Abstract
Background
l-Asparaginase (l-Asp) is an important therapeutic for childhood acute lymphoblastic leukemia (ALL). Asparaginase-associated pancreatitis (AAP) is a severe complication of l-Asp related to the dosage. We investigated the incidence of, and risk factors for, AAP in pediatric patients with ALL.
Methods
From January 2002 to December 2018, pediatric patients with ALL treated at National Taiwan University Hospital were enrolled in this study. The diagnosis of AAP was based on the criteria of the Ponte di Legno Toxicity Working Group.
Results
Of the 353 patients enrolled in this study, 14 (4.0%) developed AAP. The incidence of AAP in ALL patients was significantly higher after treatment with the 2013 protocol compared with the 2002 protocol of the Taiwan Pediatric Oncology Group (9.5% vs. 1.3%). Multivariate analysis showed that a high peak l-Asp dose intensity (>45,000 U/m2/month) and older age at diagnosis (>6.8 years) were independently predictive of AAP development.
Conclusions
The incidence of acute pancreatitis in childhood ALL was correlated more strongly with the peak dose intensity than with the cumulative dose of l-Asp. These results could be used to reduce the treatment-related complications of ALL.
Impact
-
l-Asparaginase is an important therapeutic for childhood acute lymphoblastic leukemia, and the accumulated dosage of l-asparaginase is considered as a major risk factor of asparaginase-associated pancreatitis.
-
This article demonstrated that the incidence of pancreatitis correlates with the dose-intensity of l-asparaginase, but not the accumulated dosage.
-
Identification of patient group with high risk of pancreatitis could lead to early diagnosis and reduce the complication.
-
This finding could aid in developing further new protocol or therapeutic strategy design to reduce treatment-related complications and improve clinical outcomes of childhood acute lymphoblastic leukemia.
Similar content being viewed by others
Introduction
Medication-related pancreatitis accounts for 5–19.9% of all cases of pediatric acute pancreatitis.1 l-Asparaginase (l-Asp) has been used to treat lymphoblastic malignancies in children since 1970. The cytotoxic effect of l-ASP is caused by near-total depletion of circulating asparagine. Unlike healthy cells, malignant lymphoblasts are incapable of synthesizing asparagine, and asparagine depletion induces apoptosis.2 The serum asparagine level is restored to normal within 2 weeks after discontinuing l-Asp.
Acute lymphoblastic leukemia (ALL) is the most common pediatric hematological malignancy. Combination chemotherapy has increased the 5-year survival rate of ALL patients to >85% in developed countries.3 As a result, chemotherapy-related toxicity and complications now account for one-third of all deaths among pediatric patients with ALL.4 l-Asp is an important anti-leukemic agent used for childhood ALL, and its early cessation due to complications is associated with reduced event-free survival.5,6
Asparaginase-associated pancreatitis (AAP) is a severe and potentially life-threatening treatment-related complication with an incidence of 2–18% in pediatric patients with ALL. The crude incidence of AAP is correlated with the average cumulative dosage and the planned treatment duration of l-Asp.7 Older age is also a significant risk factor for AAP.8,9 The pathophysiology of AAP is unclear. AAP may worsen prognosis by causing severe complications, postponement of scheduled chemotherapy, and unintended discontinuation of l-Asp. Sakaguchi. et al tried to prevent AAP by administering octreotide before l-Asp treatment in patients who develop AAP after primary exposure. However, more than half of these patients still developed AAP,10 similar to the AAP incidence following re-exposure to l-Asp without prophylaxis (25–63%).11,12,13 There is no method for predicting the occurrence of AAP.
To improve the clinical outcomes of pediatric patients with ALL, it is important to reduce treatment-related complications and increase the safety of l-Asp administration. The Taiwan Pediatric Oncology Group (TPOG) designed a new protocol for pediatric ALL in 2013 (TPOG 2013 protocol); in this protocol, the l-Asp dosage was increased to improve therapeutic outcomes compared with the TPOG 2002 protocol.14,15 However, the effect of this increased l-Asp dosage on the incidence of AAP in pediatric patients with ALL is unknown. Therefore, we investigated the incidence of, and risk factors for, AAP in pediatric patients with ALL treated with these two protocols.
Subjects and methods
Patient population and study design
From January 2002 to December 2018, patients <18 years old diagnosed with ALL and treated at National Taiwan University Hospital were enrolled in this study. The patients’ clinical data were obtained retrospectively by review of the medical records. The diagnosis of AAP was based on fulfilment of two of the following three Ponte di Legno Toxicity Working Group criteria:16 (a) symptoms of acute pancreatitis, such as epigastralgia with/without radiation to the back, (b) serum amylase or lipase levels more than threefold the upper limit of normal, and (c) abdominal sonography, computed tomography, or magnetic resonance imaging findings indicative of acute pancreatitis.17 Patients who developed AAP within 50 days of l-ASP therapy were identified. This study was approved by the Institutional Review Board of National Taiwan University Hospital.
Treatment with l-Asp
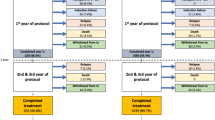
The TPOG 2002 and 2013 protocols stratified patients into standard risk, high risk, and very high risk groups based on the clinical characteristics at diagnosis (age, immunophenotype, cytogenetics, tumor burden, central nervous system involvement, and bone marrow minimal residual disease at induction days 15 and 42, measured by flow cytometry.14,15) In the TPOG 2002 protocol, risk stratification was based on the clinical characteristics of patients at diagnosis, and patients were assigned to receive the appropriate risk-adaptive treatment. In the TPOG 2013 protocol, patients received induction chemotherapy for 14 days, and bone marrow minimal residual disease was assessed on days 15 and 42. The induction regimen after day 15 was determined according to the minimal residual disease on day 15. Risk stratification in the TPOG 2013 protocol was dependent on not only the clinical parameters at diagnosis but also the bone marrow minimal residual disease on days 15 and 42. The differences between the two protocols in dosage and therapeutic duration are listed in Table 1. Details of the protocol are provided in Additional File 1. We calculated the l-Asp dosage as indicated in the standard protocol.
Statistical analysis
Statistical analysis was performed using STATA software (v. 14; StataCorp LP, College Station, TX) and MedCalc (v. 17.5; MedCalc Software, Ostend, Belgium). Differences in categorical variables between groups were analyzed by Fisher’s exact test or the chi-squared test. Student’s t-test was used to compare continuous variables between groups. We defined the peak dose intensity of l-Asp as the maximum l-Asp administered in 1 month and compared it with the cumulative dosage of l-Asp as an independent risk factor for AAP. Receiver operating characteristic (ROC) analysis was used to evaluate the optimal cut-off values of the identified risk factors. Two-sided P values <0.05 were considered indicative of significance.
Results
Patient characteristics
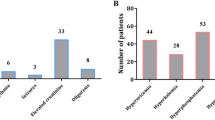
Three hundred seventy-nine pediatric patients diagnosed with ALL were identified during the study period. Twenty-six patients were excluded because of incomplete clinical data. The remaining 353 patients were included in the analysis. Among them, 237 (67.1%) patients were treated with the TPOG 2002 protocol and the other 116 with the TPOG 2013 protocol. The incidences of AAP were 1.3% (3/237) and 9.5% (11/116) in patients treated with the TPOG 2002 and 2013 protocols, respectively (Table 2). Of the 353 patients, 14 (4.0%) had AAP during treatment and were classified as the AAP group. The distributions of sex, immunophenotype, risk stratification, and disease outcome were not significantly different between the AAP and non-AAP groups (Table 2).
Age at ALL diagnosis was significantly older in the AAP group than the non-AAP group (P = 0.006). The mean l-Asp cumulative dose throughout the treatment course was significantly higher in the AAP group than the non-AAP group (203,642.9 vs. 118,073.7 U/m2, P = 0.004).
The characteristics of the 14 ALL patients with AAP are summarized in Table 3. All developed AAP in the induction or reinduction phase, and the induction and reinduction phases involved the highest l-Asp dosage of the treatment protocol. Although five (35.7%) patients were admitted to the intensive care unit because of an unstable clinical condition, no pancreatitis-related deaths occurred. Four (28.6%) patients developed serious complications related to AAP, two (14.3%) had a pancreatic pseudocyst, one (7.1%) had necrotizing pancreatitis, and one (7.1%) had both a pseudocyst and necrotizing pancreatitis. Patient no. 8 had the longest nil per os period (158 days), due to recurrent episodes of pancreatitis after feeding. Severe pancreatic duct damage was responsible for this phenomenon. He received total parenteral nutrition at home via a port-A catheter for 5 months, after which full enteral feeding was resumed. Two (14.3%) patients died from ALL relapse, which was not directly related to AAP. None of the patients needed long-term insulin treatment.
Predictors of AAP
To compare the ability of the peak dose intensity and cumulative dose of l-Asp to predict AAP development, we analyzed the l-Asp dose regimen in each protocol. We grouped the patients according to the l-Asp dosage and calculated the incidence of AAP. The peak dose intensity of l-Asp was defined as the maximum l-Asp dose administered within 1 month (U/m2/month). As shown in Fig. 1, the incidence of AAP tended to increase with the l-Asp dosage. No patient developed AAP in the group receiving the lowest l-Asp dosage (30,000 U/m2/month), whereas the AAP incidence reached 15.6% in the group with the highest l-Asp peak dose intensity and cumulative l-Asp dosage.
The relationship between the cumulative l-Asp dosage and AAP incidence was non-linear because an unexplained decrease in the AAP incidence (1.3%) was observed in the group receiving a cumulative l-Asp dosage of 135,000 U/m2 (Fig. 1b). By contrast, each escalation of the peak dose intensity caused an increase in AAP incidence (Fig. 1a).
The ROC analysis identified the optimal cut-off l-Asp dosage and age as risk factors for AAP (Fig. 2). The area under the curve was largest for age >6.8 years and l-Asp dose intensity >45,000 U/m2/month, with sensitivities of 78.6% and 78.6% and specificities of 60.2% and 69.2%, respectively. We included these two variables in a multivariate analysis of the risk factors for AAP. The odds ratios for age > 6.8 years and l-Asp peak dose intensity > 45,000 U/m2 were 5.86 (95% confidence interval (CI): 1.42–24.25) and 9.36 (95% CI: 2.54–35.65) (Table 4). Therefore, age at diagnosis >6.8 years and l-Asp peak dose intensity >45,000 U/m2 were independently predictive of AAP development in ALL patients during treatment.
Discussion
l-Asp is an essential component of combination chemotherapies for childhood ALL.16 The improved survival outcomes of childhood ALL are attributed, at least in part, to increased use of intense and prolonged l-Asp therapy.18,19,20 By contrast, failure to receive the full course of l-Asp therapy due to treatment-related toxicity is associated with poor survival outcomes in children with ALL.5,20 l-Asp activity ≥0.1 IU/mL indicates adequate asparagine depletion.21 A prolonged course of high-dose l-Asp improves the clinical outcomes in children with ALL.18,19 In response, a higher dose and duration of l-Asp therapy were used in the TPOG 2013 protocol compared with the TPOG 2002 protocol in Taiwan. In this study, the incidence of AAP was significantly higher in pediatric patients with ALL treated with the TPOG 2013 protocol (9.5%) than in those treated with the TPOG 2002 protocol (1.3%). Multivariate analysis confirmed the peak dose intensity of l-Asp as an independent risk factor for AAP in pediatric patients with ALL.
An increased cumulative l-Asp dosage and prolonged treatment duration are associated with an increased AAP incidence.7 However, according to a pharmacokinetic (PK) and pharmacodynamic study by Asselin et al.,22,23 the activity of injected l-Asp dropped to 0.1–0.5 IU/mL at 7 days after injection, and the half-life of Escherichia coli Asp was 1.28 ± 0.35 days. It is unreasonable to estimate the risk of developing AAP according to the cumulative l-Asp dose, which was administered during the induction and reinduction phases, with an interval of approximately 20 weeks. The patients in our cohort developed AAP during induction and reinduction therapy, but not after TPOG 2013 maintenance week 20, when they received the largest cumulative l-Asp dosage (Fig. 3). Kearney et al.12 observed that AAP tends to develop within the first 10 weeks after l-Asp administration, and their regimen included subsequent low-dose l-Asp for 20–30 weeks. The Asp dose intensity represents the total amount of Asp received during a finite period, thus reflecting the dosage, frequency, and duration of Asp administration. For example, the Asp dose intensity was 40,000 U/m2/month during maintenance therapy, less than that during the induction and reinduction phases. Therefore, we propose that the peak dose intensity of l-Asp is a stronger risk factor for AAP compared with the cumulative l-Asp dosage.
Each L-asparaginase administration in TPOG protocol is visualized by arrows. The different box pattern below time line represent the induction and reinduction phase of treatment. The timing of pancreatitis occurred are marked by stars, and all of which were located near the end of induction and reinduction.
The low AAP incidence in the group receiving 135,000 U/m2 l-Asp (Fig. 1b) could be explained by the model of l-Asp dose intensity. The high-risk group of TPOG 2002 received a total l-Asp dose of 135,000 U/m2, but the peak l-Asp dose intensity was 45,000 U/m2/month (Table 1). This suggests that the difference in the l-Asp dose intensity outweighs the difference in cumulative l-Asp dosage when determining the risk of AAP.
Therapeutic protocols use different preparations of l-Asp. In our cohort, E. coli- and Erwinia chrysanthemi-derived l-Asp were used interchangeably. E. coli l-Asp is used as a first-line treatment because it is more cost-effective; it is substituted with Erwinia l-Asp in patients allergic to E. coli l-Asp. According to a head-to-head comparative study,24 E. coli l-Asp caused more coagulation abnormalities compared with Erwinia l-Asp (30.2% vs. 11.9%) but resulted in similar incidences of other toxicities, including pancreatitis, allergy, and liver toxicities. Pegylated asparaginase (Peg-Asp) has a longer half-life than those of the above two preparations of l-Asp.23,25 The AAP incidence of Peg-Asp is typically <10%,26 but one study reported a 10-fold increase in AAP incidence with Peg-Asp (2500 U/m2/dose) compared with E. coli l-Asp (6000 U/m2/dose) (1.9% vs. 18%).27 However, that study did not provide detailed protocols, precluding any comparison. According to the PK and pharmacodynamic study by Panetta et al.,28 an l-Asp dosage of 6000 U/m2 thrice weekly (as in the TPOG 2013 protocol) was equivalent to 3500 U/m2 Peg-Asp weekly, and both preparations had beneficial effects on asparagine depletion in cerebrospinal fluid. These different PK properties of l-asp and Peg-Asp suggest different effects on Asp depletion.23 However, the duration of asparaginase depletion may also depend on the dose schedule. In this study, the incidence of acute pancreatitis in childhood ALL was correlated more strongly with the peak dose intensity than with the cumulative dosage of l-Asp. This suggests that a higher dose intensity of l-Asp prolongs asparaginase depletion and increases the risk of AAP.
Several issues regarding the Asp dose intensity remain unresolved. We did not investigate the biomarkers contributing to the increased incidence of AAP because of the retrospective nature of this study. We typically use the nadir serum asparaginase activity to assess the treatment effect of l-Asp, but increased nadir serum asparaginase activity was not correlated with the incidence of AAP.29 Furthermore, the cut-off of 54,000 U/m2/month may not be applicable to other regimens or ethnicities. The genetic background and the cytotoxic agents administered with l-Asp could alter the critical Asp dose intensity responsible for AAP development.
Patient age at diagnosis of ALL is a risk factor for AAP. Adult ALL protocols typically lack intensive l-Asp treatment, in part because of toxicity concerns. In previous pediatric studies, the risk of AAP increased more than twofold in patients >9 years old.5,9 We performed ROC analysis to identify the cut-off age instead of using predefined age groups. The cut-off (age > 6.8 years) was an independent risk factor in the multivariate analysis.
Regarding confounding factors, physician awareness is a major concern. Proactive surveillance is crucial to avoid missing AAP symptoms, especially in younger children. We follow similar monitoring methods for patients with ALL treated according to the TPOG 2002 or 2013 protocol, i.e., weekly follow-up of serum amylase and lipase levels during l-Asp administration irrespective of symptoms. Therefore, physician awareness likely had little impact on our findings.
Although the pathogenesis of AAP is unclear, it likely involves interplay between the l-asp dosage and host genetic factors. Genetic variants of CPA2, ULK2, RGS6, ASNS, MYBBP1A, IL16, SPEF2, PRSS1, and PRSS2 are reportedly associated with an increased risk of AAP and other complications such as thrombosis and allergic reaction.7,11,17,30,31 Asparaginase synthase (ASNS) is an endogenous enzyme highly expressed in pancreatic acinar cells, and it could replenish the intracellular asparagine depleted by asparaginase. Mukherjee et al.32 reported that l-asp-induced apoptosis is more severe in pancreatic acinar cells with ASNS knockdown. Furthermore, overexpression of ASNS protected against l-Asp-induced apoptosis. This suggests a relationship between genetic variations and the risk of AAP. In this retrospective study, we did not perform genetic analyses and thus could not identify the genes related to AAP development. Investigation of genetic factors implicated in AAP would provide insight into the pathogenesis of the complications related to l-Asp.
The management of AAP is similar to that of pancreatitis of other etiologies. However, there is no consensus on whether to re-challenge with l-Asp after recovery. Almost half of patients with AAP experience AAP relapse after re-exposure to l-Asp,16,33 but the risk of early CNS relapse should also be considered. Physicians usually attempt l-Asp re-challenge in select cases: mild pancreatitis cases, cases in which amylase and lipase levels returned to less than threefold the upper limit of normal within 48 h, and cases without complications such as pseudocysts or necrotizing pancreatitis.20 In our cohort, no patient was re-exposed to l-Asp after developing AAP. Identifying patients at risk is crucial for preventing AAP. Although monitoring of l-Asp is not recommended in the standard of care for ALL, it may prevent adverse effects.34
Exploration of the genetic risk factors for AAP is needed. A prospective randomized control study considering host genetic factors is needed to compare different l-Asp dose intensity age- and risk-specific regimens. To enhance cost-effectiveness, l-Asp activity should be monitored proactively and the l-Asp dosage adjusted in patients at risk of AAP.
Conclusion
We evaluated the incidence of, and risk factors for, AAP in pediatric patients with ALL treated with two different TPOG protocols. Patients treated with the newer protocol had a significantly higher incidence of AAP. A higher peak dose intensity of l-Asp and an older age at diagnosis were independently predictive of a higher risk of AAP development during treatment. These findings will enable identification of high-risk patients, thereby allowing adjustment of the peak dose intensity of l-Asp, monitoring for prevention or early intervention, and formulation of novel therapeutic protocols to reduce treatment-related complications and improve the clinical outcomes of childhood ALL.
References
Suzuki, M., Sai, J. K. & Shimizu, T. Acute pancreatitis in children and adolescents. World J. Gastrointest. Pathophysiol. 5, 416–426 (2014).
Tallal, L. et al. E. coli L-asparaginase in the treatment of leukemia and solid tumors in 131 children. Cancer 25, 306–320 (1970).
Pui, C. H., Mullighan, C. G., Evans, W. E. & Relling, M. V. Pediatric acute lymphoblastic leukemia: where are we going and how do we get there? Blood 120, 1165–1174 (2012).
Prucker, C. et al. Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Munster Study Group. Leukemia 23, 1264–1269 (2009).
Silverman, L. B. et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 97, 1211–1218 (2001).
Gupta, S. et al. Impact of asparaginase discontinuation on outcome in childhood acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J. Clin. Oncol. 38, 1897–1905 (2020).
Liu, C. et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J. Clin. Oncol. 34, 2133–2140 (2016).
Raja, R. A. et al. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the Nopho All2008 protocol. Br. J. Haematol. 165, 126–133 (2014).
Moghrabi, A. et al. Results of the Dana-Farber Cancer Institute all consortium protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109, 896–904 (2007).
Sakaguchi, S., Higa, T., Suzuki, M., Fujimura, J. & Shimizu, T. Prophylactic use of octreotide for asparaginase-induced acute pancreatitis. Int. J. Hematol. 106, 266–268 (2017).
Wolthers, B. O. et al. Asparaginase-associated pancreatitis: a study on phenotype and genotype in the Nopho All2008 Protocol. Leukemia 31, 325–332 (2017).
Kearney, S. L. et al. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr. Blood Cancer 53, 162–167 (2009).
Samarasinghe, S. et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, Ukall 2003. Br. J. Haematol. 162, 710–713 (2013).
Liang, D. C. et al. Long-term results of Taiwan Pediatric Oncology Group Studies 1997 and 2002 for childhood acute lymphoblastic leukemia. Leukemia 24, 397–405 (2010).
Yeh, T. C. et al. Treatment of childhood acute lymphoblastic leukemia with delayed first intrathecal therapy and omission of prophylactic cranial irradiation: results of the Tpog-All-2002 Study. Cancer 124, 4538–4547 (2018).
Wolthers, B. O. et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: an observational Ponte Di Legno Toxicity Working Group Study. Lancet Oncol. 18, 1238–1248 (2017).
Ben Tanfous, M. et al. Polymorphisms of asparaginase pathway and asparaginase-related complications in children with acute lymphoblastic leukemia. Clin. Cancer Res. 21, 329–334 (2015).
Amylon, M. D. et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: A Pediatric Oncology Group Study. Leukemia 13, 335–342 (1999).
Pession, A. et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J. Clin. Oncol. 23, 7161–7167 (2005).
Hijiya, N. & van der Sluis, I. M. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk. Lymphoma 57, 748–757 (2016).
Pieters, R. et al. L-Asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer 117, 238–249 (2011).
Asselin, B. L. et al. Measurement of serum L-asparagine in the presence of L-asparaginase requires the presence of an L-asparaginase inhibitor. Cancer Res. 51, 6568–6573 (1991).
Asselin, B. & Rizzari, C. Asparaginase pharmacokinetics and implications of therapeutic drug monitoring. Leuk. Lymphoma 56, 2273–2280 (2015).
Duval, M. et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group Phase 3 Trial. Blood 99, 2734–2739 (2002).
Raja, R. A., Schmiegelow, K. & Frandsen, T. L. Asparaginase-associated pancreatitis in children. Br. J. Haematol. 159, 18–27 (2012).
Stock, W. et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an Expert Panel. Leuk. Lymphoma 52, 2237–2253 (2011).
Alvarez, O. A. & Zimmerman, G. Pegaspargase-induced pancreatitis. Med. Pediatr. Oncol. 34, 200–205 (2000).
Panetta, J. C. et al. Comparison of native E. coli and Peg asparaginase pharmacokinetics and pharmacodynamics in pediatric acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 86, 651–658 (2009).
Place, A. E. et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (Dfci 05-001): a randomised, open-label phase 3 trial. Lancet Oncol. 16, 1677–1690 (2015).
Abaji, R. et al. Whole-exome sequencing identified genetic risk factors for asparaginase-related complications in childhood all patients. Oncotarget 8, 43752–43767 (2017).
Wolthers, B. O. et al. Trypsin-encoding Prss1-Prss2 variations influence the risk of asparaginase-associated pancreatitis in children with acute lymphoblastic leukemia: a Ponte Di Legno Toxicity Working Group Report. Haematologica 104, 556–563 (2019).
Mukherjee, A. et al. Asparagine synthetase is highly expressed at baseline in the pancreas through heightened perk signaling. Cell Mol. Gastroenterol. Hepatol. 9, 1–13 (2020).
Rank, C. U. et al. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: results from the Nopho All2008 treatment of patients 1-45 years of age. J. Clin. Oncol. 38, 145–154 (2020).
Cooper, S. L. et al. Universal premedication and therapeutic drug monitoring for asparaginase-based therapy prevents infusion-associated acute adverse events and drug substitutions. Pediatr. Blood Cancer 66, e27797 (2019).
Funding
This study is supported by grants from the Ministry of Science and Technology, Taiwan (MOST) (109-2314-B-002-129, 110-2314-B-002-088-MY3 [to H-H-C.]). The remaining authors received no external funding. The funder did not participate in the work.
Author information
Authors and Affiliations
Contributions
C.-B.C., the first author of the study, is responsible for the study design, data management, manuscript writing, and data analysis. H.-H.C. and J.-F.W. are responsible for long-term patients follow, recruitment, study design, critical review of the article, and is the principal investigator of this study. S-W.C., Y.-L.Y., M.-Y.L., S.-T.J., H.-L.C., Y.-H.N, D.-T.L., and M.-H.C. are responsible for long-term patients follow, recruitment, and critical review of the manuscript. All authors have seen and approved the final version. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, CB., Chang, HH., Chou, SW. et al. Acute pancreatitis in children with acute lymphoblastic leukemia correlates with L-asparaginase dose intensity. Pediatr Res 92, 459–465 (2022). https://doi.org/10.1038/s41390-021-01796-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01796-w
This article is cited by
-
Prediction of chemotherapy-related complications in pediatric oncology patients: artificial intelligence and machine learning implementations
Pediatric Research (2023)
-
Does L-asparaginase dose intensity correlate with acute pancreatitis in acute lymphoblastic leukemia patients?
Pediatric Research (2022)