Abstract
Perioperative use of probiotics serves as efficient prophylaxis against postoperative infections after liver transplantation, yet data on long-term effects of pre-transplant probiotic intake is lacking. The aim of this study was to assess the effects of pre-transplant probiotic administration on long-term results of liver transplantation. This was secondary analysis of a randomized trial. Patients were randomized to receive either 4-strain probiotic or placebo before liver transplantation. Five year graft survival was set as the primary end-point. Secondary end-points comprised serum bilirubin and C-reactive protein (CRP) concentration, international normalized ratio (INR), serum transaminases and gamma-glutamyl transferase (GGT) activity. Study group comprised 44 patients, of whom 21 received probiotics and 23 received placebo with 5-year graft survival of 81.0% and 87.0%, respectively (p = 0.591). Patients in the probiotic arm exhibited lower INR (p = 0.001) and CRP (p = 0.030) over the first 6 post-transplant months. In the absence of hepatitis B or C virus infection, pre-transplant administration of probiotics also reduced aspartate transaminase activity (p = 0.032). In the intervention arm, patients receiving probiotics for under and over 30 days had 5-year graft survival rates of 100% and 66.7%, respectively (p = 0.061). Duration of probiotic intake > 30 days was additionally associated with increased INR (p = 0.031), GGT (p = 0.032) and a tendency towards increased bilirubin (p = 0.074) over first 6 post-transplant months. Pre-transplant administration of probiotics has mild positive influence on 6-month allograft function, yet should not exceed 30 days due to potential negative effects on long-term outcomes. (ClinicalTrials.gov Identifier: NCT01735591).
Similar content being viewed by others
Introduction
Alterations in gut microbiota are involved in pathogenesis and progression of various chronic liver diseases through a complex system of interactions, commonly referred to as the gut-liver axis1,2,3. The spectrum of diseases at least partially dependent on gut dysbiosis includes non-alcoholic fatty liver disease, alcoholic liver disease, and hepatocellular carcinoma, among other4,5,6,7. Administration of probiotics is therefore being extensively studied to target the gut dysbiosis in order to improve or slow the progression of various chronic liver diseases.
The positive effects of probiotic intake reported for patients with liver cirrhosis include improvement of liver function, attenuation of portal hypertension, decreased severity of hepatic encephalopathy episodes, attenuation of systemic inflammation, and improvement in immune response8,9,10. In patients with non-alcoholic fatty liver disease, probiotic interventions were associated with improvement of both biochemical and morphological measures of disease severity11,12. A recent meta-analysis revealed that in general, administration of probiotics improves liver function tests, particularly in a setting of underlying liver disease, the use of synbiotics, and longer duration of intervention13. The underlying mechanisms of action comprise inhibition of lipopolysaccharide/toll-like receptor pathway through decreased endotoxin concentrations, improvement of intestinal vascular barrier, and inhibition of growth of pathogenic species in the intestine lumen14,15. Notably, studies on animal models revealed that administration of probiotics ameliorate ischemia–reperfusion injury and improves liver redox status16,17. Further, the negative effects of lipopolysaccharide challenge, partially resembling portal reperfusion in liver transplantation, were attenuated by probiotics18.
The aforementioned benefits of probiotic intake point towards their potential usefulness in liver transplant setting. Given in the perioperative period, probiotics were proven to decrease the rate of postoperative infections without any remarkable risk of adverse effects19,20,21,22,23,24. Further, a single randomized trial performed in our department revealed that continuous administration of probiotics in the pre-transplant period improves early biochemical parameters of graft function and injury, namely serum bilirubin concentration and transaminases activity24. There is, however, no data whether long-term pre-transplant probiotic intake has any effects on post-transplant patient outcomes. While potential protective effects of probiotics may improve liver transplant results by decreasing infection rate and ameliorating ischemia–reperfusion injury, reversion of gut dysbiosis occurs as early as 6 months post-transplantation even without interventions to modulate gut microbiota25. The aim of this study was to evaluate the effects of continuous pre-transplant probiotic intake on long-term outcomes of patients after deceased donor liver transplantation.
Methods
This is a post-hoc analysis of the randomized controlled trial for which data on primary and secondary outcome measures were published previously24. Of the 55 patients enrolled in the trial, 44 patients who underwent liver transplantation in the Department of General, Transplant and Liver Surgery in the period between December 2012 and April 2015 were included. The patients were recruited in the period between November 2012 and March 2015. All participants provided informed consent before inclusion in the study. The study was approved by the local ethics committee of the Medical University of Warsaw (KB196/2011). All the methods were in accordance with the Declaration of Helsinki and national regulations. No organs were procured from prisoners. All organs were procured by the transplant team of the Department of General, Transplant and Liver Surgery of the Medical University of Warsaw.
Specific details on recruitment, randomization, blinding, and sample size calculation were provided in the previous paper24. The criteria for inclusion in the trial were age of at least 18 years, liver cirrhosis, established underlying liver disease, and inclusion on liver transplant waiting list. Patients were excluded in case of immunosuppressive treatment before transplantation, presence of malignancy, renal function impairment, cystic fibrosis, and human immunodeficiency virus infection.
Participants were randomly allocated with a 1:1 ratio to intervention and control groups based on drawing a sealed envelope containing intervention code performed by the investigators. Randomization was blocked (n = 40) and stratified by Child-Turcotte-Pugh class. Patients in the intervention group received 3 × 109 colony-forming units of Lactococcus lactis PB411 (50.0%), Lactobacillus casei PB121 (25.0%), Lactobacillus acidophilus PB111 (12.5%), and Bifidobacterium bifidum PB211 (12.5%) in capsules (ProBacti 4 Enteric, Institut Rosell, Canada) daily from inclusion until transplantation. Patients in the control group received placebo in the form of capsules of identical appearance and taste to that administered in the intervention group once daily from inclusion until transplantation. Placebo consisted of bulking and anti-caking agents in the probiotic capsules: potato starch, cellulose, and magnesium stearate. One capsule per day of either placebo or probiotic was administered. Compliance was assessed by collecting empty boxes and by patient interviews. Patients, surgeons, and other care-providers were blinded to randomization results until the end of the original study.
The primary factor of interest was duration of pre-transplant probiotic intake. Patients were categorized into short-term intake group (< 30 days) and long-term intake group (> 30 days). The primary outcome measure for this post-hoc analysis was graft survival, defined as time from transplantation to retransplantation or patient death irrespective of the cause (combined end-point) and censored at the time of last follow-up. Secondary outcome measures included changes of several laboratory measures over 6-month post-transplantation period, including serum bilirubin concentration, activity of aspartate (AST) and alanine (ALT) transaminase, gamma-glutamyl transferase (GGT), international normalized ratio (INR) for prothrombin time, and c-reactive protein (CRP) concentration.
Quantitative variables were presented as medians with interquartile ranges (IQR) or means with 95% confidence intervals (95% CIs). Qualitative variables were presented as numbers with frequencies. Fisher’s exact test and Mann Whitney U test were used for intergroup comparison of baseline characteristics, as appropriate. Kaplan–Meier estimator was used to calculate graft survival and log-rank test was used to evaluate differences between survival curves. Mixed models with repeated measurements were applied to find differences in laboratory values. Effect sizes were calculated as differences of least squares means of mixed models. In the entire cohort analyses, mixed models analyses were adjusted for donor risk index. The level of significance was set to 0.05. All p values were two-sided. Statistical analyses were computed using SAS v. 9.4 (SAS Institute, Cary, NC) and STATISTICA v. 13.1 (Dell Inc., Tulsa, USA).
Results
The study cohort included 21 patients receiving probiotics (intervention group) and 23 patients receiving placebo (control group) in the pre-transplantation period (Table 1). There were no significant differences between group regarding recipient age (p = 0.735), recipient gender (p = 0.724), Child-Turcotte-Pugh class (p = 0.785), model for end-stage liver disease (p = 0.310), hepatitis C virus infection rate (p = 0.999), hepatitis B virus infection rate (p = 0.481), alcoholic liver disease rate (p = 0.521), duration of graft ischemia (p = 0.897), donor age (p = 0.991), donor risk index (p = 0.948), and caval anastomosis technique (p = 0.999). However, patients in the probiotics group had significantly lower rate of hepaticojejunostomies (p = 0.049). Probiotic intake lasted < 30 and > 30 days in 9 patients (42.9%) and 12 patients (57.1%) in the intervention group, respectively, with the corresponding rates of placebo intake of 30.4% (7 of 23) and 69.6% (16 of 23) in the control group, respectively (p = 0.533). Median duration of pre-transplant probiotic and placebo intake was 44 and 41 days, respectively.
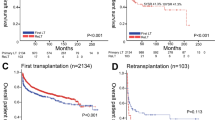
Median duration of follow-up was 63.4 months. There were 5 deaths. Retransplantations were performed in 3 patients. In the entire study cohort, graft survival rates were 88.6% at 1 year and 84.1% at 3 and 5 years. Five-year graft survival was similar in patients in the intervention (81.0%) and control (87.0%) groups (p = 0.591; Fig. 1a). However, 5-year graft survival rate of patients in the intervention group receiving probiotics for > 30 days was 66.7% as compared to 100% of those receiving probiotics for < 30 days (p = 0.061; Fig. 1b).
Notably, all of the three patients who died in the subgroup receiving probiotics for > 30 days had hepatitis C virus reinfection, including two with fibrosing cholestatic hepatitis. Both of these two patients died in the course of allograft failure. The cause of death for the third of these patients was neuroinfection and myositis that occurred during therapy with interferon and ribavirin.
Patients in the intervention group had significantly lower INR (p = 0.001) and CRP concentration (p = 0.030) throughout the first 6 month post-transplantation (Fig. 2; Table 2).
AST, ALT, GGT and bilirubin were non-significantly lower in patients receiving probiotics as compared to those receiving placebo (Fig. 2).
In a subgroup of patients without hepatis C or B viruses infection, pre-transplant probiotic intake was additionally associated with significantly lower AST (p = 0.032). However, patients receiving probiotics for > 30 days before transplantation had higher INR (p = 0.031) and GGT activity (p = 0.032) and a tendency towards increased bilirubin concentration (p = 0.074) over the first 6 post-transplant months than patients receiving probiotics for < 30 days (Table 3).
Discussion
Previously published results on the original primary and secondary outcome measures of this study pointed towards a remarkable reduction in postoperative infection rates and amelioration of ischemia–reperfusion injury with continuous pre-transplant administration of the probiotic regimen24. Notably, the intervention seemed to have a favorable safety profile, given no major adverse events related to probiotic intake and similar rates of the reported gastrointestinal symptoms in the probiotic and placebo groups. However, the results of this post-hoc analysis indicate that despite its clinically irrelevant positive effects regarding INR and systemic inflammation, as reflected by CRP concentration, prolonged pre-transplant intake of probiotics may exert negative effects on post-transplant patient outcomes.
Perioperative administration of probiotics in patients undergoing liver transplantation is being increasingly recognized as a preventive measure against postoperative infections19,20,21,22,23,24,26. While one previous randomized trial assessed the effects of continuous pre-transplant probiotic intake on early post-transplant outcomes, the long-term consequences of such intervention remained unknown24. Given the protective effects on ischemia–reperfusion injury and modulation of gut microbiota at the time of transplantation, continuous pre-transplant probiotic intake was hypothesized to have positive effects on long-term allograft function, especially in the context of liver function improvement in a population of cirrhotic patients8.
No benefits regarding graft survival associated with pre-transplant probiotic administration in general were observed. Despite small numbers, patients receiving probiotics for more than 30 days prior to transplantation had remarkably lower graft survival rate at 5 years than those with probiotic intake not exceeding 30 days, with the difference being on the verge of significance. While there is no clear explanation of this phenomenon, patients with probiotic intake > 30 days additionally had significantly higher INR and GGT as compared to those receiving probiotics for < 30 days, in contrast to the general difference between the intervention and control groups. This may suggest that, unexpectedly, prolonged administration of probiotics before liver transplantation has a negative effect on allograft function. The potential reasons may be related to the modulatory effect on gut-liver axis of the long-term probiotics intervention. Although the present study provided no data on the effects of pre-transplant probiotic administration < 30 days on gut microbiota, 10-week intervention was previously found to be associated with increase in the abundance of Bacteroides and Enterococcus24. Increased Enterococcus counts are known to alter liver function in a population of liver transplant candidates3. Importantly, all deaths in patients receiving probiotics for more than 30 days occurred in the setting of recurrent hepatitis C virus infection. Gut dysbiosis characterized by increased Bacteroides counts and decreased bacterial diversity are found in the course of hepatitis C virus infection27,28. Finally, increased Lactobacillus and Bifidobacterium counts, the bacterial strains included in the regiment, are also known to be increased in the setting of hepatitis C virus-dependent hepatic injury29. Accordingly, as the lower diversity of gut microbiota with increased abundance of Bacteroides, Lactobacillus, and Bifidobacterium may be implicated in the pathogenesis of hepatitis C virus infection, administration of the probiotic regimen utilized in the present study may unexpectedly worsen this state of gut dysbiosis at the time of transplantation, yet this hypothesis remains to be elucidated.
The results of this study are insufficient to provide any clear evidence on casuality between prolonged intake of probiotics and inferior graft survival. However, given the patient numbers, the difference in graft survival rates on the verge of statistical significance cannot be omitted and should be considered as an argument for cautious administration of probiotics before liver transplantation. The positive effects of probiotic intake before liver transplantation with respect to lower INR values over the first 6 months after transplantation are unlikely to reflect improved allograft function, given the effect size not exceeding 0.1. However, the difference with respect to INR may indirectly reflect changes of gut microbiota composition related to pre-transplant probiotics, as vitamin K metabolism is influenced by intestinal bacteria30. Accordingly, increased INR in patients receiving probiotics for more than 30 days may point towards the negative effects of prolonged administration on gut dysbiosis, in line with increased Bacteroides abundance reported previously24. Notably, increased abundance of Bacteroides and associated enhanced immune response was previously reported as one of benefits of probiotic administration31. In the setting of liver disease, this in fact may be a negative consequence of probiotic intake as illustrated by a recent study indicating aggravation of pro-inflammatory response related to higher abundance of these bacteria in the gut32. Nevertheless, probiotic intake in general was associated with lower CRP concentrations in all patients and with lower AST activity in patients without hepatitis B or C virus infection, which points towards its mild protective effect on the allograft. This is in line with the results of previous studies indicating improvement in liver function tests and reduced systemic inflammation associated with administration of probiotics12,13. The observed differences yet do not appear to be clinically relevant.
The present study has several limitations. First, this is a post-hoc analysis utilizing relatively small numbers of patients. Second, there were no data on post-transplant changes in the composition of gut microbiota, which limits the ability to explain the potential reasons for negative consequences of prolonged probiotic intake. Further, the duration of pre-transplant probiotic administration was not random, as it was directly related to the waiting time. However, the selection bias should influence the results in the opposite fashion, as patients with shorter waiting time are expected to be in worse condition and have higher clinical urgency for transplantation. Finally, the results of this post-hoc analysis remain limited to the probiotic regimen containing 3 × 109 colony-forming units of Lactococcus lactis PB411 (50.0%), Lactobacillus casei PB121 (25.0%), Lactobacillus acidophilus PB111 (12.5%), and Bifidobacterium bifidum PB211 (12.5%).
In conclusion, the results of the present study indicate that probiotic intake for more than 30 days before liver transplantation may exert negative effects on post-transplant outcomes. This may be considered as a safety alert, especially for patients undergoing transplantations for hepatitis C virus-related cirrhosis.
Data availability
The data used for this study are available from the authors upon a reasonable request.
References
Wiest, R., Albillos, A., Trauner, M., Bajaj, J. S. & Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103 (2017).
Tripathi, A. et al. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 15, 397–411 (2018).
Grąt, M. et al. The relevance of intestinal dysbiosis in liver transplant candidates. Transpl. Infect. Dis. 17, 174–184 (2015).
Puri, P. & Sanyal, A. J. The intestinal microbiome in nonalcoholic fatty liver disease. Clin. Liver Dis. 22, 121–132 (2018).
Brandi, G. et al. Microbiota, NASH, HCC and the potential role of probiotics. Carcinogenesis 38, 231–240 (2017).
Shen, T. D., Pyrsopoulos, N. & Rustgi, V. K. Microbiota and the liver. Liver Transpl. 24, 539–550 (2018).
Grąt, M. et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transplant. Proc. 48, 1687–1691 (2016).
Dhiman, R. K. et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 147, 1327–1337 (2014).
Horvath, A. et al. Randomised clinical trial: the effects of a multispecies probiotic vs. placebo on innate immune function, bacterial translocation and gut permeability in patients with cirrhosis. Aliment. Pharmacol. Ther. 44, 926–935 (2016).
Rincón, D. et al. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 34, 1504–1512 (2014).
Kobyliak, N. et al. A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J. Gastrointestin. Liver Dis. 27, 41–49 (2018).
Mofidi, F. et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br. J. Nutr. 117, 662–668 (2017).
Khalesi, S. et al. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: a systematic review and meta-analysis. Eur. J. Nutr. 57, 2037–2053 (2018).
Xue, L. et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 7, 45176 (2017).
Ewaschuk, J. B. et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 295, 1025–1034 (2008).
Nardone, G. et al. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 299, 669–676 (2010).
Xing, H. C. et al. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J. Gastroenterol. Hepatol. 21, 647–656 (2006).
Zhao, D. et al. Dietary supplementation with Lactobacillus casei alleviates lipopolysaccharide-induced liver injury in a porcine model. Int. J. Mol. Sci. 18, 2535 (2017).
Rayes, N. et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 74, 123–127 (2002).
Rayes, N. et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation—a randomized, double-blind trial. Am. J. Transplant. 5, 125–130 (2005).
Eguchi, S. et al. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: a prospective randomized study. Am. J. Surg. 201, 498–502 (2011).
Zhang, Y. et al. Probiotic use in preventing postoperative infection in liver transplant patients. Hepatobiliary Surg. Nutr. 2, 142–147 (2013).
Sawas, T., Al Halabi, S., Hernaez, R., Carey, W. D. & Cho, W. K. Patients receiving prebiotics and probiotics before liver transplantation develop fewer infections than controls: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 13, 1567–1574 (2015).
Grąt, M. et al. Effects of continuous use of probiotics before liver transplantation: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. 36, 1530–1539 (2017).
Bajaj, J. S. et al. Alterations in gut microbial function following liver transplant. Liver Transpl. 24, 752–761 (2018).
Jorgenson, M. R. et al. Efficacy and safety of probiotics and synbiotics in liver transplantation. Pharmacotherapy https://doi.org/10.1002/phar.2130 (2018).
Inoue, T. et al. Gut dysbiosis associated with hepatitis C virus infection. Clin. Infect. Dis. 67, 869–877 (2018).
Aly, A. M., Adel, A., El-Gendy, A. O., Essam, T. M. & Aziz, R. K. Gut microbiome alterations in patients with stage 4 hepatitis C. Gut Pathog. 8, 42 (2016).
Heidrich, B. et al. Intestinal microbiota in patients with chronic hepatitis C with and without cirrhosis compared with healthy controls. Liver Int. 38, 50–58 (2018).
Karl, J. P. et al. Fecal concentrations of bacterially derived vitamin K forms are associated with gut microbiota composition but not plasma or fecal cytokine concentrations in healthy adults. Am. J. Clin. Nutr. 106, 1052–1061 (2017).
Núñez, I. N., Galdeano, C. M., de LeBlanc Ade, M. & Perdigón, G. Evaluation of immune response, microbiota, and blood markers after probiotic bacteria administration in obese mice induced by a high-fat diet. Nutrition 30, 1423–1432 (2014).
Ponziani, F. R. et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 69, 107–120 (2019).
Acknowledgements
This research was funded with budgetary resources for science for the years 2012–2015 as a scientific project of the program named “Diamond Grant” of the Ministry of Science and Higher Education of the Republic of Poland (DI2011025641). Michał Grąt was supported by the Foundation for Polish Science (FNP) with START stipend (START 032.2018) and by the Ministry of Science and Higher Education of the Republic of Poland Young Researcher stipend (571/STYP/14/2019). No other funding is associated with the presented research. The funding body had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
M.G.: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, writing—original draft, final approval of manuscript. K.G.: conceptualization, data curation, inclusion of patients in the study, follow-up, writing—review & editing, final approval of manuscript. M.K.: data curation, methodology, supervision, writing—review & editing, final approval of manuscript. Z.L.: conceptualization, formal analysis, methodology, writing—review & editing, final approval of manuscript. M.K.: data curation, writing—review & editing, final approval of manuscript. Ł.M.: data curation, writing—review & editing, final approval of manuscript. W.P.: data curation, writing—review & editing, final approval of manuscript. K.Z.: data curation, supervision, writing—review & editing, final approval of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grąt, M., Grąt, K., Krawczyk, M. et al. Post-hoc analysis of a randomized controlled trial on the impact of pre-transplant use of probiotics on outcomes after liver transplantation. Sci Rep 10, 19944 (2020). https://doi.org/10.1038/s41598-020-76994-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76994-3
This article is cited by
-
Nutritional Optimization of Patients Undergoing Liver Transplantation
Current Treatment Options in Gastroenterology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.