Abstract
Acute lymphoid leukemia is a childhood cancer that in high-income countries has event-free survival rates of 80% and global survival rates of 90%. In Brazil these rates are under 70%. This difference may be due to the implementation of supportive care, including the assessment of asparaginase (ASNase) activity. ASNase may cause hypersensitivity reactions and silent drug inactivation. For this reason, ASNase activity monitoring is an essential tool to ensure an effective treatment. Our aim was to implement an ASNase activity measurement technique at a hospital setting. samples from children who were given Escherichia coli-derived ASNase were collected. The results of the analyses conducted in our laboratory Hospital de Clínicas de Porto Alegre were compared to those of two institutions: Centro Infantil Boldrini and University of Munster. 262 samples were assessed. The results of the first analyses were compared with those obtained at Centro Infantil Boldrini and showed an ICC of 0.954. Thirty samples were sent to the University of Munster and presented an ICC was 0.960. Our results, when compared to those of national and international centers, showed an excellent agreement. The study was able to implement an ASNase activity test to monitor the treatment.
Similar content being viewed by others
Introduction
Acute lymphoid leukemia (ALL) is the most common neoplasm in children. Over the years, improvements in treatment have been observed with the use of multicenter protocols. Whereas high-income countries have event-free survival rates of 80% and global survival rates of 90%, in Brazil these rates are under 70%1. This difference is due to several factors, including supportive care with adequate monitoring of the drugs used in the protocols. Asparaginase (ASNase) activity is one of the aspects lacking assessment within our reality in Brazil. Within recent years monitoring increasingly recognized as important to manage ASNase therapy, as it provides a means of identifying patients with sub-ideal activity and provides the information necessary to make adjustments in treatment2,3.
ASNase is an enzyme derived from bacteria that has an anti-leukemic function by catalyzing the hydrolysis of the amino acid l-asparagine in l-aspartic acid and ammonia, and is considered a crucial/essential component of therapies for leukemias4,5. Three different forms have been developed: one is derived from Escherichia coli, another is derived from Erwinia chrysantemi, and a third formulation, pegylated asparaginase (PEG ASNase), which is a conjugation of E. coli with polyethylene glycol and was created to reduce the immunogenic potential4,6,7. Among the adverse effects of ASNase, clinical hypersensitivity reactions were found to occur as a result of the production of anti-ASNase antibodies. These antibodies may also cause rapid enzyme inactivation without clinical signs, referred to as silent inactivation. This phenomenon may generate sub-therapeutic ASNase concentrations leading to a greater chance of relapsed disease8,9. Because of these facts, ASNase monitoring is important to predict future allergic reactions or to alert to silent inactivation1,5,10,11.
The most sensitive and reproducible method with established clinical use is the assessment of ASNase activity. In addition to being related to the level of asparagine depletion, it has the best correlation with clinical efficacy. For these reasons, it is the method currently indicated for regular use in patient care. Today, regimens that achieve ASNase activity ≥ 0.1 IU/mL are considered to be effective and indicative of a better prognosis11,12,13,14.
Understanding the reasons for differences in cure rates in ALL patients between Brazil and high-income countries is essential. Several studies postulate that obtaining an effective treatment requires monitoring the activity of medications6,10,15. In this study, our aim was to implement an ASNase activity technique with safety, quality, and reproducibility at a hospital setting in Brazil, in order to improve the quality of care for our patients.
Methods
Patients and methods
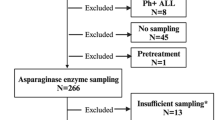
In total, 262 samples were collected from 19 children who were given E. coli-derived ASNase according to the Berlin-Frankfurt-Münster BFM 2009 ALL protocol at Hospital de Clínicas de Porto Alegre (HCPA), a university hospital in Porto Alegre, southern Brazil, between April 2017 and December 2017. A minimum 2 mL of blood sample in ethylenediaminetetraacetic acid (EDTA) were collected 24 h and 48 h after each ASNase infusion. Samples were centrifuged at 3670 rpm for 10 min and stored a maximum of 2 h after collection. All procedures and protocols were approved by the Research Ethics Committee of the Hospital de Clinicas de Porto Alegre (CEP/HCPA) under the number CAAE 69093817.4.0000.5327. Furthermore, all experiments were carried out respecting the appropriate guidelines and all individuals who participated in the study confirmed informed consent, including those who were under 18, who had the informed consent of legal guardians.
Comparative analysis of results
To confirm the successful set up/establishment of the AHA method at our hospital Seventeen analyses were performed at Hospital Boldrini (HB, Campinas, Brazil), which performed the tests only on animals, to practice the technique and establish the critical points of the protocol. Subsequently, 30 samples were sent to the University of Munster (UM, Munster, Germany), a European center of excellence in the analysis of ASNase activity.
The activity of 262 samples was evaluated in our center, based on the reactions of the below-described method, which were classified according to the activity level as above or below 0.1 IU/mL16. The method of analysis used by all centers to determine enzyme activity was based on a technique described by Lanvers et al.10 which uses aspartic acid B-hydroxamate (AHA) as a substrate for the quantification of ASNase derived from E. coli, Erwinia chrysanthemi, and PEG ASNase in human plasma. For determination of ASNase activity, we used E.coli ASNase concentrations between 0.0025 IU/mL and 0.1 IU/mL for the standard curve. Samples with activity > 0.1 IU/mL were repeated on a standard curve with concentrations between 0.1 and 1 IU/mL of.E.coli ASNase. Twenty µl of plasma were diluted with 180 µL of a 2 mM AHA solution dissolved in Tris buffer, pH 7.3 (0.015 M), supplemented with 0.015% (w/v) bovine serum albumin (BSA), and incubated for 30 min at 37 °C. Then, 50 µL of the resulting supernatant were added to a new plate to react with 200 µL of oxin reagent, which consisted of one part of 2% 8-hydroxyquinoline dissolved in absolute ethanol (w/v) and three parts of 1 M sodium carbonate solution. After heating the plate at 95 °C for 1 min and cooling it down for exactly 10 min, absorbance was measured at 690 nm in a SpectraMax M3 equipment (Fig. 1). The standards and samples were analyzed in duplicate, and the control was read in triplicate.

adapted from Lanvers et al.10.
Mechanism of action of asparaginase and reaction of the indooxin method, based on the hydrolysis of l-aspartic b-hydroxamate (AHA), to determine enzyme activity. Figure
Precision was determined by analyzing plasma samples from the standard curve in concentrations (1; 0.5 and 0.15 IU/mL) with five repetitions (intraday precision = analyzes performed on the same day; inter-day precision = analyzes performed on different days with up to 5 days apart). The acceptance criterion used is the coefficient of variation (CV) < 20% for precision10.
Results
Table 1 shows the results of the 262 samples taken 24 and 48 after the infusion and expresses the results in median and interquartile range. The results of the first 17 analyses were compared to those obtained at HB. The results are shown in Table 2 and had an intraclass correlation coefficient (ICC) of 0.954.
Thirty samples from patients who were given ASNase were then sent to the UM in Germany. The results obtained there are shown in Table 3 and compared to the results obtained at our institution. ICC was 0.960, showing agreement. The precision varied between 5.39 and 5.70% for samples analyzed on the same day (intra-day) and between 5.43 and 6.01% for samples analyzed on different days (inter-days), as shown in Table 4.
Discussion
ASNase is an important component of the treatment of children with ALL. However, it may cause complications because of some side effects of the enzyme; also, it may be inactivated by the production of antibodies, leading to decreased treatment effectiveness17. Adequate levels of ASNase activity are known to result in depletion of asparagine and are of critical importance for patients undergoing treatment for ALL17,18. Accurate measurement of serum asparagine in patients may be hindered by continuous enzymatic hydrolysis, since ex vivo metabolism may continue to occur after blood collection, providing an erroneous reading19,20.
For this reason, Albersten et al.21 recommended monitoring the activity levels of the enzyme ASNase and measuring the levels of antibodies. However, measurement of antibodies is not correlated with clinical repercussion for the treatment of each patient, as there is no clear association between antibody titer and level of enzyme activity22. Thus, measurement of ASNase activity levels, as reported in several studies, can be used as a substitute for standardizing asparagine depletion during therapy12,23.
To measure the level of substrate conversion, several quantification methods were developed based on the determination of aspartate or ammonia (NH3) that was produced. The released NH3 can be measured by methods involving reactions with colorimetric reagents, such as Nessler or indophenol, followed by spectrophotometric determination24. These methods detect amounts of ASNase in human plasma as low as 0.02 IU/mL10,25. The Nessler method exhibits good reproducibility and high level of detection but requires caution because it involves the use of highly toxic reagents. Besides, reaction temperature and color equilibration time affect the color development of the solution, which contributes to variation in the results for this method24.
As asparagine depletion is still observed at ASNase concentrations of 0.02 IU/mL, more sensitive methods are needed to determine ASNase activity in human plasma and detect silent inactivation10. Methods for determining aspartate include high-performance liquid chromatography (HPLC), electrophoresis assays, and colorimetric assay with hydroxylamine. Lanvers et al.10 have described the development of an indooxin method for quantification of three different preparations of ASNase in human plasma, a technique that we used in this study. The technique is based on the hydrolysis of AHA, which releases hydroxylamine, which in turn reacts with 8-hydroxyquinoline at alkaline pH. This method produces an intensely green pigment, easily detectable at a range between 690 and 710 nm. This method has a limit of detection as low as 1 × 10–5 IU/mL in human plasma but also has a limited working pH range due to hydroxylamine instability above neutrality10,24. HPLC-based methods overcome the disadvantages of colorimetric methods, showing excellent reproducibility, precision, and linearity when compared, but they have the disadvantage of resulting in higher costs, making them almost unfeasible for analysis of a large number of samples24,26. Therefore, the method described in this study is the most suitable for routine analysis.
The standardization of ASNase quantification by a simple, reliable, fast, and robust method is very useful to overcome the disadvantages of the great disparity between existing methods for determining enzyme activity. With a lack of standardized protocols and pharmaceutical quality control guidelines, standard protocols for measuring the activity of ASNase preparations are rarely reported23,24.
ICC is a measure of agreement with ability to identify identical results, being one of the most widely used statistical tools to determine the reliability of measurements. An ICC ≥ 0.75 is considered excellent for data reproducibility27. In this study, ICCs of 0.954 and 0.960 ensured agreement between the results of our center and those of HB and UM, respectively. The technique showed reproducibility and precision.
The present study was able to implement an ASNase activity test for monitoring the treatment of patients with ALL, allowing for possible adjustments. Our results, when compared to those of other centers, showed excellent agreement. Importantly, to our knowledge, no laboratory in Brazil had yet routinely monitored the biological activity of this enzyme in patients, although there is already a consensus with expert recommendations for its use.
As far as we know, this study was a pioneer in our country in the assessment of ASNase activity in humans. As previously mentioned, the data were obtained at two different and independent oncology centers in Brazil and at an international center using a well-established protocol. Several reports from European groups have indicated the importance of monitoring ASNase activity to prescribe appropriate treatment, as different methods of administration, formulations, doses, and immune responses may generate substantial variation in ASNase activity levels, as well as the interpersonal response10,18. Our study highlights the importance of following the recommendations of international experts for careful handling. The establishment of an appropriate technique allows expanding the availability of this type of assessment to children from other centers.
References
Pieters, R. et al. l-Asparaginase treatment in acute lymphoblastic leukemia: A focus on Erwinia asparaginase. Cancer 102, 238–249 (2012).
Asselin, B. et al. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leukemia Lymphoma 56, 2273–2280 (2015).
Avramis, V. I. & Tiwari, P. N. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int. J. Nanomed. 1, 241–254 (2006).
Schrey, D. et al. Therapeutic drug monitoring of asparaginase in the ALL-BFM 2000 protocol between 2000 and 2007. Pediatr. Blood Cancer. 54, 952–958 (2010).
Rizzari, C. et al. Optimizing asparaginase therapy for acute lymphoblastic leukemia. Curr. Opin. Oncol. 25, S1–S9 (2013).
Fernandez, C. A. et al. Successful challenges using native E. coli asparaginase after hypersensitivity reactions to PEGylated E. coli asparaginase. Cancer Chemother. Pharmac. 73, 1307–1313 (2014).
Petersen, W. C. et al. Comparison of allergic reactions to intravenous and intramuscular pegaspargase in children with acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 31, 311–317 (2014).
Gupta, S. et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J. Clin. Oncol. 38, 1897–1905 (2020).
Vrooman, L. M. et al. Post induction dexamethasone and individualized dosing of Escherichia coli l-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: Results from a randomized study—Dana-Farber Cancer Institute ALL Consortium Protocol 00–01. J. Clin. Oncol. 31, 1202–1210 (2013).
Lanvers, C. et al. Analytical validation of a microplate reader-based method for the therapeutic drug monitoring of l-asparaginase in human serum. Anal. Bioanal. Chem. 309, 117–126 (2002).
Van Der Sluis, I. M. et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica 101, 279–285 (2016).
Panosyan, E. H. et al. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children’s Cancer Group Study CCG-1961. J. Pediatr. Hematol. Oncol. 26, 217–226 (2004).
Barba, P. et al. Asparaginase use for the treatment of acute lymphoblastic leukemia. Med. Clin. 148, 225–231 (2017).
Salzer, W. et al. Asparaginase activity levels and monitoring in patients with acute lymphoblastic leukemia. Leuk. Lymphoma 59, 1797–1806 (2018).
Cecconello, D. K. et al. Asparaginase: An old drug with new questions. Hematol. Transf. Cell Therap. 1379, 30142–30147 (2019).
Cecconello, D. K. et al. Monitoring asparaginase activity in middle-income countries. Lancet Oncol. 2045, 30584–30589 (2018).
Shrivastavaa, A. et al. Recent developments in l-asparaginase discovery and its potential as anticancer agent Conflict of interest: Authors declare no conflict of interest. Crit. Rev. Oncol. Hematol. 100, 1–1 (2016).
Asselin, B. et al. Measurement of serum l-asparagine in the presence of Lasparaginase requires the presence of an l-asparaginase inhibitor. Cancer Res. 51, 6568–6573 (1991).
Gentili, D. et al. Determination of l-asparagine in biological samples in the presence of l-asparaginase. J. Chromatogr. B Biomed. Appl. 657, 47–52 (1994).
Lanvers-Kaminsky, C. et al. Immediate cooling does not prevent the ex vivo hydrolysis of l-asparagine by asparaginase. Ther. Drug Monit. 36, 549–552 (2014).
Albersten, B. K. et al. Asparaginase treatment in infants with acute lymphoblastic leukemia; pharmacokinetics and asparaginase hypersensitivity in Interfant-06. Leuk. Lymphoma 60, 1–7 (2019).
Kloos, R. Q. et al. Allergic-like reactions to asparaginase: Atypical allergies without asparaginase inactivation. Pediatr. Blood Cancer. 63, 1928–1934 (2016).
Grigoryan, R. S. et al. Changes of amino acid serum levels in pediatric patients with higher-risk acute lymphoblastic leukemia (CCG-1961). In Vivo 18, 107–112 (2004).
Magri, A. et al. A critical analysis of l-asparaginase activity quantification methods—colorimetric methods versus high-performance liquid chromatography. Anal. Bioanal. Chem. 410, 6985–6990 (2018).
Boos, J. et al. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur. J. Cancer. 32, 1544–1550 (1996).
Nath, C. E. et al. An isocratic fluorescence HPLC assay for the monitoring of l-asparaginase activity and l-asparagine depletion in children receiving E. colil-asparaginase for the treatment of acute lymphoblastic leukaemia. Biomed. Chromatogr. 23, 152–159 (2009).
Prieto, L. et al. The evaluation of agreement on continuous variables by the intraclass correlation coefficient. J. Epidemiol. Community Health 51, 579–581 (1997).
Acknowledgements
This study was funded by Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundo de Incentivo à Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE/HCPA).
Author information
Authors and Affiliations
Contributions
M.B.M. initiated the design of this study. D.K.C., C.R. and I.W. performed laboratory tests. D.K.C. and M.B.M. wrote the manuscript. L.E.D., C.L.K., J.A.Y., P.P.Z. and A.P.A. provided important clinical insights and helped with the interpretation of variants. All authors have seen and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cecconello, D.K., Rechenmacher, C., Werlang, I. et al. Implementation of the asparaginase activity assessment technique for clinical use: experience of a Brazilian Center. Sci Rep 10, 21481 (2020). https://doi.org/10.1038/s41598-020-78549-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78549-y
This article is cited by
-
Follow our path with asparaginase activity: one technique, but different uses in clinical practice
Experimental Hematology & Oncology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.