Abstract
Background
Probiotics are commonly used after bariatric surgery. However, uncertainty remains regarding their effects. The purpose of this systematic review was to assess the effect of probiotics in patients with morbid obesity undergoing bariatric surgery.
Methods
PubMed, Cochrane Library, Embase, Science Direct, and Web of Science were searched from inception to April 4, 2023. No language restrictions were applied. Relevant randomized controlled trials and controlled clinical trials were included. We used the aggregated data extracted from the trials and assessed the heterogeneity. When severe heterogeneity was detected, a random effect model was used. All stages of the review were done by independent authors.
Results
We screened 2024 references and included 11 randomized controlled trials and controlled clinical trials. Compared with the protocol groups, probiotics showed significant effects on regulating aspartate amino transferase level (MD = −4.32 U/L; 95% CI [−7.10, −1.53], p = 0.002), triglycerides (MD = −20.16 mg/dL; 95% CI [−34.51, −5.82], p = 0.006), weight (MD = −1.99 kg; 95% CI [−3.97, −0.01], p = 0.05), vitamin B12 (MD = 2.24 pg/dL; 95% CI [−0.02, 4.51], p = 0.05), dietary energy (MD = −151.03 kcal; 95% CI [−215.68, −86.37], p < 0.00001), dietary protein (MD = −4.48 g/day, 95% CI [−8.76, −0.20], p = 0.04), dietary carbohydrate (MD = −34.25 g/day, 95% CI [−44.87, −23.62], p < 0.00001), and dietary fiber (MD = −2.17 g/day, 95% CI [−3.21, −1.14], p < 0.0001). There were no severe side effects related to probiotics.
Conclusions
Our meta-analysis suggested that probiotics may delay the progression of liver function injury, improve lipid metabolism, reduce weight, and reduce food intake, although the effects on other indicators were insignificant. Probiotics may be helpful for patients undergoing bariatric surgery. The review was registered on PROSPERO (International prospective register of systematic reviews): CRD42023407970. No primary source of funding.
Similar content being viewed by others
Introduction
Obesity is a multifactorial disease that accumulates excess body fat and leads to negative health effects [1]. Over the past 40 years, the global prevalence of obesity has increased dramatically, from 3% to 11% in men and from 6% to 15% in women over the same period [2]. Moreover, compared to normal weight, obesity is associated with significantly higher all-cause mortality [3, 4]. With social lifestyles change, the number of patients with obesity is increasing constantly, placing a serious burden on public health [4, 5].
Obesity remains a largely refractory disease to dietary and pharmacological treatment, but responds well to bariatric surgery generally [6]. Bariatric surgery was reported to result in significant weight loss and may induce remission or improvement in obesity-related risks and complications [7, 8].
In recent years, the field of probiotics has been booming. Probiotics are active microorganisms that are beneficial to the host people. Obesity is associated with reduced gut microbial diversity and high rates of micronutrient deficiency [9]. What’s more, oral probiotics can modulate the structure of intestinal microbiome and the altered gut microbiome may influence inflammatory pathways, glucose and lipid metabolism in the host [10,11,12]. In addition, alterations in the gut microbiome were shown to affect these host responses in other settings [13, 14]. Therefore, probiotics were suggested as a therapeutic strategy in patients with obesity for being effective in reducing body mass index and waist circumference [1].
An extensive systematic review on the effects of probiotics on patients with morbid obesity undergoing bariatric surgery has not been conducted. This systematic review and meta-analyses of RCTs aimed to extensively assess the effects of probiotics supplementation in patients with morbid obesity undergoing bariatric surgery.
Methods
A protocol of the study had been registered in PROSPERO database of systematic review protocols on March 26, 2023, with identification number CRD42023407970.
Searches and selection strategy
We searched electronic databases of PubMed, Cochrane Library, Embase, Science Direct and Web of Science, from inception to March 14, 2023, using a combination of subject terms and free words. There were no language or date restrictions. Search terms included: probiotics, probiotic, probiotic*, prebiotics, prebiotic, prebiotic*, synbiotics, synbiotic, synbiotic*, randomized controlled trial, bariatric surgery, RYGB (Roux-en-Y gastric bypass), LSG (Laparoscopic Sleeve Gastrectomy), SG (Sleeve Gastrectomy), OAGB (One-Anastomosis Gastric Bypass). Additionally, references of included studies as well as any systematic review, meta-analysis, and practice guideline relevant to the topic were checked manually to identify studies that were not captured by the online electronic searches. All of the above work was done by independent researchers and was approved by third reviewers.
Inclusion criteria
Studies were selected for inclusion by two independent reviewers and the work was approved by third reviewers. Here are the inclusion criteria: (A) The participants were adults (≥18 years) with morbid obesity (BMI ≥ 40 or as BMI ≥ 35 with accompanying obesity related co-morbidities such as type 2 diabetes, hypertension, obstructive sleep apnea, and others [15]) who received any kind of bariatric surgery (B)The patients were subjected to probiotics at any dose and for any duration. Probiotics were defined as “living microorganisms which when administered in adequate amounts confer a health benefit on the host [16]”. The patients did not use any antibiotics prior to the beginning of the study. (C) The study design was a randomized controlled clinical trial (RCT) or a controlled clinical trial (CCT). (D) The study compared any type of probiotics or synbiotics (a combination of probiotics and prebiotics) with placebo, digestive enzymes, care as usual, and no intervention.
Exclusion criteria
Studies that included patients who had undergone any other gastrointestinal procedures were excluded. Studies comparing probiotics with other interventions rather than placebo were also excluded, as they would also affect the results.
Screenings and data extraction
Identified references were checked for duplications using Endnote software. Screening of titles, abstracts, and full texts was also done by using Endnote. After meeting the inclusion and exclusion criteria, the included studies were reviewed using a standardized template. The subsequent data were extracted: (A) basic features: author, publication year, study design, number of participants, intervention, and outcomes. (B) methods: randomization, allocation concealment, blindness, data integrity, selective reporting, and other biases; (C) intervention measures: specific medication, dose, treatment duration; (D) outcome biomarkers: liver function: serum ALT (Alanine Aminotransferase), AST (Aspartate Aminotransferase), GGT (Glutamyl Transpeptidase); glycemic parameters: plasma glucose, insulin, HbA1c (Hemoglobin A1c), HOMA-IR (Homeostatic Model Assessment for Insulin), QUICKI(Quantitative Insulin Sensitivity Check Index); blood lipid levels: TC (Total Plasma Cholesterol), TG (Triglyceride), HDL (High-Density Lipoprotein), LDL (Low-Density Lipoprotein); inflammatory factor levels: serum IL-6 (Interleukin-6)levels, TNF-a (Tumor Necrosis Factor-a)levels, CRP (C-reactive protein); general measures: %EWL (%Excess Weight Loss), BMI (Body Mass Index), weight, waist circumstance (WC); serum vitamin B12, 25-hydroxy vitamin D3, folate; food intake: dietary energy, dietary protein, dietary cholesterol, dietary fat, dietary fiber; ferritin, Hb (Hemoglobin), BES (Binge Eating Score), YFBS (The Yale Food Addiction Scale), GSRS (Gastric Symptom Rating Scale), and adverse events. Both phases were preceded by pilot screenings to ensure common understanding of inclusion criteria. All the above work was done by two independent researchers and was approved by third reviewers.
Data collection, risk of bias assessment and analysis
Independent researchers evaluated the quality of the literature and were approved by third reviewers. Evaluation aspects included whether: (A)random sequences were properly generated; (B) the distributions of hidden were properly used; (C) subjects and intervention providers were properly blinded; (D) evaluators of the results were properly blinded; (E) the completeness of outcome data was properly maintained; (F) selective reporting was properly conducted (assessed by comparing outcomes specified in the methodology compared to those reported in the results section.); (G) other biases were properly disposed. According to the above specific evaluation criteria, the included studies were categorized as ‘low risk’, ‘high risk’ or ‘unclear risk’. Any disagreements were discussed and resolved with Professor Wang.
Statistical data analysis
We used the Review Manager 5.4 software to perform the data analysis. The effects of probiotics on selected parameters were mostly analyzed using mean difference (MD) with standard deviation (SD). When the study’s authors did not provide SDs of mean differences, we calculated the SDs of outcomes using the following formula: SD² change = SD² baseline + SD² final – (2*correlation coefficient*SD baseline*SD final), assuming that the correlation coefficient is 0.5 [17]. A P ≤ 0.05 was considered statistically significant. To investigate statistical heterogeneity, we visually assessed the forest plots and examined the heterogeneity. Heterogeneity between studies was analyzed using a chi-square test. When I² was >50% or P < 0.1, high heterogeneity was indicated, and random effects model was used; otherwise, the fixed effects model was used.
Results
Literature retrieval, research characteristics, and methodological quality assessment
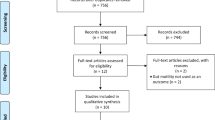
A total of 2024 documents were included based on the search strategy. Finally, 11 studies [18,19,20,21,22,23,24,25,26,27,28] between 2009 and 2022, including 559 patients (279 patients in the probiotic group and 280 patients in the placebo group) were included in the analysis by assessing the full text of articles eligible for detailed assessment. The study of Chen [29] and Fernandes [30] et al. tended to meet the inclusion criteria, but they were excluded for the subjects were small and low quality. Sample sizes ranged from 29 [20] to 80 [22]. The process diagram was shown in Fig. 1. The characteristics of all included RCTs [18,19,20,21,22,23,24,25,26,27,28] were summarized in Table 1, with their methodological quality highlighted in Fig. 2. The durations of the probiotics were 3 months after bariatric surgery except the studies of Woodard and Han et al. The studies of Karbaschian and Mokhtari et al. were from 4 weeks before surgery to 12 weeks after bariatric surgery. Adequate random sequence generation was reported in 11 trials. Allocation concealment was reported in 10 trials. Adequate blinding of participants and personnel for objective outcomes was achieved in 10 of 11 trials. Adequate blinding of outcome assessments was achieved in 7 trials. Attrition bias was reported in 5 trials and reporting bias was not reported. Study protocols were registered in 5 [21, 22, 24, 25, 28] of 11 trials. The study by Han et al. [21] comprised 3 groups: symbiotic, prebiotic, and placebo; thus, in accordance with our methods, we only included prebiotic and placebo groups.
Effects of probiotics on liver function levels
Three studies assessed data on ALT at the end of the third month (The following descriptions, unless otherwise stated, indicate that the results were measured three months after bariatric surgery) postoperatively [18, 22, 28]. There was no significant difference between both groups (80 vs 83, MD = −3.67 U/L; 95% CI [−8.16, 0.82], p = 0.11), with no significant heterogeneity (I² = 0%, P = 0.75) (Fig. 3A). Three studies reported data on AST postoperatively [18, 22, 28]. There was a significant difference between both groups (80 vs 83, MD = −4.32 U/L; 95% CI [−7.10, −1.53], p = 0.002), with no significant heterogeneity (I² = 0%, P = 0.42) (Fig. 3B). Two studies reported data on GGT postoperatively [22, 28]. There was no significant difference between both groups (65 vs 63, MD = −0.53 U/L; 95% CI [−8.12, 7.06], p = 0.89), with no significant heterogeneity (I² = 0%, P = 0.49) (Fig. 3C).
Effects of probiotics on glycemic parameters
Four studies assessed changes in blood glucose from baseline [18, 22, 24, 28]. There was no significant difference between both groups (126 vs 119, MD = −1.52 mg/dL; 95% CI [−7.20, 4.15], p = 0.60), with no significant heterogeneity (I² = 0%, P = 0.98) (Fig. 4A). Four studies reported data in insulin from postoperatively [18, 19, 24, 28]. There was no significant difference between both groups (101 vs 99, MD = 1.12 mU/L; 95% CI [−1.53, 3.78], p = 0.41), with no significant heterogeneity (I² = 0%, P = 0.57) (Fig. 4B). Four studies reported data on HbA1c postoperatively [18, 19, 22, 28]. There was no significant difference between both groups (80 vs 83, MD = −0.09%; 95% CI [−0.27, 0.10], p = 0.37), with a no significant heterogeneity (I² = 0%, P = 0.93). (Fig. 4C). Four studies assessed data on HOMA-IR postoperatively [18, 22, 24, 28]. There was no significant difference between both Groups (126 vs 119, MD = −0.05; 95% CI [−0.83, 0.74], p = 0.91), with no significant heterogeneity (I² = 0%, P = 0.76) (Fig. 4D). Two studies assessed data on QUICKI postoperatively [18, 24]. There was no significant difference between both groups (61 vs 56, MD = 0.00; 95% CI [−0.01, 0.01], p = 1.00), with no significant heterogeneity (I² = 0%, P = 1.00) (Fig. 4E).
A Four studies reported data on plasma glucose and there was no significant difference between both groups. B Four studies reported data on insulin and there was no significant difference between both groups. C Four studies reported data on HbA1c and there was no significant difference between both groups. D Four studies reported data on HOMA-IR and there was no significant difference between both groups. E Two studies reported data on QUICKI and there was no significant difference between both groups.
Effects of probiotics on blood lipid levels
Five studies assessed data on TC from baseline postoperatively [18, 19, 22, 24, 28]. There was no significant difference between both groups (141 vs 139, MD = −6.18 mg/dL; 95% CI [−13.58, 1.22], p = 0.10), with no significant heterogeneity (I² = 0%, P = 0.62) (Fig. 5A). Five studies assessed data on TG from baseline postoperatively [18, 19, 22, 24, 28]. There was a significant difference between both groups (141 vs 139, MD = −20.16 mg/dL; 95% CI [−34.51, −5.82], p = 0.006), with no significant heterogeneity (I² = 0%, P = 0.94) (Fig. 5B). Five studies assessed data on HDL-C from baseline postoperatively [18, 19, 22, 24, 28]. There was no significant difference between both groups (141 vs 139, MD = 1.49 mg/dL; 95% CI [−0.52, 3.51], p = 0.15), with no significant heterogeneity (I² = 0%, P = 0.98) (Fig. 5C). Five studies assessed data on LDL from baseline postoperatively [18, 19, 22, 24, 28]. There was no significant difference between both groups (141 vs 139, MD = −4.46 mg/dL; 95% CI [−11.01, 2.10], p = 0.18), with no significant heterogeneity (I² = 0%, P = 0.85) (Fig. 5D).
A Five studies reported data on TC and there was no significant difference between both groups. B Five studies reported data on TG and there was a significant difference between both groups. C Five studies reported data on HDL and there was no significant difference between both groups. D Five studies reported data on LDL and there was no significant difference between both groups.
Effects of probiotics on inflammatory factors levels
Three studies assessed data on IL-6 from baseline postoperatively [22, 25, 28] (The result of Sherf-Dagan included is 6 months postoperatively). There was no significant difference between both groups (88 vs 86, MD = 0.24 pg/mL; 95% CI [−0.75, 1.23], p = 0.64), with no significant heterogeneity (I² = 0%, P = 0.38) (Table 2). Two studies assessed data on TNF-α from baseline [22, 24] (The result of Sherf-Dagan included is 6 months postoperatively). There was no significant difference between both groups (63 vs 63, MD = −5.64 pg/mL; 95% CI [−15.78, 4.49], p = 0.28), with a high level of heterogeneity (I² = 87%, P = 0.005) (Table 2). Four studies assessed data on CRP from baseline postoperatively [19, 22, 24, 28]. There was no significant difference between both groups (103 vs 106, MD = 1.40 mg/L; 95% CI [−1.33, 4.13], p = 0.32), with a significant heterogeneity (I² = 58%, P = 0.07) (Table 2).
Effects of probiotics on general measure
Meta-analysis of seven studies did not indicate a significant effect of probiotics supplementation on %EWL (180 vs 193, MD = 1.89%; 95% CI [−2.19, 5.97], p = 0.36) [18,19,20, 22,23,24, 27], with a significant heterogeneity (I² = 63%, P = 0.01) (Fig. 6A). Seven studies assessed data on BMI from baseline postoperatively [18,19,20, 22, 24, 26, 28]. There was a significant difference between both groups (191 vs 187, MD = −2.89 kg/m²; 95% CI [−0.32, 6.09], p = 0.08), with a significant heterogeneity (I² = 97%, P < 0.00001) (Fig. 6B). Four studies assessed data in weight from baseline postoperatively [20, 24, 26, 28]. There was a significant difference between both groups (98 vs 94, MD = −1.99 kg; 95% CI [−3.97, −0.01], p = 0.05), with no significant heterogeneity (I² = 18%, P = 0.30) (Fig. 6C). Four studies assessed data in WC from baseline postoperatively [18, 22, 24, 28]. There was no significant difference between both groups (126 vs 119, MD = −0.16 cm; 95% CI [−3.08, 2.76], p = 0.91), with no significant heterogeneity (I² = 62%, P = 0.05) (Fig. 6D).
A Seven studies reported data on %EWL and there was no significant difference between both groups. B Seven studies reported data on BMI and there was no significant difference between both groups. C Four studies reported data on weight and there was a significant difference between both groups. D Four studies reported data on WC and there was no significant difference between both groups.
Effects of probiotics on Vitamin B12, 25-hydroxy Vitamin D3, folate, ferritin and Hb
Four studies assessed data on serum vitamin B12 from baseline postoperatively [18, 19, 23, 24] (The result of Woodard et al. included is 6 months postoperatively.). There was a significant difference between both groups (93 vs 98, MD = 2.24 pg/dL; 95% CI [−0.02, 4.51], p = 0.05), with no significant heterogeneity (I² = 30%, P = 0.23) (Fig. 7A). Three studies assessed data on serum 25-hydroxy vitamin D3 from baseline postoperatively [18, 19, 24]. There was no significant difference between both groups (76 vs 76, MD = 7.34 mg/dL; 95% CI [−0.67, 15.35], p = 0.07), with no significant heterogeneity (I² = 40%, P = 0.19) (Fig. 7B). Two studies assessed data on serum folate from baseline postoperatively [18, 24]. There was no significant difference between both groups (61 vs 56, MD = 0.48 nmol/L; 95% CI [−0.98, 1.93], p = 0.52), with no significant heterogeneity (I² = 0%, P = 0.63) (Table 2). Two studies assessed data on serum ferritin from baseline postoperatively [22, 28]. There was no significant difference between both groups (57 vs 62, MD = −14.5 ng/mL; 95% CI [−39.59, 10.58], p = 0.26), with no significant heterogeneity (I² = 0%, P = 0.83) (Table 2). Two studies assessed data on hemoglobin (Hb) from baseline postoperatively [22, 28]. There was no significant difference between both groups (57 vs 62, MD = −0.11 g/dL; 95% CI [−0.40, 0.18], p = 0.45), with no significant heterogeneity (I² = 18%, P = 0.27) (Table 2).
Effects of probiotics on dietary energy, protein, cholesterol, carbohydrate, fat, fiber
Two studies assessed data on dietary energy postoperatively [24, 27]. There was a significant difference between both groups (57 vs 62, MD = −151.03 kcal, 95% CI [−215.68, −86.37], p < 0.00001), with no significant heterogeneity (I² = 0%, P = 0.40) (Fig. 8A). Two studies assessed data on dietary protein postoperatively [24, 27]. There was a significant difference between both groups (57 vs 62, MD = −4.48 g/day, 95% CI [−8.76, −0.20], p = 0.04), with no significant heterogeneity (I² = 43%, P = 0.19) (Fig. 8B). One study assessed data on dietary cholesterol postoperatively [24]. There was no significant difference between both groups (23 vs 23, MD = −32.91 mg/day, 95% CI [−77.26, 11.44], p = 0.15) (Fig. 8C). Two studies assessed data on dietary carbohydrate postoperatively [24, 27]. There was a significant difference between both groups (57 vs 62, MD = −34.25 g/day, 95% CI [−44.87, −23.62], p < 0.00001), with no significant heterogeneity (I² = 0%, P = 0.55) (Fig. 8D). Two studies assessed data on dietary fat postoperatively [24, 27]. There was no significant difference between both groups (57 vs 62, MD = −0.90 g/day, 95% CI [−4.56, 2.76], p = 0.63), with no significant heterogeneity (I² = 48%, P = 0.16) (Fig. 8E). Two studies assessed data on dietary fiber postoperatively [24, 27]. There was a significant difference between both groups (57 vs 62, MD = −2.17 g/day, 95% CI [−3.21, −1.14], p < 0.0001), with no significant heterogeneity (I² = 0%, P = 0.33) (Fig. 8F).
A Two studies reported data on dietary energy and there was a significant difference between both groups. B Two studies reported data on dietary protein and there was a significant difference between both groups. C One study reported data on dietary cholesterol and there was no significant difference between both groups. D Two studies reported data on dietary carbohydrate and there was a significant difference between both groups. E Two studies reported data on dietary fat and there was no significant difference between both groups. F Two studies reported data on dietary fiber and there was a significant difference between both groups.
Effects of probiotics on gastrointestinal symptoms scores, BES, YFBS
There was a significant difference between both groups in the evolutions of GSRS [27] (34 vs 39, MD = 0.34, 95% CI [−0.46, −0.22], p < 0.00001) (Table 2). Calos ’s study [26] showed that after give probiotics supplementation to patients with morbid obesity undergoing bariatric surgery 3 months and 1 year later, the BES and the YFAS were lower in the probiotics groups (BES (3 M): 37 vs 32, MD = −1.12, 95% CI [−1.93, −0.31], p = 0.007; BES (12 M): 22 vs 22, MD = −1.40, 95% CI [−2.40, −0.40], p = 0.006; YFAS (3 M): 37 vs 32, MD = −3.29, 95% CI [−6.86, −0.28], p = 0.07; YFAS (12 M): 22 vs 22, MD = −5.06, 95% CI [−9.51, −0.61], p = 0.03) (Table 2).
Adverse events
No studies reported severe adverse events. Adverse events were reported in 5 studies [21,22,23, 27, 30]. In Han’s study [21], one patient suffered from nausea or vomiting and two patients suffered from diarrhea in probiotic group (n = 41). In the digestive enzyme group (n = 42), five patients suffered from nausea or vomiting, one patient suffered from diarrhea and one suffered from constipation (33 vs 31, MD = 0.39, 95% [0.09, 1.65], p = 0.2) (Table 2). In 4 studies, no adverse events associated with the interventions were observed [22, 23, 27, 30].
Discussion
This systematic review, assessing the effects of oral probiotics supplementation in patients with morbid obesity undergoing bariatric surgery, included 11 randomized clinical trials encompassing 559 participants. The results suggested that it was statistically significant of probiotics in reducing AST, TG, weight, food intake and vitamin B12, which may help inform clinical physicians and patients concerning the use of probiotics.
Different probiotics had different effects on the results under different intervention durations and intervention doses. Regretfully, we were not able to select the most effective dose of probiotics by comparing our present data. However, from the analysis, we proposed that the probiotics supplementation, especially Lactobacillus and Bifidobacterium, may be beneficial in the patients with morbid obesity undergoing bariatric surgery, for 9 studies used either Lactobacillus or Bifidobacterium except the study of Kazzi and Han et al. And the durations of probiotics supplementation mostly were 3 months postoperatively except the duration of Han and Woodard et al. were 6 months (Results related with serum TNF-a and IL-6 levels in Sherf-Dagan were also 6 months) [31, 32].
We found that probiotics consumption for 3 months decreased serum AST in patients with morbid obesity undergoing bariatric surgery. NAFLD is one of the complications associated with obesity [33], and is characterized by hepatic lipid accumulation, lipotoxicity, insulin resistance, gut dysbiosis and inflammation [34]. An association between probiotics and NAFLD had been reported that the gut-liver axis was established by the portal vein which enabled direct transport of gut-derived products to the liver [35] and various metabolites produced by the gut microbiota may impact the liver and thus modulate the susceptibility of NAFLD [36, 37]. Probiotics may improve liver function through various mechanisms such as modification of the gut microbiota, reducing appetite, intestinal permeability, and modulating the immune system [11, 38,39,40]. Probiotics had a significant effect on the function of the mucosal immune systems [41], modulating different signaling pathways involved in inflammatory and antioxidant processes, thus providing therapeutic effects [42, 43]. Probiotics were considered as a novel strategy for the management of NAFLD [44]. In our meta-analysis, we found that probiotics consumption for 3 months significantly reduced AST levels in patients with morbid obesity who underwent bariatric surgery. In the study of Kazzi et al., the probiotics were Bacillus. It was reported in the study of Salem et al. that the released exopolysaccharide by Bacillus alleviated CCl4 -induced liver injury in mice by lowering the activities of AST levels [45]. In the study of Crommen and Sherf-Dagan et al., the bacterium types included Lactobacillus and Bifidobacterium. It was reported Lactobacillus and Bifidobacterium can protect against NAFLD, and restored liver functions to normal levels [46, 47]. This suggested that probiotics may be used as an adjunct therapy for patients with morbid obesity undergoing bariatric surgery so to help in alleviating liver damage in NAFLD.
We also found that probiotics consumption for 3 months resulted in a significant decrease in serum TG level. In the studies included, the bacterium types included in probiotics were Lactobacillus and Bifidobacterium except the probiotics type of Kazzi et al. was Bacillus. Obesity was considered one of the common secondary causes of hyperlipidemia [48]. Bariatric surgery can improve intestinal flora, and probiotics may improve intestinal flora on the basis of bariatric surgery [27, 49]. Altered intestinal flora can affect lipid metabolism in various mechanisms. In the study of Aziz et al., it was reported that Lactobacillus restored lipolytic gene expression [46]. Lactobacillus and Bifidobacterium can improve the short-chain fatty acids (SCFAs) which had been shown to improve lipid signaling [50, 51]. Lactobacillus may affect TC levels by secreting biological inhibitors, such as cholesterol lipid coenzyme A inhibitors, to inhibit the formation of key enzymes or precursors in the cholesterol biosynthesis pathway [52]. Bacillus may alter bile acid composition and alleviate high-carbohydrate diet-induced hepatic lipid accumulation [53]. After related literature searches, we found that there were relatively fewer studies on how probiotics affected serum TG, LDL and HDL compared to TC, thus more in-depth studies could be conducted in this area in the future.
We also found that probiotics consumption for 3 months resulted in a significant decrease in weight. Probiotics modulated microbiota in a way to increase bile salt hydrolase activity, which in turn increased taurine abundance in the gut that stimulated tight junctions and suppressed gut leakiness [54], thus reducing inflammation [55]. The reduction in inflammation led to increased concentrations of leptin, glucagon-like peptide 1, and pancreatic polypeptide in the intestine, which leads to a reduction in food intake due to an increase in satiety [56,57,58]. A recent study reported Fusimonas intestini, highly colonized in humans with obesity and hyperglycemia, can produce long-chain fatty acids and facilitate diet-induced obesity consequently [59]. The study of Liang et al. and Karl et al. reported that Lactobacillus [60] and Bifidobacterium [61] can promote SCFAs which may increase energy expenditure through induction of thermogenesis in brown adipose tissue as well as browning of the white adipose tissue [62], contributing to the weight loss. In our meta-analysis, the studies of weight all included Lactobacillus and Bifidobacterium. Therefore, we proposed that the probiotics supplementation, especially Lactobacillus and Bifidobacterium may alter the component of intestinal microbiome and alter the SCFAs levels and finally contribute to the opposite direction of weight change. There were also studies showing that the intake of probiotics could lead to significant weight reductions, either maintaining habitual lifestyle habits or in combination with energy restriction and/or increased physical activity for an average of 12 weeks and specific strains belonging to the genus Lactobacillus and Bifidobacterium were the most used and showed the best results in reducing body weight [63, 64]. To be more precise, Lactobacillus and Bifidobacterium probiotics could contribute to weight loss in patients with morbid obesity undergoing bariatric surgery, suggesting probiotics might be a complement for patients with morbid obesity undergoing bariatric surgery.
Meta-analysis indicated that dietary energy, protein, carbohydrate and fiber were statistically reduced in the probiotics group compared to the placebo group. In Calos ’s study, they confirmed that the BES and YFAS statistically reduced in experiment group contrasting to the placebo group [26]. The probiotics type all included Lactobacillus and Bifidobacterium. The gut-brain axis can be altered by diet [65]. Several studies reported that probiotics may affect food appetite. Favorable effects of probiotics had been shown on regulating adiponectin, leptin, secretion and desire to eat [66]. It was reported that Lactobacillus tended to reduce energy intake [67]. Therefore, probiotics may present an appetite altering effect, contributing to a reduction of food intake, leading to an improvement in lipids metabolism and weight loss.
Significant improvements in vitamin B12 was also found in our meta-analysis. The bacterium included in the meta-analysis were mainly Lactobacillus and Bifidobacterium. The bacterium in the study of Kazzi et al. was Bacillus. Probiotics consumption may be an appropriate strategy to improve vitamin B12 status through intestinal microbiota modulation. Lactobacillus and Bifidobacterium were related to vitamin B12 metabolite transport systems [68]. It was reported that Lactobacillus supplement ameliorated vitamin B12 deficiency as an adjunctive therapy in canine clinical practice. Bacillus was a bacterium that had been used in the past for the industrial production of vitamin B12 [69]. Thus, we proposed the probiotics, including Lactobacillus, Bacillus and Bifidobacterium were beneficial in improving the vitamin B12 levels in patients with morbid obesity undergoing bariatric surgery.
The adverse events reported in patients with morbid obesity undergoing bariatric surgery were insufficient. We could not clearly confirm whether probiotics would contribute to adverse events. But in the meta-analysis, 559 patients included, no severe adverse events were reported. After weight loss surgery, patients may experience a variety of post-operative complications, such as vomiting or diarrhea, even without the use of probiotics [70, 71]. The results of probiotics may be overshadowed by the side effects of bariatric surgery. Thus, it was not easy to determine whether the use of probiotics itself might cause side effects to the patient, or whether it might alleviate the side effects of bariatric surgery.
And this review also showed reductions in many other indicators, such as glycemic parameters, inflammatory factors levels, and an increase in serum 25-hydroxy vitamin D3 level, although they were not statistically significant. There were several possible reasons for these statistically insignificant effects. Firstly, the duration of probiotics supplementation was not adequate to produce an effect. Secondly, the intestinal microbiota composition in individuals with morbid obesity was not favorable to produce a significant result. Thirdly, the statistical power of this review might have been insufficient to demonstrate the effects of small change. Nevertheless, as related research progresses, the results may one day present significant. And many preclinical medical studies indicated that probiotics may affect these indicators through many mechanisms. Therefore, these indicators should be attended to in the next update. It was unclear whether probiotics could improve glycemic parameters and morbid obesity was often associated with insulin resistance [72]. In recent years, a number of preclinical studies reported a complex interplay between probiotics and the gut microbiota within the gut environment. Probiotics had antioxidant effects, which can scavenge free radicals and increase the sensitivity of tissues to insulin [73]. An altered gut environment may increase the secretion of short-chain fatty acids and thus stimulate the secretion of some glucose-lowering hormones [74, 75]. Reactive oxygen species (ROS) played vital roles in intestinal inflammation [76]. Probiotics may eliminate ROS to alleviate the oxidation and inflammation through the inhibition of NLRP3 inflammasome and increase the secretion of immunoglobulin A [77, 78]. Moreover, probiotics may also restore damaged intestinal epithelium barriers so to reduce the inflammatory factors induced by other harmful bacteria [79]. Mallard’s study supported the view that the association between obesity and lower serum 25-hydroxy vitamin D3 might be due to a reversed causality for an increasement of adiposity, leading to reduced concentrations of circulating 25-hydroxy vitamin D3 [80]. Probiotics consumption might be an appropriate strategy to improve 25-hydroxy vitamin D3 status through intestinal microbiota modulation [37, 81, 82]. It was reported that in animal models, probiotics prevented bone loss by regulating bone resorption in osteoclasts and bone formation by osteoblasts [82]. In humans, osteoblasts may regulate 25-hydroxy vitamin D levels and calcium absorption [81]. Besides, probiotics can improve the level of other nutrients in the body to some extent [83]. It was reported that application of special lactic acid bacteria, especially strains that produce folic acid and riboflavin as well as immune-stimulating strains, can be used as adjuvant therapy for patients suffering from various inflammatory diseases [84]. In addition, Bahareh’s results suggest that, although varying degrees of efficacy, the intake of certain probiotics in healthy subjects was associated with the status of certain other micronutrients, such as calcium, folate, iron and zinc [83, 85]. Therefore, probiotics may lead to improved nutrient absorption in patients with morbid obesity undergoing bariatric surgery and more relevant studies are needed.
This meta-analysis has several strengths. A comprehensive literature search was conducted, involving 5 electronic databases and manual searches of relevant studies. Therefore, it is unlikely that eligible studies were neglected. 6 eligible studies [19, 21, 22, 26,27,28] that were not included in any previous reviews [83, 86, 87] were also identified. Additionally, a pilot phase prior to data abstraction was implemented to test the extraction, thereby increasing the systematicity and accuracy of the data. In studies with more than 1 compassion or control group, only the groups including the live microbiome were included as the probiotics group. In addition, the GRADE approach was used to formally assess the quality of evidence.
Our meta-analysis also has some limitations: the number of RCTs included in the study was relatively small and bias was inevitable, so the quality of the literature was reduced. However, RCTs reduced the bias to some extent.
Despite the above-mentioned shortcomings, the reliability of this meta-analysis was strengthened by minimized incorporation of biased literature, rigorous data extraction, and strong statistical analysis by teamwork. The results of this study are still worthy of clinical reference.
Conclusion
This study comprehensively evaluated outcome indicators associated with probiotics in the treatment of patients with morbid obesity undergoing bariatric surgery. Compared with previously published studies, we included more studies, providing a comprehensive analysis and evaluation of outcome indicators. The meta-analysis demonstrated that probiotics among patients with morbid obesity undergoing bariatric surgery had a beneficial effect on many indicators including the regulation of AST, TG, weight, food intake, and vitamin B12. Probiotics may be beneficial in patients with morbid obesity undergoing bariatric surgery. However, additional high-quality RCTs will be necessary for the future to further clarify the therapeutic effects of probiotics in patients with morbid obesity undergoing bariatric surgery.
Data availability
The data used in this publication are readily available from original source papers included in the systematic review.
References
Tome-Castro XM, Rodriguez-Arrastia M, Cardona D, Rueda-Ruzafa L, Molina-Torres G, Roman P. Probiotics as a therapeutic strategy in obesity and overweight: a systematic review. Benef Microbes. 2021;12:5–15.
Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7:231–40.
Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories. JAMA. 2013;309:71–82.
Jacobsen E, Boyers D, Manson P, Avenell A. A systematic review of the evidence for non-surgical weight management for adults with severe obesity: what is cost effective and what are the implications for the design of health services? Curr Obes Rep. 2022;11:356–85.
Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14. https://doi.org/10.3390/ijerph14040435.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery a systematic review and meta-analysis. JAMA. 2004;293:1724–8.
Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021;397:1830–41.
Alsumali A, Al-Hawag A, Samnaliev M, Eguale T. Systematic assessment of decision analytic models for the cost-effectiveness of bariatric surgery for morbid obesity. Surg Obes Relat Dis. 2018;14:1041–59.
Ciobarca D, Catoi AF, Copaescu C, Miere D, Crisan G. Bariatric surgery in obesity: effects on gut microbiota and micronutrient status. Nutrients. 2020;12. https://doi.org/10.3390/nu12010235.
Chen HT, Huang HL, Li YQ, Xu HM, Zhou YJ. Therapeutic advances in non-alcoholic fatty liver disease: a microbiota-centered view. World J Gastroenterol. 2020;26:1901–11.
Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–40.
Ding Q, Hu Y, Fu Y, Qian L. Systematic review and meta-analysis of the correlation between intestinal flora and gestational diabetes mellitus. Ann Palliat Med. 2021;10:9752–64.
Davidson SJ, Barrett HL, Price SA, Callaway LK, Dekker Nitert M. Probiotics for preventing gestational diabetes. Cochrane Database Syst Rev. 2021;4:CD009951.
Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15–21.
Di Lorenzo N, Antoniou SA, Batterham RL, Busetto L, Godoroja D, Iossa A, et al. Clinical practice guidelines of the European Association for Endoscopic Surgery (EAES) on bariatric surgery: update 2020 endorsed by IFSO-EC, EASO and ESPCOP. Surg Endosc. 2020;34:2332–58.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. 2022:Available from www.training.cochrane.org/handbook.
Ramos MRZ, de Oliveira Carlos L, Wagner NRF, Felicidade I, da Cruz MR, Taconeli CA, et al. Effects of Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 supplementation on nutritional and metabolic parameters in the early postoperative period after Roux-en-Y gastric bypass: a randomized, double-blind, placebo-controlled trial. Obes Surg. 2021;31:2105–14.
Kazzi F, Daher N, Zimmerman G, Garcia M, Schmidt N, Scharf K. Effect of bacillius coagulans and galactomannans on obese patients undergoing sleeve gastrectomy, a randomized-controlled clinical trial. Altern Ther Health Med. 2021;27:138–45.
Ramos MRZ, Felicidade I, de Oliveira Carlos L, Wagner NRF, Mantovani MS, de Lima LVA, et al. Effect of probiotic supplementation on plasma metabolite profile after Roux-Y gastric bypass: a prospective, randomized, double-blind, placebo-controlled clinical trial. Int J Obes (Lond). 2022;46:2006–12.
Han ML, Lee MH, Lee WJ, Chen SC, Almalki OM, Chen JC, et al. Probiotics for gallstone prevention in patients with bariatric surgery: a prospective randomized trial. Asian J Surg. 2022;45:2664–9.
Sherf-Dagan S, Zelber-Sagi S, Zilberman-Schapira G, Webb M, Buch A, Keidar A, et al. Probiotics administration following sleeve gastrectomy surgery: a randomized double-blind trial. Int J Obes (Lond). 2018;42:147–55.
Woodard GA, Encarnacion B, Downey JR, Peraza J, Chong K, Hernandez-Boussard T, et al. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: a prospective randomized trial. J Gastrointest Surg. 2009;13:1198–204.
Karbaschian Z, Mokhtari Z, Pazouki A, Kabir A, Hedayati M, Moghadam SS, et al. ProbiOtic Supplementation In Morbid Obese Patients Undergoing One Anastomosis Gastric Bypass-mini Gastric Bypass (OAGB-MGB) surgery: a randomized, double-blind, placebo-controlled, clinical trial. Obes Surg. 2018;28:2874–85.
Mokhtari Z, Karbaschian Z, Pazouki A, Kabir A, Hedayati M, Mirmiran P, et al. The effects of probiotic supplements on blood markers of endotoxin and lipid peroxidation in patients undergoing gastric bypass surgery; a randomized, double-blind, placebo-controlled, clinical trial with 13 months follow-up. Obes Surg. 2019;29:1248–58.
Carlos LO, Ramos MRZ, Wagner NRF, Freitas LAC, Felicidade I, Campos ACL. Probiotic supplementation attenuates binge eating and food addiction 1 year after Roux-En-Y gastric bypass: a randomized, double-blind, placebo-controlled trial. Arq Bras Cir Dig. 2022;35:e1659.
Wagner NRF, Ramos MRZ, de Oliveira Carlos L, da Cruz MRR, Taconeli CA, Filho AJB, et al. Effects of probiotics supplementation on gastrointestinal symptoms and SIBO after Roux-en-Y gastric bypass: a prospective, randomized, double-blind, placebo-controlled trial. Obes Surg. 2021;31:143–50.
Crommen S, Rheinwalt KP, Plamper A, Simon MC, Rosler D, Fimmers R, et al. A specifically tailored multistrain probiotic and micronutrient mixture affects nonalcoholic fatty liver disease-related markers in patients with obesity after mini gastric bypass surgery. J Nutr. 2022;152:408–18.
Chen JC, Lee WJ, Tsou JJ, Liu TP, Tsai PL. Effect of probiotics on postoperative quality of gastric bypass surgeries: a prospective randomized trial. Surg Obes Relat Dis. 2016;12:57–61.
Fernandes R, Beserra BTS, Mocellin MC, Kuntz MGF, Rosa JSd, Miranda RCDd, et al. Effects of Prebiotic and Synbiotic Supplementation on Inflammatory Markers and Anthropometric Indices After Roux-en-Y Gastric Bypass A Randomized, Triple-blind, Placebo-controlled Pilot Study. J Clin Gastroenterol. 2016;50:208–17.
Mu J, Guo X, Zhou Y, Cao G. The effects of probiotics/synbiotics on glucose and lipid metabolism in women with gestational diabetes mellitus: a meta-analysis of randomized controlled trials. Nutrients. 2023;15:16.
Zhu M, Wang X, Wang K, Zhao Z, Dang Y, Ji G, et al. Lingguizhugan decoction improves non-alcoholic steatohepatitis partially by modulating gut microbiota and correlated metabolites. Front Cell Infect Microbiol. 2023;13:17.
Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15:261–73.
Tilg H, Adolph TE, Dudek M, Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nat Metab. 2021;3:1596–607.
Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–77.
Safari Z, Gerard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76:1541–58.
Hu H, Lin A, Kong M, Yao X, Yin M, Xia H, et al. Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J Gastroenterol. 2020;55:142–58.
Mohamad Nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, Wong Z, et al. The effect of probiotics (MCP((R)) BCMC((R)) Strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients. 2021;13. https://doi.org/10.3390/nu13093192.
Noormohammadi M, Ghorbani Z, Lober U, Mahdavi-Roshan M, Bartolomaeus TUP, Kazemi A, et al. The effect of probiotic and synbiotic supplementation on appetite-regulating hormones and desire to eat: a systematic review and meta-analysis of clinical trials. Pharmacol Res. 2023;187:106614.
Wang T, Zheng N, Luo Q, Jiang L, He B, Yuan X, et al. Probiotics lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front Immunol. 2019;10:1235.
Maldonado Galdeano C, Cazorla SI, Lemme Dumit JM, Velez E, Perdigon G. Beneficial effects of probiotic consumption on the immune system. Ann Nutr Metab. 2019;74:115–24.
Vincenzi A, Goettert MI, Volken de Souza CF. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-alpha) signaling and gene expression. Cytokine Growth Factor Rev. 2021;57:27–38.
Zhou X, Wang J, Zhou S, Liao J, Ye Z, Mao L. Efficacy of probiotics on nonalcoholic fatty liver disease: a meta-analysis. Medicine (Baltimore). 2023;102:e32734.
Ebrahimi-Mousavi S, Alavian SM, Sohrabpour AA, Dashti F, Djafarian K, Esmaillzadeh A. The effect of daily consumption of probiotic yogurt on liver enzymes, steatosis and fibrosis in patients with nonalcoholic fatty liver disease (NAFLD): study protocol for a randomized clinical trial. BMC Gastroenterology. 2022;102. https://doi.org/10.1186/s12876-022-02176-2.
Salem GEM, Azzam SM, Nasser MAF, El Malah T, Abd El-Latief HM, Khan RH, et al. Bacterial protease alleviate chronic liver fibrosis induced by thioacetamide through suppression of hepatic stellate cells consequently decrease its proliferative index. Int J Biol Macromol. 2023;239:124243.
Aziz M, Hemeda SA, Albadrani GM, Fadl SE, Elgendey F. Ameliorating effect of probiotic on nonalcoholic fatty liver disease and lipolytic gene expression in rabbits. Sci Rep. 2023;13:6312.
Huo R, Chen Y, Li J, Xu Q, Guo J, Xu H, et al. Altered gut microbiota composition and its potential association in patients with advanced hepatocellular carcinoma. Curr Oncol. 2023;30:1818–30.
Closs C, Ackerman M, Masson W, Lobo M, Molinero G, Lavalle-Cobo A, et al. Effectiveness of Roux-en-Y gastric bypass vs sleeve gastrectomy on lipid levels in type 2 diabetes: a meta-analysis. J Gastrointest Surg. 2022;26:1575–84.
Boscaini S, Leigh SJ, Lavelle A, Garcia-Cabrerizo R, Lipuma T, Clarke G, et al. Microbiota and body weight control: weight watchers within? Mol Metab. 2022;57:101427.
Yoon SJ, Yu JS, Min BH, Gupta H, Won SM, Park HJ, et al. Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front Microbiol. 2023;14:1129904.
Oyabambi AO, Olaniyi KS. Sodium butyrate aggravates glucose dysregulation and dyslipidemia in high fat-fed Wistar rats. Metabol Open. 2023;17:100226.
Tomaro-Duchesneau C, Jones ML, Shah D, Jain P, Saha S, Prakash S. Cholesterol assimilation by Lactobacillus probiotic bacteria: an in vitro investigation. Biomed Res Int. 2014;2014. https://doi.org/10.1155/2014/380316.
Luo Y, Li M, Wang T, Zhou NN, Qiao F, Du ZY, et al. Bacillus cereus alters bile acid composition and alleviates high-carbohydrate diet-induced hepatic lipid accumulation in Nile Tilapia (Oreochromis niloticus). J Agric Food Chem. 2023;71:4825–36.
Ahmadi S, Wang S, Nagpal R, Wang B, Jain S, Razazan A, et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020;5:18.
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12:578386.
Candia PD, Prattichizzo F, Garavelli S, Alviggi C, Cava AL, Matarese G. The pleiotropic roles of leptin in metabolism, immunity, and cancer. J Exp Med. 2021;218. https://doi.org/10.1084/jem.20191593.
Carvalho BM, Saad MJ. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm. 2013;2013:986734.
Radaic A, Kapila YL. The oralome and its dysbiosis: new insights into oral microbiome-host interactions. Comput Struct Biotechnol J. 2021;19:1335–60.
Takeuchi T, Kameyama K, Miyauchi E, Nakanishi Y, Kanaya T, Fujii T, et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity. Cell Metab. 2023;35:361–75 e9.
Liang T, Xie X, Wu L, Li L, Yang L, Jiang T, et al. Metabolism of resistant starch RS3 administered in combination with Lactiplantibacillus plantarum strain 84-3 by human gut microbiota in simulated fermentation experiments in vitro and in a rat model. Food Chem. 2023;411:135412.
Mahalak KK, Firrman J, Narrowe AB, Hu W, Jones SM, Bittinger K, et al. Fructooligosaccharides (FOS) differentially modifies the in vitro gut microbiota in an age-dependent manner. Front Nutr. 2022;9:1058910.
Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. 2020;9:179–92.
Alvarez-Arrano V, Martin-Pelaez S. Effects of probiotics and synbiotics on weight loss in subjects with overweight or obesity: a systematic review. Nutrients. 2021;13:18.
Perna S, Ilyas Z, Giacosa A, Gasparri C, Peroni G, Faliva MA, et al. Is probiotic supplementation useful for the management of body weight and other anthropometric measures in adults affected by overweight and obesity with metabolic related diseases? A systematic review and meta-analysis. Nutrients. 2021;13:18.
Navarro-Tapia E, Almeida-Toledano L, Sebastiani G, Serra-Delgado M, Garcia-Algar O, Andreu-Fernandez V. Effects of microbiota imbalance in anxiety and eating disorders: probiotics as novel therapeutic approaches. Int J Mol Sci. 2021;22:43.
Legrand R, Lucas N, Dominique M, Azhar S, Deroissart C, Le Solliec MA, et al. Commensal Hafnia alvei strain reduces food intake and fat mass in obese mice-a new potential probiotic for appetite and body weight management. Int J Obes (Lond). 2020;44:1041–51.
NabizadehAsl L, Sendur SN, Ozer B, Lay I, Erbas T, Buyuktuncer Z. Acute and short-term effects of Lactobacillus paracasei subsp. paracasei 431 and inulin intake on appetite control and dietary intake: a two-phases randomized, double blind, placebo-controlled study. Appetite. 2022;169:105855.
Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, et al. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:21.
Moore SJ, Mayer MJ, Biedendieck R, Deery E, Warren MJ. Towards a cell factory for vitamin B12 production in Bacillus megaterium: bypassing of the cobalamin riboswitch control elements. N Biotechnol. 2014;31:553–61.
Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, Adam A, Neto MG, Badurdeen D, et al. Efficacy and safety of endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18:1043–53 e4.
Chierici A, Chevalier N, Iannelli A. Postoperative morbidity and weight loss after revisional bariatric surgery for primary failed restrictive procedure: a systematic review and network meta-analysis. Int J Surg. 2022;102:106677.
Tong Y, Xu S, Huang L, Chen C. Obesity and insulin resistance: pathophysiology and treatment. Drug Discov Today. 2022;27:822–30.
Ko SH, Kim HS. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. 2020;12:202.
Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31:35–51.
You H, Tan Y, Yu D, Qiu S, Bai Y, He J, et al. The therapeutic effect of SCFA-mediated regulation of the intestinal environment on obesity. Front Nutr. 2022;9:886902.
Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan Y, et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics. 2021;11:1845–63.
Zhao H, Lu Z, Lu Y. The potential of probiotics in the amelioration of hyperuricemia. Food Function. 2022;13:2394–414.
Zhou J, Li M, Chen Q, Li X, Chen L, Dong Z, et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13:14.
Rose EC, Odle J, Blikslager AT, Ziegler AL. Probiotics, prebiotics and epithelial tight junctions: a promising approach to modulate intestinal barrier function. Int J Mol Sci. 2021;22:18.
Mallard SR, Howe AS, Houghton LA. Vitamin D status and weight loss: a systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials. Am J Clin Nutr. 2016;104:1151–9.
Vandenplas Y, Huys G, Daube G. Probiotics: an update. J Pediatr. 2015;91:6–21.
Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273–84.
Swierz MJ, Storman D, Staskiewicz W, Gorecka M, Jasinska KW, Swierz AM, et al. Efficacy of probiotics in patients with morbid obesity undergoing bariatric surgery: a systematic review and meta-analysis. Surg Obes Relat Dis. 2020;16:2105–16.
LeBlanc JG, Levit R, Savoy de Giori G, de Moreno, de LeBlanc A. Application of vitamin-producing lactic acid bacteria to treat intestinal inflammatory diseases. Appl Microbiol Biotechnol. 2020;104:3331–7.
Barkhidarian B, Roldos L, Iskandar MM, Saedisomeolia A, Kubow S. Probiotic supplementation and micronutrient status in healthy subjects: a systematic review of clinical trials. Nutrients. 2021;13:3001.
Daghmouri MA, Chaouch MA, Yang W, Akremi S, Jaoua H, Fadhel KB, et al. Probiotics in bariatric surgery ensure greater lipids and glycemic profile with no effect on anthropometric measurements and inflammatory markers: a systematic review and meta-analysis of RCT. Surg Open Dig Adv. 2022. https://doi.org/10.1016/j.soda.2022.100061.
Zhang Y, Yan T, Xu C, Yang H, Zhang T, Liu Y. Probiotics can further reduce waist circumference in adults with morbid obesity after bariatric surgery: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2021;2021:5542626.
Acknowledgements
The authors acknowledge Jiaping Sui from Peking Union Medical College and Mingyang Shen from The Fourth Affiliated Hospital of China Medical University for their assistance in the library search.
Author information
Authors and Affiliations
Contributions
YTW and YW conceived and designed the study. YTW, YW, YWZ, JJX, and XDL participated in databases searches. YTW, YW, XCL, SS, and SRW conducted the screening process. YTW, YW, LRK, KYY, and JJX conducted the quality assessment process. YTW, YW, YWZ, and JLW pulled data. YTW, YW, YYY, and JFS performed all statistical analyses. YWZ, YTW, YW, and XDL interpreted results of the analysis. YTW wrote the first manuscript draft and was critically revised by YTW, YW, YWZ, JJX, XDL, XCL, SS, SRW, JLW, YYY, and JFS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Zheng, Y., Kuang, L. et al. Effects of probiotics in patients with morbid obesity undergoing bariatric surgery: a systematic review and meta-analysis. Int J Obes 47, 1029–1042 (2023). https://doi.org/10.1038/s41366-023-01375-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01375-5