeated every 4 weeks. Patients assigned to GS arm received gemcitabine (1000 mg/m2) via infusion on days 1 and 8. S-1 was administered orally twice daily (60 mg/day for a body surface area [BSA] < 1.25 m2, 80 mg/day for a BSA between 1.25 and < 1.50 m2, and 100 mg/day for a BSA ≥ 1.50 m2) on days 1–14. This regimen was repeated every 3 weeks.
Statistical analysis
OS and PFS were estimated by the Kaplan–Meier method. Hazard ratios and corresponding 95% confidence intervals [CIs] were estimated using the Cox regression hazard model. CIs of RR and clinically significant AEs were calculated by Clopper-Pearson method. Statistical analyses were performed with SAS version 9.4. All statistical analyses were conducted at the JCOG Data Center.
Results
Patient characteristics
Of all registered 354 patients in JCOG1113, 60 patients aged ≥ 75 years were classified into the elderly group (20 in GC arm and 40 in GS arm), and 294 patients aged < 75 years were classified into the non-elderly group (155 in GC arm and 139 in GS arm). One patient in the GC arm was determined to be ineligible after registration; the patient was included in the efficacy analysis but excluded from the safety analysis (Fig. 1). Patient baseline characteristics were similar between the two groups (Table 1), and they were also similar between the treatment arms (GC arm and GS arm) in the elderly group.
Treatment compliance
Patient compliance with each treatment is shown in Table 2. There were no remarkable differences in dose reduction or percent planned dose between elderly and non-elderly patients within treatment arms. In elderly patients, those metrics were also similar between the treatment arms. Reasons for the termination of protocol treatment were similar between elderly and non-elderly patients (proportion of disease progression; 32.2% in elderly patients and 38.4% in non-elderly patients; incidence of AEs or patient’s refusal associated with AEs; 16.9% in elderly patients and 14.9% in non-elderly patients). In elderly patients, those were similar between treatment arms (proportion of disease progression; 36.8% in the GC arm and 30.0% in the GS arm; incidence of AEs or patient’s refusal associated with AEs, 15.8% in the GC arm and 17.5% in the GS arm).
Adverse events
The prevalence of all-grade AEs and clinically significant AEs were similar between elderly and non-elderly patients. In 59 elderly patients (19 in GC arm and 40 in GS arm), Grade ≥ 3 hematological adverse events were more frequent in the GC arm compared to the GS arm. Considering all-Grade non-hematological adverse events, nausea (26.3%), and vomiting (15.8%) were more frequent in the GC arm, while anorexia (40.0%), fever (35.0%), oral mucositis (25.0%) and diarrhea (22.5%) were more frequent in the GS arm (Table 3). Clinically significant AEs were observed in 25.0% and 32.5% and Grade ≥ 3 clinically significant AEs were observed in 15.0% and 10.0% of elderly patients in the GC and the GS arms, respectively.
In JCOG1113, three treatment-related deaths occurred in the GC arm, while none occurred in the GS arm. Of these deaths, one (disseminated intravascular coagulation from cholangitis and/or liver abscess) was an elderly patient.
Efficacy
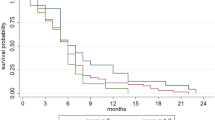
The median OS of the elderly patients was 16.2 (95% CI 11.5–20.1) months, whereas that of the non-elderly patients was 13.4 (95% CI 12.4–15.2) months (HR 0.959, 95% CI 0.709–1.299). There were no remarkable differences in OS between elderly patients and non-elderly patients. In elderly patients, the median OS in the GC arm was 12.7 (95% CI 7.5–20.1) months versus 17.7 (95% CI 15.1–21.5) months in the GS arm (HR 0.693, 95% CI 0.391–1.227). The median PFS was 5.7 (95% CI 2.7–9.9) months in the GC arm and 8.5 (95% CI 4.3–12.7) months in GS arm (HR 0.650, 95% CI 0.371–1.137) (Fig. 2). Among elderly patients with measurable lesions, the RRs were 50.0% (8/16; 95% CI 24.7%–75.4%) in the GC arm and 30.0% (9/30; 95% CI 14.7%–49.4%) in the GS arm. There were no remarkable differences in OS, PFS, or RR between treatment arms in elderly patients.
Overall survival and progression-free survival (intention-to-treat population). (A) Overall survival for elderly and non-elderly patients. (B) Overall survival for elderly patients in the GC and GS group. (C) Progression-free survival for elderly patients in the GC and GS group. Vertical lines on curves indicate patients censored on the date of their last follow-up. GC, gemcitabine plus cisplatin; GS, gemcitabine plus S-1.
Second-line treatment
A total of 46 (78.0%) elderly patients received second-line treatment. In the GC arm, 13 patients (68.4%) received second-line chemotherapy, mainly S-1 monotherapy (8 patients). In the GS arm, 33 patients (82.5%) received second-line chemotherapy, mainly GC (20 patients; Table 4).
Discussion
This subgroup analysis indicates that survival benefits with gemcitabine-based combination chemotherapy were similar between elderly and non-elderly patients. Furthermore, the frequency of all-Grade AEs was also similar between elderly and non-elderly patients. Our present results suggest that elderly patients with good general condition are expected to achieve clinical outcomes with the combination chemotherapy comparable to non-elderly patients treated for BTC. In elderly patients, OS and PFS of GS arm was tended to be better than that of GC arm, and Grade ≥ 3 hematological adverse events were observed more frequently in GC arm.
Several studies have investigated the difference of survival benefits by age in patients with BTC who received palliative chemotherapy. McNamara et al. reported the results of meta-analysis of 13 trials of systemic therapy for BTC, including 1163 patients6. The meta-analysis included 260 patients (22%) ≥ 70 years of age and the primary analysis demonstrated that PFS for those < 70 and ≥ 70 years was 6.0 and 5.0 months, and OS was 10.2 and 8.8 months, respectively. Their multivariable analysis of those data demonstrated that age was not associated with either PFS or OS. Similarly to our present study, they concluded that survival in elderly patients treated with combination chemotherapy is similar to that of non-elderly patients. Additionally, Horgan et al. reported results from a large retrospective study of elderly patients with BTC that included 913 patients9. The study included 321 patients ≥ 70 years of age and they concluded that active therapy is associated with similar survival benefit regardless of age. While some retrospective studies showed similar results10,11, other studies showed discrepant results that elderly patients treated with palliative chemotherapy for BTC had a poorer prognosis than non-elderly patients12. Only "fit" elderly patients are enrolled to clinical trials, however, many elderly patients are not fit in the real world. Therefore, indication of aggressive treatments for elderly patients has remained controversial and selection bias may have influenced these discrepant results. In these previous studies, the age 70 years was selected as a cut-off value to define the ‘elderly’ population. However, now that the global population is aging, the age of patients with BTC is also increasing, especially in Japan, a lot of patients with BTC are over 75 years old2. There is an increasing need to develop standard treatment for patients at an older age than before. Therefore, in this exploratory analysis, we adopted 75 years as a cut-off value, an older age than the age selected in the previous studies.
Elderly patients with cancer are a heterogeneous population. Aggressive treatment may be difficult for some elderly patients to tolerate; however, many fit elderly patients may not be adequately treated based only on their chronological age. Thus, in recent years, a comprehensive assessment of elderly patients is considered to be important to promote more individualized therapeutic approaches in geriatric oncology. A comprehensive geriatric assessment (CGA) is a multidisciplinary evaluation of an elderly person, not only regarding physical status, but also functional, cognitive, and psychosocial status13. Several studies have shown that CGA can identify patients at an increased risk for mortality and be useful in making decisions concerning appropriate treatment for elderly patients with cancer14,15,16. Similarly, decisions regarding treatment of elderly patients for BTC should not be dictated by chronological age alone, and multidisciplinary assessment should be considered to determine which patients may benefit from combination chemotherapy.
Notably, elderly patients treated with GS had a trend towards improved OS and PFS compared with those treated with GC in the present study. In terms of toxicities, Grade ≥ 3 hematological adverse events were observed more frequently in elderly patients treated with GC than in those treated with GS. In consideration of the above, GS is more suitable for elderly patients with low hematopoietic function levels at baseline. Additionally, GC requires hydration to reduce renal toxicity; however, elderly patients with cardiac and/or renal disease are unsuitable to receive large volume fluid infusions. Therefore, GS is more convenient for elderly patients as it does not require hydration, thereby reducing in-hospital time. However, the specific mechanisms leading to favorable outcomes with GS in elderly patients remains unclear and further study is needed. On the other hand, predefined clinically significant AEs such as anorexia, oral mucositis, nausea and vomiting were observed more frequently in elderly patients treated with GS than in those treated with GC. Elderly patients are more likely to suffer from dehydration due to poor oral intake, therefore it may be better not to administer GS to elderly patients with unstable oral intake. Imaoka et al. analyzed the clinical outcomes of elderly patients treated with gemcitabine alone, S-1 alone, or GS in prospective trials enrolling patients with unresectable pancreatic cancer that included 90 patients ≥ 70 years of age treated with GS17. There were three treatment-related deaths (interstitial lung disease, cerebrovascular disorder and an unknown cause associated with myelosuppression) in elderly patients treated with GS and two of the three patients were ≥ 80 years. There are only few reports of GS treatment for elderly patients and elderly candidates for GS therapy should be carefully selected.
One limitation of our study was the lack of international standardization, as all registered patients were from Japanese institutions. More severe gastrointestinal toxicities associated with S-1 have been reported among patients outside Asia18,19. Thus, these results should be considered carefully if GS is used to treat Western patients. Another limitation was the lack of quality of life (QoL) assessment. Maintaining QoL is a key goal of therapy and it affects the treatment decisions of many patients. Bridgewater et al. reported that the survival advantage of GC compared to gemcitabine alone was not associated with an improvement or deterioration of the QoL in the ABC-02 trial20. In the advanced disease setting, we need to discuss treatment goals with elderly patients, as QoL has been shown to be of greater importance than survival among elderly patients21. Additionally, our analysis was limited by the inclusion of a small number of elderly patients from 75 to 79 years old and lack of adequate statistical power. Therefore, detailed subgroup analysis was not meaningful and favorable outcomes associated with GS in elderly patients should be interpreted with caution.
In conclusion, the clinical outcomes of combination chemotherapy in elderly patients with advanced BTC were comparable to those in non-elderly patients. From the view point of the trend toward better prognosis, less hematological toxicities, and unnecessariness of hydration, GS may be a more favorable treatment than GC for elderly patients with advanced BTC.
References
Miyakawa, S. et al. Biliary tract cancer treatment: 5584 results from the biliary tract cancer statistics registry from 1998 to 2004 in Japan. J. Hepatobiliary Pancreat. Surg. 16, 1–7 (2009).
Cancer Information Service, National Cancer Center, Japan. Cancer Registry and Statistics. Ministry of Health, Labour and Welfare, National Cancer Registry (2016).
Lepage, C. et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: results of EUROCARE-5. Eur. J. Cancer. 51, 2169–2178 (2015).
Brandi, G., Farioli, A., Astolfi, A., Biasco, G. & Tavolari, S. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget 6, 14744–14753 (2015).
Corrigan, L. R., Bracken-Clarke, D. M. & Horgan, A. M. The challenge of treating older patients with pancreaticobiliary malignancies. Curr. Probl. Cancer. 42, 59–72 (2018).
McNamara, M. G. et al. Systemic therapy in younger and elderly patients with advanced biliary cancer: sub-analysis of ABC-02 and twelve other prospective trials. BMC Cancer 17, 262 (2017).
Valle, J. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281 (2010).
Morizane, C. et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann. Oncol. 30, 1950–1958 (2019).
Horgan, A. et al. Patterns of care and treatment outcomes in older patients with biliary tract cancer. Oncotarget 6, 44995–45004 (2015).
Lee, B. et al. Older adults with biliary tract cancer: treatment and prognosis. J. Am. Geriatr. Soc. 60, 1862–1871 (2012).
Kou, T. et al. Comparative outcomes of elderly and non-elderly patients receiving first-line palliative chemotherapy for advanced biliary tract cancer. J. Gastroenterol. Hepatol. 29, 403–408 (2014).
Sasaki, T. et al. Prognostic factors in patients with advanced biliary tract cancer receiving chemotherapy. Cancer Chemother. Pharmacol. 67, 847–853 (2011).
Rubenstein, L. Z., Stuck, A. E., Siu, A. L. & Wieland, D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J. Am. Geriatr. Soc. 39, 8S-16S (1991).
Extermann, M. & Hurria, A. Comprehensive geriatric assessment for older patients with cancer. J. Clin. Oncol. 25, 1824–1831 (2007).
Horgan, A. M. et al. Impact and feasibility of a comprehensive geriatric assessment in the oncology setting: a pilot study. Am. J. Clin. Oncol. 35, 322–328 (2012).
Kenis, C. et al. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Ann. Oncol. 24, 1306–1312 (2013).
Imaoka, H. et al. Clinical outcome of elderly patients with unresectable pancreatic cancer treated with gemcitabine plus S-1, S-1 alone, or gemcitabine alone: subgroup analysis of a randomised phase III trial, GEST study. Eur. J. Cancer. 54, 96–103 (2016).
Haller, D. G. et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J. Clin. Oncol. 26, 2118–2123 (2008).
Chuah, B. et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between Caucasian and East Asian patients. Cancer Sci. 102, 478–483 (2011).
Bridgewater, J. et al. Quality of life, long-term survivors and long-term outcome from the ABC-02 study. Br. J. Cancer. 114, 965–971 (2016).
Yellen, S. B., Cella, D. F. & Leslie, W. T. Age and clinical decision making in oncology patients. J. Natl. Cancer Inst. 86, 1766–1770 (1994).
Acknowledgements
The authors are grateful to the members of the JCOG Data Center and JCOG Operations Office for their support in preparing the manuscript (Dr. Tadayoshi Hashimoto) and oversight of the study management (Dr. Haruhiko Fukuda).
Funding
The study was supported in part by the National Cancer Center Research and Development Funds (23-A-22, 26-A-4, 29-A-3, 2020-J-3), AMED under Grant Number JP 16ck0106350, and the Grant-in-Aid for Clinical Cancer Research (H22-ganrinsho-ippan-013) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Consortia
Contributions
I.Y. wrote the main manuscript text and J.M. prepared all figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
CM reports grants and personal fees from Yakult, MSD, J-Pharma, AstraZeneca and Taiho, grants from ONO, Eisai, Merck biopharma and Daiichi Sankyo, personal fees from Teijin, Novartis and Abbvie, outside the submitted work. TO reports grants and personal fees from Taiho and Bristol-Myers Squibb, personal fees from Yakult, Pfizer, Takeda and Eli Lilly, during the conduct of the study; grants and personal fees from AstraZeneca, Eisai, MSD and Dainippon Sumitomo, grants from Baxter, personal fees from Nippon Servier, Meiji Seika, Shire, AbbVie, Incyte, ONO, Daiichi Sankyo, Takara Bio, Chugai, Teijin, Nippon Shinyaku, Novartis, Bayer and Mundipharma, outside the submitted work. JM reports grants from Ministry of Health, Labour and Welfare (MHLW), Japan and Japan AMED, during the conduct of the study; personal fees from Chugai and Taiho, outside the submitted work. MU reports grants and personal fees from Taiho, AstraZeneca, Merck Serono, MSD, Daiichi Sankyo, Ono and Chugai, grants from Astellas, Eisai, Dainippon Sumitomo and Incyte, personal fees from Nihon Servier, outside the submitted work. MI reports personal fees and other from Eisai, MSD, Eli Lilly, Yakult and ASLAN, personal fees from GSK, other from Merck Serono, Ono, J-Pharma and AstraZeneca, during the conduct of the study; personal fees and other from EA Pharma, Yakult, Nihon Servier, Chugai, Bristol-Myers Squibb, Novartis, Bayer and Takeda, personal fees from Teijin, Astellas, Sumitomo Dainippon, Otsuka and Taiho, other from Pfizer, Merus N.V., Ono, Delta-Fly Pharma and Chiome Bioscience, outside the submitted work. NO reports personal fees from Taiho, Eli Lilly, Kyowa Hakko Kirin, Eisai, Bayer, Chugai, J-Pharma, Takeda and GSK, outside the submitted work. SS reports grants from Yakult, Incyte, AstraZeneca and Delta-Fly Pharma, outside the submitted work. NM reports grants from MHLW, Japan and Taiho, during the conduct of the study; grants and personal fees from Novartis, MSD and Yakult, grants from NanoCarrier, Dainippon Sumitomo, ASLAN, Incyte and Ono, personal fees from AstraZeneca and Teijin, outside the submitted work. TN reports grants and personal fees from Taiho, Chugai, Ono, Bristol Myers Squibb, Eli Lilly, grants from MSD, Dainippon sumitomo and AstraZeneca, outside the submitted work. YK reports grants from Beigene, Incyte, Ono, Takeda, Daiichi Sankyo and Eisai, personal fees from Chugai, Sanofi, Bristol-Myers Squibb, Taiho, Eli Lilly and Yakult, personal fees and non-financial support from AstraZeneca, outside the submitted work. YK reports personal fees from Takeda, Merck Biopharma, Eli Lilly, Taiho and Yakult, outside the submitted work. TY reports personal fees from Eli Lilly, outside the submitted work. HI reports personal fees from Taiho, Eli Lilly, Yakult, Daiichi Sankyo, Kyowa Hakko Kirin, Mochida and Hospira Japan, during the conduct of the study; personal fees from Yakult and Taiho, outside the submitted work. JF reports grants from the National Cancer Center and the MHLW, Japan, during the conduct of the study; grants from Ono, MSD, Merck Bio, J-Pharma, Taiho, Takeda, Chugai, Astra Zeneca, Yakult, Eisai, Daiichi Sankyo, Mochida, Sanofi, Sumitomo Dainippon, Bayer, Astellas and Incyte, personal fees from Ono, Bayer, Eisai, Eli Lilly, MSD, Yakult, Chugai, Novartis, Astra Zeneca, Pfizer, Takeda, Taiho, Sanofi, Mylan EPD, EA Pharma, Kyowa Hakko Kirin, Daiichi Sankyo, Teijin, Servier Japan and Incyte, outside the submitted work. All other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamada, I., Morizane, C., Okusaka, T. et al. The clinical outcomes of combination chemotherapy in elderly patients with advanced biliary tract cancer: an exploratory analysis of JCOG1113. Sci Rep 12, 987 (2022). https://doi.org/10.1038/s41598-021-04550-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04550-8
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.