Abstract
Prognosis of patients with advanced extrahepatic cholangiocarcinoma (eCCA) is poor. The current standard first-line treatment is systemic chemotherapy (CT) with gemcitabine and a platinum derivate. Additionally, endobiliary radiofrequency ablation (eRFA) can be applied to treat biliary obstructions. This study aimed to evaluate the additional benefit of scheduled regular eRFA in a real-life patient cohort with advanced extrahepatic cholangiocarcinoma under standard systemic CT. All patients with irresectable eCCA treated at University Hospital Bonn between 2010 and 2020 were eligible for inclusion. Patients were stratified according to treatment: standard CT (n = 26) vs. combination of eRFA with standard CT (n = 40). Overall survival (OS), progression free survival (PFS), feasibility and toxicity were retrospectively analyzed using univariate and multivariate approaches. Combined eRFA and CT resulted in significantly longer median OS (17.3 vs. 8.6 months, p = 0.004) and PFS (12.9 vs. 5.7 months, p = 0.045) compared to the CT only group. While groups did not differ regarding age, sex, tumor stage and chemotherapy treatment regimen, mean MELD was even higher (10.1 vs. 6.7, p = 0.015) in the eRFA + CT group. The survival benefit of concomitant eRFA was more evident in the subgroup with locally advanced tumors. Severe hematological toxicities (CTCAE grades 3 – 5) did not differ significantly between the groups. However, therapy-related cholangitis occurred more often in the combined treatment group (p = 0.031). Combination of eRFA and systemic CT was feasible, well-tolerated and could significantly prolong survival compared to standard CT alone. Thus, eRFA should be considered during therapeutic decision making in advanced eCCA.
Similar content being viewed by others
Introduction
Biliary tract cancer, representing 3% of all gastrointestinal malignancies, is a rare disease with an incidence of 2–3/100,000 in the Western world1,2,3. The only curative treatment is radical surgery, but due to a locally advanced or metastatic stage most patients are eligible for palliative therapies only4. Despite the suggested survival benefits in the randomized phase III BILCAP trial by adjuvant administration of capecitabine for resected intrahepatic cholangiocarcinoma, high rates of disease recurrence are still contributing to a poor overall prognosis5,6,7.The pivotal phase III ABC-02 trial established the current palliative systemic first-line chemotherapy (CT) standard with gemcitabine and cisplatin8. A large number of trials investigating other combined chemotherapies or the addition of a third agent to gemcitabine and cisplatin (e.g., nab-paclitaxel, S1) failed to improve survival benefit to gemcitabine plus platinum derivate9. In 2021, pemigatinib, the first targeted therapy for patients with unresectable cholangiocarcinoma previously treated with fibroblast growth factor receptor 2 (FGFR2) fusion or rearrangement has been approved based on the results of the phase II FIGHT-202 trial10. Further trials using checkpoint inhibitors and other targeted therapies (e.g. pembrolizumab, nivolumab, anlotinib) are ongoing and results are eagerly awaited9.
In eCCA, concomitant endoscopic placement of biliary metal or plastic stents is an established procedure to ensure biliary drainage and to reduce the risk of obstructive cholangitis11. To improve local tumor control and biliary strictures, local ablative therapies, such as endobiliary radiofrequency ablation (eRFA) or photodynamic therapy (PDT), are applied individually.
eRFA uses a high frequency alternating current applied via a bipolar probe to generate heat that induces localized tissue necrosis12,13. Similarly, in patients with small intrahepatic cholangiocarcinoma (iCCA), percutaneous thermal ablation through RFA or microwave ablation has been shown to be safe and effective in terms of survival14. Studies have also supplied evidence that eRFA prolongs stent patency in cases of eCCA, which may be beneficial in improving survival15,16,17. However, available evidence remains insufficient, as it is mainly derived from retrospective studies with a limited number of patients with malignant biliary obstruction of diverse etiology. Some data is available for eRFA in the setting of eCCA18,19,20. To the best of our knowledge, only one study has evaluated the efficacy of eRFA in eCCA limited to Bismuth type I and II and distal cholangiocarcinoma using a prospective cohort design. Yang et al. reported a significantly longer overall survival (OS) in the eRFA + stent group compared to the stent-only group (13.2 ± 0.6 vs. 8.3 ± 0.5 months; p < 0.001)21. However, patients receiving CT were excluded, hence data evaluating possible synergism of eRFA in combination with current standard of care CT are lacking. Thus, the aim of this study was to evaluate the benefit of concomitant eRFA in combination with systemic CT compared to CT alone in a real-life cohort of patients with advanced eCCA.
Materials and methods
Patient population
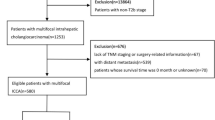
All patients diagnosed with non-curative resectable biopsy-proven eCCA between 2010 and 2020 at the University Hospital of Bonn, Germany, who received palliative systemic first-line CT with gemcitabine ± platinum derivate and who were treated with endobiliary stenting were eligible for inclusion (Fig. 1). Patients were stratified according to treatment: combination eRFA + CT (n = 40) or standard CT only (n = 26). Diagnosis was based on histological (n = 64) or cytological (n = 2) validation. Patients were considered inoperable because of advanced stage of disease (vascular invasion corresponding T4 stage of TNM classification or distant metastasis corresponding N2 and/or M1 stages of TNM classification) or poor performance status due to relevant comorbidities. Patients were treated with systemic CT if performance status, hepatic and renal function were considered sufficient. Concomitant eRFA was offered to every patient with obstructive biliary symptoms and informed consent was obtained. Therapy decisions were made following consensus decision by our interdisciplinary tumor board and in agreement with the individual patient wishes, especially considering toxicities of CT.
Therapeutic procedures
As first-line standard CT, a combination of gemcitabine (1000 mg/m2) and cisplatin (25 mg/m2) was applied. Unfit patients were offered gemcitabine monotherapy and, in case of renal impairment, cisplatin was replaced by oxaliplatin (80 mg/m2). Second-line therapies with FOLFIRI (folinic acid, fluorouracil and irinotecan), capecitabine or cetuximab were applied in 16.6% of patients.
Bile duct stenting was performed via endoscopic retrograde cholangiography (ERC) to treat and prevent cholestasis. Plastic stents (7Fr or 10Fr double-pigtail-stents, ENDO-FLEX, Voerde, Germany) were routinely replaced after 8–12 weeks or earlier in case of cholangitis or progressive cholestasis. When anatomically feasible, self-expanding metal stents (covered or uncovered 10 mm Wallstent™, Boston Scientific, Marlborough, MA, USA) were applied in case of recurrent early dysfunction of plastic stents or if patient performance did not allow scheduled stent replacements. In four patients (eRFA + CT: 2, CT: 2), bile duct stenting via ERC was not possible and cholestasis was treated with percutaneous transhepatic cholangiodrainage (PTCD). A further 11 patients received PTCD during follow-up due to altered anatomy following surgery or disease progression (Table 2). In 38 patients (95%), eRFA was performed through ERC and in two patients (5%), percutaneously. After removal of plastic stents and debris, the 8Fr RFA probe (Habib EndoHPB Bipolar Radiofrequency Catheter, Boston Scientific, Marlborough, MA, USA) was placed into the strictured duct using a guidewire. Cylindrical ablation over a length of 25 mm was performed for 90 s (VIO 200, Soft Coag mode, effect 8, 10 W, ERBE, Tübingen, Germany). The electrode was allowed to cool down for 60 s before being moved. Stepwise ablation from proximal to distal was performed in strictures longer than 25 mm. After eRFA, plastic stents were inserted to ensure adequate decompression of the stricture and bile drainage. If feasible, eRFA was repeated every 3–4 months.
Data collection and study design
This is a single institution retrospective analysis. Baseline parameters (Table 1) were recorded prior to therapy. Patients were followed until death or end of observation period in May 2020. Patients lost to follow-up were censored at date of last visit. Tumor response was assessed by computer tomography and/or magnetic resonance imaging, which were performed regularly every 2–3 months. CT toxicity was recorded according to the common terminology criteria for adverse events version 4.03 (CTCAE) for grades 3–5. Median OS (mOS) was defined as the time range from application of first tumor-specific therapy until death. Median progression free survival (PFS) was defined as the time range from first tumor-specific therapy until progressive disease or death.
This study was approved by the Ethics Committee of the Medical Faculty of the University of Bonn (No. 341/17) and was conducted in accordance to the Declaration of Helsinki. Written, informed consent was obtained from the patients before therapy beginning.
Statistical analysis
Normal distribution of continuous variables was tested with the Kolmogorov–Smirnov test. Differences in continuous variables, expressed as medians and first and third quartiles, were assessed using Student unpaired t test or non-parametric Mann–Whitney test, as appropriate. Categorical variables, expressed as absolute frequencies and percentages, were compared using Pearson’s Chi squared test or Fisher exact test, as appropriate. Survival was compared by log-rank test and transcribed into Kaplan–Meier diagrams. Survival is presented as median and with 95% confidence interval (CI). Univariate and multivariate analyses were performed using Cox regression forward conditional models. Parameters with p-values ≤ 0.1 in univariate analysis were included in multivariate analysis. Results are expressed as hazard ratio (HR) and 95% confidence interval. Two-tailed p-values ≤ 0.05 were considered statistically significant. SPSS version 22 (IBM Corporation, Armonk, NY, USA, https://www.ibm.com/products/spss-statistics) was used for statistical analysis.
Results
Baseline and therapy characteristics
Between 2010 and 2020, 66 patients fulfilled the inclusion criteria: 26 (39.4%) patients were treated with CT alone and 40 (60.6%) patients received a combined therapy with CT and concomitant eRFA. Baseline characteristics are shown in Table 1. Hilar CCA Bismuth types III and IV were the predominant tumor localization in both groups (77.5% for combination group and 73.1% for CT alone). Patients receiving eRFA + CT had a worse liver function determined by higher MELD score (p = 0.015) than patients receiving CT at time of diagnosis. There were no other significant baseline differences between the combination group and the CT alone group.
All patients treated with CT received either a combination of gemcitabine and platinum derivates (cisplatin, oxaliplatin) or gemcitabine monotherapy in first-line CT. There were no significant differences in protocols, number of received cycles of CT or applied second-line CT between the two groups.
During therapy, patients received bile duct stenting or percutaneous transhepatic cholangiography interventions (PTCD) at regular intervals. If feasible, eRFA was repeated every 3–4 months. However, the total number of ablation procedures varied considerably (1–21 procedures) due to clinical performance, progression of disease, and patient decision. Overall, we performed 126 eRFAs, 55% of all patients treated with eRFA received more than one ablation, while 12.5% received more than five procedures.
A total of 20 (30.3%) patients were treated with PDT at least once, with even distribution between the combination group and the CT alone group (p = 0.947).
Therapy characteristics are shown in Table 2.
Analysis of survival
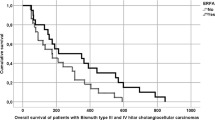
The median OS was 17.3 months (95% CI 10.9, 23.8) in the combination group and 8.6 months (95% CI 4.9, 12.4) in the CT alone group. (PFS) was 12.9 months (95% CI 7.8, 18.0) and 5.7 months (95% CI 4.0, 7.4) in the combination and the CT alone group, respectively. OS and PFS were significantly longer in the combined therapy group, determined by log-rank tests (p = 0.004 and p = 0.045, respectively). Kaplan‐Meier analysis of OS and PFS for the combination group vs. CT alone group is shown in Fig. 2a,b.
Kaplan–Meier survival analysis, with Log-Rank P. (a) Overall survival: eRFA + CT vs. CT alone. (b) Progression free survival: eRFA + CT vs. CT alone. (c) Overall survival of patients with non-metastatic disease: eRFA + CT vs. CT alone. (d) Overall survival of patients with metastatic disease: eRFA + CT vs. CT alone. CT chemotherapy, eRFA endobiliary radiofrequency ablation.
Subgroup analysis
A subgroup analysis of patients with locally advanced disease vs. patients with metastatic disease revealed a survival benefit for the former when treated with combined CT + eRFA. Median OS was 20.9 months (95% CI 17.9, 24.0) for the combination group vs. 12.4 months for the CT alone group (95% CI: 3.7, 21.0) for non-metastatic disease and 15.0 months (95% CI 4.7, 25.3) vs. 8.6 months (95% CI 4.3, 13.0) for patients with metastatic disease. Comparison by log-rank test showed a significant survival benefit for the combination group in locally advanced stage (p = 0.043) that disappeared in the presence of extrahepatic metastases (p = 0.116), (Fig. 2c,d).
Univariate and multivariate analysis
The parameters identified as significant predictors of survival by univariate analysis are shown in Table 3. In a multivariate Cox regression analysis, combined eRFA with CT (HR: 0.422, 95% CI 0.218, 0.816, p = 0.010) and initial surgery with tumor resection (HR: 0.201, 95% CI 0.068, 0.596, p = 0.004) remained significant independent predictors for survival.
Toxicity
Distribution of adverse events (AE) and toxicity is shown in Table 4. Cholangitis was the most frequently observed adverse event during therapy, with more episodes in the combination group (p = 0.031). Interestingly, there were no significant differences in the frequency of post-interventional cholangitis and other typical intervention-related complications, such as bleeding, pancreatitis, abscess or biloma formation between the combination group and the CT alone group. Hematological toxic effects occurred equally in both groups receiving CT. No further significant differences were found between the two groups.
Discussion
In this retrospective study, we found that endobiliary RFA in combination with systemic CT was a feasible and safe treatment regimen in our cohort of patients with unresectable eCCA that was associated with a significantly prolonged median survival (17.3 vs. 8.6 months; p = 0.004) and PFS (12.9 vs. 5.7 months; p = 0.045) compared to current standard treatment with systemic CT alone.
Locally advanced or metastatic cholangiocarcinoma are difficult to manage and limited to palliative treatment options that aim to improve patient survival and quality of life. The current standard first-line treatment option for irresectable eCCA is systemic CT with gemcitabine ± platinum-based agents8.
However, the median OS is still less than one year in studies evaluating standard first-line CT, while in studies with second-line therapies, an OS up to 12.1 months has been reported22,23.
In addition to systemic treatment, advanced eCCA requires the endoscopic management of malignant bile duct strictures with the goal of optimal biliary drainage in order to avoid cholestasis and cholangitis. This can be done effectively through the implantation of biliary plastic or metal stents. To date, local ablative tumor therapy with PDT or eRFA has not been generally recommended for palliative treatment of eCCA. However, there is some evidence that these techniques could prolong stent patency and thus improve overall survival. In a recent retrospective study from our group, we found that PDT combined with CT resulted in significantly longer OS than CT alone24. However, phototoxicity of the photosensitizer is not acceptable for all patients, limiting the use of PDT. Furthermore, a laser is required for PDT, which is not available in all endoscopy units. In contrast, eRFA has no systemic side effects, since the effect of local, high temperatures is limited to the surrounding tissue and neither additional equipment nor specific drugs are needed.
Since Steel et al. reported on the use of eRFA for the treatment of malignant biliary obstruction in 2011, several further studies have demonstrated the safety and the improved maintenance of the bile duct system through eRFA and the influence of eRFA on survival of unresectable eCCA17,18,21,25,26,27,28,29(Table 5). However, all these studies focus on the efficacy of eRFA compared to stenting alone, disregarding the influence of current standard systemic CT, by excluding patients with CT or by matching controls with equal CT status. Accordingly, the safety and the efficacy of eRFA in combination with palliative CT for the treatment of unresectable eCCA remains unclear to date.
Consecutively, we aimed in our analysis to compare the outcome of additional combined eRFA with standard CT vs. standard CT alone. The median survival of the eRFA CT combination group (17.3 months), where the majority of patients had a Bismuth type III and IV hilar CCA, is slightly longer than most results of the already published studies (Table 5).
Furthermore, the combination therapy with eRFA and CT was a significant independent predictor of prolonged survival in the univariate as well as in the multivariate analysis, supporting the significant log-rank test result for OS for combination therapy vs. CT alone. These findings correspond to a combination of the results from Yang et al., whose multivariate analysis revealed eRFA as a main protecting factor improving patient survival, and from Sharaiha et al. and Liang et al., whose multivariate analysis presented CT as a significant predictor of improved survival17,21,27.
Contrary to our previous promising results, PDT was not associated with prolonged survival in this study24. However, with the availability of eRFA in our center, patients requested more eRFA for intraductal treatment of eCCA due to less side effects (phototoxicity). Hence, eRFA partly replaced PDT as first-line approach and PDT was only performed when eRFA failed, when it was technically impossible or when it was requested explicitly by the patient as first-line treatment. This kind of negative selection bias might explain the observed inefficacy of PDT. Prospective randomized studies comparing PDT and eRFA as treatment approaches for intraductal therapy of eRFA are urgently needed.
Compared to the results of the phase III ABC-02 trial, which reported a median survival of 11.7 months for gemcitabine and cisplatin, and the trial of Dierks et al., which reported a 9.5 and 9.6 months OS for their CT groups, our eRFA + CT combination group had a longer OS of 17.3, which we regard as promising data reinforcing the possible beneficial role of eRFA for patients with eCCA8,30.
The results of the present study provide evidence for the feasibility and tolerability of the combination of eRFA and CT, resulting in no relevant differences in frequency of hematologic toxic events compared to CT alone. Hence, no difference in dose adjustment of CT was observed. The pooled rate of adverse events after eRFA is reported with 17% (95% CI 10%, 25%)31. We found a comparable complication rate of 15% for the combination group. Analysis of cholangitis, the most common adverse event in CCA, showed a higher frequency of therapy-related cholangitis for the combination group compared to chemotherapy alone (p = 0.031). This might be explained by the fact that eRFA-induced necrotic tissue leads to the occlusion of biliary stents. Furthermore, a selection bias cannot be excluded for the combination group, in whose patients obstructive cholangitis is seen more often due to primary eRFA-indication-giving biliary obstruction. No differences were found concerning any ERCP-related complication (p = 0.539), which is somewhat surprising due to the significant difference in median applied ERC interventions in the combination group (eight interventions vs. three interventions, p < 0.001).
In agreement with Xia et al., our subgroup analysis revealed a significantly improved survival through combination therapy in non-metastatic eCCA (20.9 vs. 12.4 months, p = 0.043), while the effect disappeared in the presence of metastatic disease (15.0 vs. 8.6 months, p = 0.116)29. These findings suggest a benefit for the combination therapy in eCCA with non-metastatic status, but a reduced influence in patients with M1 status. Future studies are required to evaluate in more detail the systemic effect of eRFA, providing information on more precise selection criteria for treatment with eRFA in patients with unresectable eCCA.
The beneficial effect of eRFA for eCCA reported in this study is also in line with the increasing evidence reported for the use of local therapy (e.g., RFA) in the therapy of intrahepatic cholangiocarcinoma (iCCA). Brandi et al. made an interesting amendment for optimization for RFA effectiveness. Their retrospective study identified intrahepatic tumor lesions < 20 mm as an independent prognostic parameter for longer progression-free survival after percutaneous ultrasound-guided RFA. Additionally, the number of overall nodules treated with RFA as well as the sum of diameter of nodules at the moment of first RFA were significant parameters affecting overall survival32. Therefore, it might be reasonable to perform additive intrahepatic RFA in patients with intrahepatic lesions < 20 mm.
In summary, our study is limited by its retrospective single center design and as therapy decisions were made based on clinical judgement, a selection bias cannot be completely excluded. However, with a cumulative number of 125 eRFA treatments and 40 patients receiving eRFA, it displays one of the largest data records of eRFA for the therapy of unresectable eCCA. Furthermore, our two groups were well balanced in terms of baseline characteristics and our study is the first to show that eRFA in combination with systemic CT is a safe and beneficial treatment regimen for the heterogenous group of patients with unresectable and mainly hilar eCCA and that it can significantly prolong the OS compared to current standard treatment with systemic CT only. To provide a general recommendation for this promising treatment option in patients with eCCA, prospective randomized confirmatory studies are urgently needed.
Data availability
The data used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Marcano-Bonilla, L., Mohamed, E. A., Mounajjed, T. & Roberts, L. R. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin. Clin. Oncol. 5, 61. https://doi.org/10.21037/cco.2016.10.09 (2016).
Walter, D. et al. Cholangiocarcinoma in Germany: Epidemiologic trends and impact of misclassification. Liver Int. 39, 316–323. https://doi.org/10.1111/liv.13954 (2019).
von Hahn, T. et al. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand. J. Gastroenterol. 46, 1092–1098. https://doi.org/10.3109/00365521.2011.589472 (2011).
Doherty, B., Nambudiri, V. E. & Palmer, W. C. Update on the diagnosis and treatment of cholangiocarcinoma. Curr. Gastroenterol. Rep. 19, 2. https://doi.org/10.1007/s11894-017-0542-4 (2017).
Primrose, J. N. et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 20, 663–673. https://doi.org/10.1016/s1470-2045(18)30915-x (2019).
Rizzo, A. & Brandi, G. BILCAP trial and adjuvant capecitabine in resectable biliary tract cancer: Reflections on a standard of care. Expert Rev. Gastroenterol. Hepatol. 15, 483–485. https://doi.org/10.1080/17474124.2021.1864325 (2021).
DeOliveira, M. L. et al. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 245, 755–762. https://doi.org/10.1097/01.sla.0000251366.62632.d3 (2007).
Valle, J. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281. https://doi.org/10.1056/NEJMoa0908721 (2010).
Rizzo, A. & Brandi, G. First-line chemotherapy in advanced biliary tract cancer ten years after the ABC-02 trial: “and yet it moves!”. Cancer Treatm. Res. Commun. 27, 100335. https://doi.org/10.1016/j.ctarc.2021.100335 (2021).
Abou-Alfa, G. K. et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 21, 671–684. https://doi.org/10.1016/S1470-2045(20)30109-1 (2020).
O’Brien, S. et al. Comparing the efficacy of initial percutaneous transhepatic biliary drainage and endoscopic retrograde cholangiopancreatography with stenting for relief of biliary obstruction in unresectable cholangiocarcinoma. Surg. Endosc. 34, 1186–1190. https://doi.org/10.1007/s00464-019-06871-2 (2020).
Mensah, E. T., Martin, J. & Topazian, M. Radiofrequency ablation for biliary malignancies. Curr. Opin. Gastroenterol. 32, 238–243. https://doi.org/10.1097/MOG.0000000000000258 (2016).
Wadsworth, C. A., Westaby, D. & Khan, S. A. Endoscopic radiofrequency ablation for cholangiocarcinoma. Curr. Opin. Gastroenterol. 29, 305–311. https://doi.org/10.1097/MOG.0b013e32835faacc (2013).
Kim, G. H. et al. Thermal ablation in the treatment of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Eur. Radiol. https://doi.org/10.1007/s00330-021-08216-x (2021).
Sharaiha, R. Z. et al. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: Is there an added benefit?. Dig. Dis. Sci. 59, 3099–3102. https://doi.org/10.1007/s10620-014-3264-6 (2014).
Dolak, W. et al. Endoscopic radiofrequency ablation for malignant biliary obstruction: A nationwide retrospective study of 84 consecutive applications. Surg. Endosc. 28, 854–860. https://doi.org/10.1007/s00464-013-3232-9 (2014).
Sharaiha, R. Z. et al. Impact of radiofrequency ablation on malignant biliary strictures: results of a collaborative registry. Dig. Dis. Sci. 60, 2164–2169. https://doi.org/10.1007/s10620-015-3558-3 (2015).
Laquiere, A. et al. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg. Endosc. 30, 1242–1248. https://doi.org/10.1007/s00464-015-4322-7 (2016).
Wang, Y. et al. Percutaneous intraductal radiofrequency ablation in the management of unresectable Bismuth types III and IV hilar cholangiocarcinoma. Oncotarget 7, 53911–53920. https://doi.org/10.18632/oncotarget.10116 (2016).
Bokemeyer, A. et al. Endoscopic radiofrequency ablation prolongs survival of patients with unresectable hilar cholangiocellular carcinoma: A case-control study. Sci. Rep. 9, 13685. https://doi.org/10.1038/s41598-019-50132-0 (2019).
Yang, J. et al. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: A randomized trial. Endoscopy 50, 751–760. https://doi.org/10.1055/s-0043-124870 (2018).
Moik, F. et al. Benefit of second-line systemic chemotherapy for advanced biliary tract cancer: A propensity score analysis. Sci. Rep. 9, 5548. https://doi.org/10.1038/s41598-019-42069-1 (2019).
Mizrahi, J. D. et al. Multi-institutional retrospective analysis of FOLFIRI in patients with advanced biliary tract cancers. World J. Gastrointest. Oncol. 12, 83–91. https://doi.org/10.4251/wjgo.v12.i1.83 (2020).
Gonzalez-Carmona, M. A. et al. Combined photodynamic therapy with systemic chemotherapy for unresectable cholangiocarcinoma. Aliment Pharmacol. Ther. 49, 437–447. https://doi.org/10.1111/apt.15050 (2019).
Steel, A. W. et al. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest. Endosc. 73, 149–153. https://doi.org/10.1016/j.gie.2010.09.031 (2011).
Figueroa-Barojas, P. et al. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: A novel palliation technique. J. Oncol. 2013, 910897. https://doi.org/10.1155/2013/910897 (2013).
Liang, H., Peng, Z., Cao, L., Qian, S. & Shao, Z. Metal stenting with or without endobiliary radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma. J. Cancer Ther. 6(11), 12. https://doi.org/10.4236/jct.2015.611106 (2015).
Kang, H. et al. Efficacy and safety of palliative endobiliary radiofrequency ablation using a novel temperature-controlled catheter for malignant biliary stricture: A single-center prospective randomized phase II TRIAL. Surg. Endosc. 35, 63–73. https://doi.org/10.1007/s00464-020-07689-z (2021).
Xia, M. X. et al. Effect of endoscopic radiofrequency ablation on the survival of patients with inoperable malignant biliary strictures: A large cohort study. J. Hepatobiliary Pancreat. Sci. https://doi.org/10.1002/jhbp.960 (2021).
Dierks, J. et al. Translating the ABC-02 trial into daily practice: outcome of palliative treatment in patients with unresectable biliary tract cancer treated with gemcitabine and cisplatin. Acta Oncol 57, 807–812. https://doi.org/10.1080/0284186x.2017.1418532 (2018).
Zheng, X. et al. Endoscopic radiofrequency ablation may be preferable in the management of malignant biliary obstruction: A systematic review and meta-analysis. J. Dig. Dis. 17, 716–724. https://doi.org/10.1111/1751-2980.12429 (2016).
Brandi, G. et al. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: a retrospective single-center experience. Int. J. Hyperth. 37, 479–485. https://doi.org/10.1080/02656736.2020.1763484 (2020).
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a BONFOR grant from the University of Bonn and grant number 109255 from the German Cancer Aid Association (Deutsche Krebshilfe) awarded to author M.G. The funding source had no role in design, practice or analysis of this study.
Author information
Authors and Affiliations
Contributions
M.G contributed with acquisition of data, analysis and interpretation of data, drafting of the manuscript, study concept and design, C.M. contributed with acquisition of data, analysis and interpretation of data, drafting of the manuscript, study concept and design R.M., T.Z. and A.B. contributed with critical revision of the manuscript for important intellectual content. F.S. contributed with analysis and interpretation of data. M.B. contributed with data curation, study concept and design. A.V. contributed with data curation, administrative support; D.K., D.H., L.D., J.N., V.B., H.M., S.M., J.K. and C.S. contributed with critical revision of the manuscript for important intellectual content. R.M. contributed with study design, drafting of the manuscript, study supervision. T.W. contributed with clinical procedures, study design, analysis and interpretation of data, drafting of the manuscript, study supervision.
Corresponding authors
Ethics declarations
Competing interests
Author T.W. has received speaker fees from Boston Scientific, Cook-Medical and Fujifilm. Author M.G. has contributed to advisory boards for Roche, Eisai, MSD and AZ. However, these activities have no potential conflicts of interest with the manuscript. None of the other authors have any potential conflicts (financial, professional or personal) that are relevant to the manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonzalez-Carmona, M.A., Möhring, C., Mahn, R. et al. Impact of regular additional endobiliary radiofrequency ablation on survival of patients with advanced extrahepatic cholangiocarcinoma under systemic chemotherapy. Sci Rep 12, 1011 (2022). https://doi.org/10.1038/s41598-021-04297-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04297-2
This article is cited by
-
Current Standards, Multidisciplinary Approaches, and Future Directions in the Management of Extrahepatic Cholangiocarcinoma
Current Treatment Options in Oncology (2024)
-
Optimal reproduction of a porcine benign biliary stricture model using endobiliary radiofrequency ablation
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.