Abstract
JCOG1113 is a randomized phase III trial in patients with advanced biliary tract cancers (BTCs) (UMIN000001685), and gemcitabine plus S-1 (GS) was not inferior to gemcitabine plus cisplatin (GC). However, poor renal function often results in high toxicity of S-1. Therefore, we examined whether GS can be recommended for patients with low creatinine clearance (CCr). Renal function was classified by CCr as calculated by the Cockcroft-Gault formula: high CCr (CCr ≥ 80 ml/min) and low CCr (80 > CCr ≥ 50 ml/min). Of 354 patients, 87 patients on GC and 91 on GS were included in the low CCr group, while there were 88 patients on GC and 88 patients on GS in the high CCr group. The HR of overall survival for GS compared with GC was 0.687 (95% CI 0.504–0.937) in the low CCr group. Although the total number of incidences of all Grade 3–4 non-haematological adverse reactions was higher (36.0% vs. 11.8%, p = 0.0002), the number of patients who discontinued treatment was not different (14.1% vs. 16.9%, p = 0.679) for GS compared with GC in the low CCr group. This study suggests that GS should be selected for the treatment of advanced BTC patients with reduced renal function.
Similar content being viewed by others
Introduction
Biliary tract cancers (BTCs) include those in the intrahepatic bile duct (IHBD), extrahepatic bile duct (EHBD), gallbladder (GB), and ampulla of Vater (AV). Although the incidence of BTC is low, it varies according to geographic region. In Japan, BTCs other than IHBD are the 6th leading cause of cancer death, with approximately 18,000 patients1, and BTCs are generally diagnosed at an advanced stage2. Gemcitabine plus cisplatin (GC) has been the standard chemotherapy treatment for advanced/recurrent BTCs since 20103. Recently, ABC-06 showed the superiority of FOLFOX compared with active symptom control as a second-line treatment4. As targeted agents, inhibitors of fibroblast growth factor receptor (FGFR) aberrations, such as pemigatinib and futibatinib, showed promising activities in the IHBD5,6. Moreover, immune checkpoint inhibitors are being actively developed7,8,9,10. However, the recommended treatment options for unresectable or metastatic disease have been limited, and the prognosis of these patients is poor, with a median overall survival (OS) of approximately 1 year. S-1, an oral fluoropyrimidine combination drug consisting of tegafur, gimeracil, and oteracil, has achieved promising outcomes for several cancers11,12.
The Japan Clinical Oncology Group (JCOG) Hepatobiliary and Pancreatic Oncology Study Group conducted a randomized phase III trial to confirm that gemcitabine plus S-1 (GS) is not inferior to GC for unresectable or recurrent BTCs (JCOG1113). From May 2013 to March 2016, 350 patients were registered for JCOG1113. In the final analysis, GS was demonstrated not to be inferior to GC for OS (median OS, 13.4 months with GC and 15.1 months with GS; hazard ratio [HR] 0.945; 90% confidence interval [CI] 0.78–1.15; P = 0.046 for non-inferiority), with good tolerability, and was considered to be a new convenient treatment option without hydration for advanced BTCs.
Renal function needs to be considered when using cisplatin, because of its renal toxicity13. Furthermore, gimeracil, one of the components of S-1, inhibits the metabolism of fluorouracil and is excreted in urine14. The serum concentration of gimeracil increases in patients with renal dysfunction, which theoretically results in a higher serum concentration of fluorouracil. Based on this mechanism, previous studies examined the influence of renal function on the toxicity of S-1 monotherapy (80 mg/day for a body surface area [BSA] on days 1–28 followed by a 14-day rest); patients with low creatinine clearance (CCr) showed a high incidence of severe adverse events (AEs) that resulted in treatment discontinuation15,16,17. Although there are no reports that have evaluated the influence of renal function on survival outcomes for patients with advanced BTCs, high incidence of AEs may result in a poor prognosis due to a low dose intensity. However, AEs and survival outcomes for patients treated with GS have not been reported. Therefore, we examined whether GS can be recommended for patients with low CCr in JCOG1113 as an exploratory analysis.
Methods
Summary of JCOG1113
JCOG1113 is a multicentre open-label randomized phase III study that was conducted to confirm that GS is not inferior to GC for OS in patients with advanced/recurrent BTCs. The key eligibility criteria for JCOG1113 were as follows: histologically proven BTCs (adenocarcinoma or adenosquamous carcinoma of the IHBD, EHBD, GB, or AV), unresectable or recurrent disease, age 20–79 years, Eastern Cooperative Oncology Group performance status score of 0 or 1, adequate self-supported nutritional intake, no previous treatment for BTCs except surgery or biliary drainage, no previous chemotherapy or radiotherapy, adequate function of the major organs, serum creatinine ≤ 1.2 mg/dl, and CCr ≥ 50 ml/min.
Patients assigned to GC received gemcitabine (1000 mg/m2) and cisplatin (25 mg/m2) infusion on days 1 and 8; this regimen was repeated every 3 weeks. Cisplatin was administered up to 16 times (400 mg/m2) unless patients met the termination criteria. After cisplatin termination, patients received gemcitabine (1000 mg/m2) infusion on days 1, 8, and 15, and this was repeated every 4 weeks. Patients assigned to GS received gemcitabine (1000 mg/m2) infusion on days 1 and 8. S-1 was administered orally twice daily (60 mg/day for a BSA < 1.25 m2, 80 mg/day for a BSA between 1.25 and 1.50 m2, and 100 mg/day for a BSA 1.50 m2) on days 1–14. This regimen was repeated every 3 weeks.
Patients
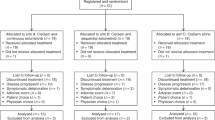
The secondary use of data from JCOG1113 was included in the written informed consent provided by all patients, and approval was obtained from the Institutional Review Board of 32 participated institution (Kanagawa Cancer Center, National Cancer Center Hospital, National Cancer Center Hospital East, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Kyorin University Faculty of Medicine, Yokohama City University Medical Center, Shizuoka Cancer Center, Saitama Cancer Center, Aichi Cancer Center Hospital, Kansai Medical University Hospital, Teikyo University School of Medicine, Kobe University Graduate School of Medicine, Teine Keijinkai Hospital, Osaka National Hospital, Jichi Medical University, National Hospital Organization Shikoku Cancer Center, Tochigi Cancer Center, National Center for Global Health and Medicine, Kyushu University, Hokkaido University Hospital, National Hospital Organization Kyushu Cancer Center, Kitasato University Hospital, Chiba Cancer Center, Sapporo-Kosei General Hospital, Kanazawa University, University of Toyama, Osaka International Cancer Institute, Chiba University, Niigata Cancer Center Hospital, Tokyo Women's Medical University, Tokai University School of Medicine, and Kindai University Faculty of Medicine). All eligible patients registered in JCOG1113 (n = 175/179 in the GC/GS arm) were included in this analysis. These patients were divided according to renal function. The study was conducted in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments. All patients provided written informed consent.
Statistical analysis
AEs related to the treatment were defined as adverse reactions (ARs). ARs and AEs were reported according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 in JCOG1113. In the present study, we defined the number of ≥ Grade 3 ARs as a mark of safety. OS was defined as the duration from the date of registration in the trial to the date of any cause of death or final follow-up. Progression-free survival (PFS) was defined as the duration from the date of registration in the trial to the date of documented disease progression or any cause of death. Response rate (RR) was defined as the proportion of CR or PR patients according to Response Evaluation Criteria in Solid Tumors version 1.1. Percentage of planned dose was defined as the ratio of the delivered dose to the planned dose.
The endpoints of the present study were OS, PFS, ≥ Grade 3 AR, and the percentage of planned dose. To examine the effects of renal function on these endpoints, we classified patients into high CCr (≥ 80 ml/min) and low CCr (< 80 ml/min) groups relative to the median value. CCr was calculated using the Cockcroft-Gault formula18 or was obtained from the actual data at the time of registration. The incidences of ARs and response rate were evaluated in each subgroup, and Clopper-Pearson 95% confidence intervals (95% CIs) were calculated. OS and PFS were estimated using the Kaplan–Meier method, and 95% CIs of the median OS and PFS were evaluated using the methods of Brookmeyer and Crowley. To assess the prognostic difference of each factor for OS and PFS, the HRs and 95% CIs were estimated using Cox’s regression model, and log-rank p-values were calculated. The percentages of planned doses for gemcitabine, cisplatin, and S-1 were expressed using the median values. These were compared with the Wilcoxon rank-sum test. ARs, total numbers of incidences of all Grade 3–4 non-haematological ARs, the numbers of patients who discontinued treatment and RRs were compared using Fisher’s exact test. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) at the JCOG Data Center.
Results
There were 87 and 88 patients in the low and high CCr groups, respectively, in the GC arm and 91 and 88 patients in the low and high CCr groups, respectively, in the GS arm. Patient characteristics in the high and low CCr groups are shown in Table 1. Median age and the percentage of female patients were higher in the low CCr group.
Safety
The incidences of clinically important Grade ≥ 3 ARs in the high and low CCr groups are shown in Table 2. The incidences of Grade ≥ 3 haematological ARs were higher in the low CCr group in each regimen, respectively. Looking at each CCr group, the incidence of Grade ≥ 3 non-haematological ARs was higher in the GS arm (36.0%; 95% CI 26.1–46.8%) than that in the GC arm (11.8%; 95% CI 5.8–20.6%) in the low CCr group (p = 0.0002). On the other hand, the incidences of Grade ≥ 3 haematological ARs, including white blood cell count decreased (35.3%/23.6%), anaemia (29.4%/7.9%), and platelet count decreased (18.8%/10.1%), were higher in the GC arm than those in the GS arm in the low CCr group. The number of patients who discontinued treatment was not different (14.1%/16.9%, p = 0.679) in the GS arm compared with the GC arm in the low CCr group. No remarkable significant differences were observed in the percentages of planned doses for gemcitabine, cisplatin, and S-1 (median), as shown in Table 3.
Efficacy
The response rates for GC and GS were 32.1% and 32.9%, respectively, in the low CCr group. In the high CCr group, they were 32.9% and 26.5%, respectively.
Kaplan–Meier curves for OS and PFS in each CCr group are shown in Fig. 1a–d. The HRs of OS and PFS for GS compared with GC were 0.800 (95% CI 0.577–1.110) and 0.687 (95% CI 0.504–0.937), respectively, in the low CCr group. They were 1.122 (95% CI 0.806–1.563) and 1.060 (95% CI 0.781–1.439), respectively, in the high CCr group.
Comparison of overall survival and progression-free survival in each CCr group according to GC and GS arm. (a) Kaplan–Meier curves of overall survival in the low CCr group. (b) Kaplan–Meier curves of progression-free survival in the low CCr group. (c) Kaplan–Meier curves of overall survival in the high CCr group. (d) Kaplan–Meier curves of progression-free survival in the high CCr group. CCr, creatinine clearance; CI, confidence interval; GC, gemcitabine plus cisplatin; GS, gemcitabine plus S-1; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Kaplan–Meier curves for OS and PFS in each regimen are shown in Fig. 2a–d. The HRs of OS and PFS for low CCr compared with high CCr were 1.112 (95% CI 0.804–1.556) and 1.081 (95% CI 0.796–1.468), respectively, for GC. The HRs of OS and PFS for low CCr compared with high CCr were 0.806 (95% CI 0.582–1.117) and 0.749 (95% CI 0.553–1.013), respectively, for GS.
Comparison of overall survival and progression-free survival in the GC and GS arms according to the low and high CCr groups. (a) Kaplan–Meier curves of overall survival for GC. (b) Kaplan–Meier curves of progression-free survival for GC. (c) Kaplan–Meier curves of overall survival for GS. (d) Kaplan–Meier curves of progression-free survival for GS. CCr, creatinine clearance; CI, confidence interval; GC, gemcitabine plus cisplatin; GS, gemcitabine plus S-1.
A multivariable analysis of OS and PFS revealed that the HRs for GS compared with GC in the low CCr group were 0.775 (95% CI 0.577–1.078; P = 0.13) and 0.658 (95% CI 0.479–0.906; P = 0.01), respectively, as shown in Table 4.
Discussion
This is the first study to compare the influence of CCr on the efficacy and safety of GC and GS in BTCs. In the future, the development of targeted agents and immune checkpoint inhibitors will progress along with that of the combination of cytotoxic agents. Thus, it is still important to understand the characteristics of the combination of cytotoxic agents. The low CCr group (< 80 ml/min) showed better PFS only in the GS arm. Moreover, response rates in the GS arm were higher in the low CCr group, and the HRs of OS and PFS for the low CCr group were better in the GS arm. Regarding safety, although the incidence of non-haematological Grade ≥ 3 ARs was higher in the GS arm than that in the GC arm, the toxicity of GS was not severe in the low CCr group. Therefore, GS may be recommended for the low CCr group.
These results may be attributed to a higher serum concentration of fluorouracil caused by gimeracil due to renal dysfunction in the low CCr group compared with the serum concentration in the high CCr group. As for efficacy, a high S-1 dose intensity is reported to be related to better results in gastric cancer19. GS in the low CCr group would have the same high S-1 dose intensity. With regard to safety, previous studies reported that renal dysfunction was associated with increases in toxicities. In the present study, increases were observed in total ≥ Grade 3 non-haematological ARs. However, the percentage of planned dose for S-1 was similar in both the low and high CCr groups, which was interpreted as good tolerability of the GS regimen despite reduced renal function. This may be attributed to the administration schedule and dose of S-1. S-1 is generally administered for 4 weeks followed by a 2-week rest. In the present GS therapy, the dose of S-1 administered was lowered by one level for 2 weeks followed by a 1-week rest. Toxicity is usually greater with administration for 4 weeks followed by a 2-week rest compared with two cycles of 2 weeks of administration followed by a 1-week rest20. Thus, in patients with renal dysfunction, S-1 may sometimes enhance antitumor effects and at other times show an increase in toxicity.
The percentage of planned dose for S-1 is an important factor in BTC patients treated with GS. In the present study, the percentage of planned dose for S-1 was not lower in the low CCr group than in the high CCr group despite the predicted higher toxicities. Regarding maintenance of the percentage of planned dose for S-1 in the low CCr group, the S-1 dosage could be determined based on renal function as well as BSA, as reported previously21. GS with a one level higher dose of S-1 (80 mg/m2 BSA per day on days 1–14) in the high CCr group may be a possibility. Previously, a phase II study in pancreatic cancer patients examined GS with a daily S-1 dose of 80 mg/m2 BSA (on days 1–14), and it showed a high response rate and modest toxicities22.
On the other hand, low CCr did not affect the efficacy and safety of GC. The renal toxicity of cisplatin is well known; however, it is accumulated toxicity. This study limited the total volume of cisplatin to ≤ 400 mg/m2/BSA, and each volume of cisplatin was 25 mg/m2/BSA. No large increases in creatine were observed for GC, at least with a total volume of cisplatin of < 400 mg.
This exploratory study had some limitations. Since this was an exploratory analysis, it did not have sufficient statistical precision. Although a prospective phase III study in patients with low CCr is necessary, this type of study is difficult to perform in patients with advanced/recurrent BTCs. In addition, this analysis was limited to patients with CCr ≥ 50 ml/min. The present analysis should not be used for patients with CCr < 50 ml/min. Moreover, the CCr value in most patients is calculated using the Cockcroft-Gault formula. This formula has a tendency to calculate a lower value for CCr than the actual value in elderly and female patients. Furthermore, this study included four sites as BTCs (gallbladder, IHBD, EHBD, ampulla of Vater). The number of patients for each site was limited, and the results should be interpreted carefully. In addition, the study was limited to an Asian population, and it is not possible to extrapolate the results directly to populations in western countries.
In conclusion, GS seemed to be superior to GC in terms of OS and PFS in the low CCr group. As for the percentage of planned dose for S-1, there was no remarkable difference between the two CCr groups despite the higher proportion of non-haematological ARs (≥ Grade 3) in the low CCr group. This study suggests that GS should be selected for the treatment of advanced BTC patients with reduced renal function.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Ministry of Health, Labour and Welfare. Vital statistics Japan. (Ministry of Health, Labour and Welfare, 2020).
Valle, J. W., Lamarca, A., Goyal, L., Barriuso, J. & Zhu, A. X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 7, 943–962 (2017).
Valle, J. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281 (2010).
Lamarca, A. et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet. Oncol. 22, 690 (2021).
Abou-Alfa, G. K. et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 21, 671–684 (2020).
Rizzo, A., Ricci, A. D. & Brandi, G. Futibatinib, an investigational agent for the treatment of intrahepatic cholangiocarcinoma: Evidence to date and future perspectives. Expert Opin. Investig. Drugs 2020, 1–8 (2020).
Ueno, M. et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: A non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol. Hepatol. 4, 611 (2019).
Yoo, C. et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother Cancer 8, 549 (2020).
Piha-Paul, S. A. et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. . Int. J. Cancer J. Int. Cancer 147, 2190–2198 (2020).
Rizzo, A. & Brandi, G. First-line chemotherapy in advanced biliary tract cancer ten years after the ABC-02 trial: “And yet it moves!”. Cancer Treat Res. Commun. 27, (2021).
Boku, N. et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: A randomised phase 3 study. Lancet Oncol. 10, 1063–1069 (2009).
Ueno, H. et al. Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J. Clin. Oncol. 31, 1640–1648 (2013).
Kobayashi, S. et al. Renal toxicity associated with weekly cisplatin and gemcitabine combination therapy for treatment of advanced biliary tract cancer. Oncology 87, 30–39 (2014).
Peters, G. J. et al. Pharmacokinetics of S-1, an oral formulation of ftorafur, oxonic acid and 5-chloro-2,4-dihydroxypyridine (molar ratio 1:0.4:1) in patients with solid tumors. Cancer Chemother. Pharmacol. 52, 1–12 (2003).
Nagashima, F., Ohtsu, A., Yoshida, S. & Ito, K. Japanese nationwide post-marketing survey of S-1 in patients with advanced gastric cancer. Gastric Cancer 8, 6–11 (2005).
Yamanaka, T. et al. Analysis of risk factors for severe adverse effects of oral 5-fluorouracil S-1 in patients with advanced gastric cancer. Gastric Cancer 10, 129–134 (2007).
Aoyama, T. et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer 16, 133–139. https://doi.org/10.1007/s10120-012-0158-1 (2013).
Cockcroft, D. W. & Gault, M. H. Prediction of creatinine clearance from serum creatinine. Nephron 16, 31–41 (1976).
Miyatani, K. et al. Late start and insufficient S-1 dose in adjuvant chemotherapy can lead to poor prognosis in stage II/III gastric cancer. Int. J. Clin. Oncol. 24, 1190–1196 (2019).
Tsukuda, M. et al. Randomized scheduling feasibility study of S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br. J. Cancer 93, 884–889 (2005).
Booka, E. et al. Development of an S-1 dosage formula based on renal function by a prospective pharmacokinetic study. Gastric Cancer 19, 876–886 (2016).
Ueno, H. et al. Multicenter phase II study of gemcitabine and S-1 combination therapy (GS Therapy) in patients with metastatic pancreatic cancer. Jpn. J. Clin. Oncol. 41, 953–958 (2011).
Acknowledgements
We are grateful to the members of the Japan Clinical Oncology Group Data Center and Operations Office for their support in preparing the manuscript and oversight of the study (Dr. Haruhiko Fukuda). We acknowledge the support of Nakanoshima Translation Center and ASCA Corporation in the editing of a draft of this manuscript.
Funding
This work was supported by the National Cancer Center Research and Development Fund (grant numbers 23-A-22, 26-A-4, 29-A-3, 2020-J-3), the Japan Agency for Medical Research and Development (grant numbers JP16ck0106079 and JP19ck0106350), and a Grant-in-Aid for Clinical Cancer Research (H22-ganrinsho-ippan-013) from the Ministry of Health, Labour and Welfare of Japan.
Author information
Authors and Affiliations
Consortia
Contributions
M.U. wrote the main manuscript text and J.M prepared all figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Ueno reports grants and personal fees from Yakult Honsha, grants and personal fees from Taiho Pharmaceutical, AstraZeneca, Merck Serono, MSD, Daiichi Sankyo and Ono Pharmaceutical, personal fees from Nihon Servier, grants from Astellas Pharma, Eisai, Sumitomo Dainippon Pharma and Incyte. Dr. Morizane reports grants and personal fees from Yakult Honsha, J-Pharma, AstraZeneca, MSD and Taiho Pharmaceutical, grants from Eisai, Merck Biopharma and Ono Pharmaceutical, personal fees from Teijin Pharma, Novartis and Abbvie. Dr. Okusaka reports grants and personal fees from Eli Lilly, Taiho Pharmaceutical, Pfizer Japan and Yakult Honsha, personal fees from Meiji Seika Pharma, grants and personal fees from Ono Pharmaceutical, Eisai, Sumitomo Dainippon Pharma, Bristol-Myers Squibb, Bayer Yakuhin, Chugai Pharmaceutical, Zeria Pharmaceutical, Daiichi Sankyo and MSD, grants from Kyowa Hakko Kirin, NanoCarrier and Baxter, personal fees from EA Pharma, FUJIFILM RI Pharma, Celgene, Teijin Pharma, Shire, AbbVie, Takeda Pharmaceutical, Mundipharma, Nihon Servier and Nippon Shinyaku. Mr. Mizusawa reports grants from the Ministry of Health, Labour and Welfare, Japan and Japan Agency for Medical Research and Development (AMED), personal fees from Chugai Pharmaceutical. Dr. Ikeda reports grants from Yakult Honsha, Ono Pharmaceutical, AstraZeneca, J-Pharma, Merck Serono, Bristol-Myers Squibb, Pfizer, Takeda Pharmaceutical and Chiome Bioscience, personal fees and grants from ASLAN, Nihon Servier, NanoCarrier, Novartis, Bayer Yakuhin, Eli Lilly, MSD and Chugai Pharmaceutical, personal fees from Taiho Phamaceutical, Sumitomo Dainippon Pharma, Teijin Pharma, Mylan, Astellas Pharma, EA Pharma, Shire, Gilead and Otsuka. Dr. Okano reports personal fees from Taiho Pharmaceutical, Eli Lilly Japan, Kyowa Hakko Kirin, Eisai, Bayer Yakuhin, Chugai Pharmaceutical, J-Pharma, Ono Pharmaceutical, Takeda Pharmaceutical and Merck BioPharma. Dr. Shimizu reports grants from Sumitomo Dainippon Pharma, Yakult Honsha, Incyte and AstraZeneca. Dr. Mizuno reports grants and personal fees from Taiho Pharmaceutical, grants from the Ministry of Health, Labour and Welfare, Japan, grants and personal fees from Novartis, AstraZeneca, MSD and Yakult Honsha, grants from NanoCarrier, Eisai, Sumitomo Dainippon Pharma, ASLAN Pharmaceuticals, Incyte and Ono Pharmaceutical, personal fees from Teijin Pharma. Dr. Takahashi reports grants from Taiho Pharmaceutical. Dr. Ishii reports personal fees from Ono Pharmaceutical, Yakult Honsha, Taiho Pharmaceutical, Eli Lilly Japan and Teijin Pharma. Dr. Furuse reports grants from the National Cancer Center Research and Development Fund and a Grant-in-Aid for Clinical Cancer Research from the Ministry of Health, Labour and Welfare of Japan, personal fees from Eisai, Bayer Yakuhin, Taiho Pharmaceutical, Ono Pharmaceutical, Novartis, Yakult Honsha, Teijin pharma, Shionogi, EA Pharma, Eli Lilly Japan, Takeda Pharmaceutical, Chugai Pharmaceutical, Mochida Pharmaceutical, Nihon Servier, Sanofi, Fujifilm Toyama Chemical, Nobelpharma, Pfizer, Sawai Pharmaceutical, Daiichi Sankyo, Sumitomo Dainippon Pharma, Merck Serono, Nippon Kayaku, MSD, Shire and Kyowa Hakko Kirin, grants from Ono Pharmaceutical, MSD, Sumitomo Dainippon Pharma, J-Pharma, Yakult Honsha, AstraZeneca, Daiichi Sankyo, Eisai, Bayer Yakuhin, Pfizer, NanoCarrier, Kyowa Hakko Kirin, Taiho Pharmaceutical, Chugai Pharmaceutical, Sanofi, Takeda Pharmaceutical, Mochida Pharmaceutical, Astellas Pharma and Eli Lilly Japan. All other authors declare no conflicts of interest in relation to the present work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ueno, M., Morizane, C., Okusaka, T. et al. Comparison of gemcitabine-based chemotherapies for advanced biliary tract cancers by renal function: an exploratory analysis of JCOG1113. Sci Rep 11, 12885 (2021). https://doi.org/10.1038/s41598-021-92166-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-92166-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.