Abstract
Hemodialysis patients are a special group of patients with higher mortality rates. Hemodialysis patients with vitamin D deficiency {plasma levels of 25-hydroxyvitamin D [25(OH)D] below 20 ng/mL} are associated with even higher mortality rates. The prognostic importance of vitamin D deficiency in hemodialysis patients with different cardiothoracic ratios (CTRs) is still unclear. This prospective study was performed in a single hemodialysis center, and 186 patients were included. This study analyzed the prognostic importance of vitamin D deficiency in hemodialysis patients with different CTRs. Vitamin D deficiency patients had a significantly higher prevalence of stroke and diabetic mellitus than those without vitamin D deficiency. In addition, the CTR was higher in patients with vitamin D deficiency than in those without vitamin D deficiency. After multivariate logistic regression, we found that CTR was the solitary factor that was independently significantly associated with vitamin D deficiency [odds ratio: 1.07, 95% confidence internal (CI): 1.01–1.13, p = 0.02]. Additionally, vitamin D deficiency was associated with all-cause mortality in patients with higher CTR after adjustment in hierarchical regression models. In conclusion, we reported that vitamin D deficiency was independently significantly associated with a higher CTR. We additionally revealed that vitamin D deficiency was an independent predicator for all-cause mortality in higher CTR hemodialysis patients.
Similar content being viewed by others
Introduction
Vitamin D deficiency {plasma levels of 25-hydroxyvitamin D [25(OH)D] below 20 ng/mL}is common in patients with chronic kidney disease (CKD) with or without dialysis. In CKD patients without dialysis, the prevalence of vitamin D deficiency is approximately 50~ 70%1,2,3,4. In CKD patients undergoing hemodialysis, the prevalence of vitamin D deficiency is approximately 50–98%5,6,7. The prevalence of vitamin D deficiency is even higher in CKD patients undergoing peritoneal dialysis, and the prevalence rates ranging from 86% to 100%7,8. The cause for the higher prevalence of vitamin D deficiency in CKD patients is not well-defined, but less exposure to sun, dietary restriction, and inefficient 25(OH)D formation are considered to be the possible causes9.
CKD patients are a special group of patients with higher mortality rates, including cardiovascular- and infection-related mortality10. In addition, diabetes mellitus (DM) is the primary cause of CKD that requires hemodialysis, accounting for nearly 50% of cases11,12. Vitamin D deficiency is linked with higher mortality risk in the general population13,14,15,16 and in CKD patients undergoing hemodialysis17,18,19. The possible reasons to explain the poor prognosis related with vitamin D deficiency are still unclear. However, some experimental studies with vitamin D receptor knock-out mice found impaired immunity, type 2 DM, and cardiovascular disease develop in those mice20,21,22, which indicates that the possible cause of the poor prognosis associated with vitamin D deficiency may be related to cardiovascular disease, infections, or comorbidity of type 2 DM. It is not surprising that CKD patients undergoing hemodialysis had higher all-cause mortality.
Several animal studies showed that vitamin D deficiency is connected with cardiac remodeling, including oxidative stress, fibrosis, apoptosis, cardiac hypertrophy, cardiac inflammation, left chamber alterations, and systolic dysfunction23,24,25. It seems that vitamin D is necessary for maintaining regular cardiac function. However, in clinical observational studies, it is difficult to define the complicated connection between vitamin D deficiency and prognosis in diabetes and hypertension hemodialysis patients. Patange et al. found that vitamin D deficiency is linked with diastolic dysfunction and left ventricular mass increase in children with CKD26. In addition, arterial wall stiffness was also found in children with vitamin D deficiency27. Cardiac disorders in pediatric CKD patients and vitamin D deficiency could provide information about the possible pathophysiology of vitamin D deficiency and mortality.
The cardiothoracic ratio (CTR) by a chest X-ray has been considered as a cardiac enlargement index, which indicates cardiac hypertrophy and volume overload28. The association between vitamin D deficiency and CTR in hemodialysis patients is still undefined. The prognostic importance of vitamin D deficiency in hemodialysis patients with cardiac hypertrophy and fluid overload is still uncertain. Therefore, we managed a cohort study to assess the relationship between CTR, vitamin D deficiency, and long-term mortality in hemodialysis patients with a follow-up period of approximate 5 years. The purpose of this present single dialysis center study was to evaluate the clinical characteristics of hemodialysis patients with vitamin D deficiency and to survey the predictive value of vitamin D deficiency on long-term prognosis in patients with different cardiac hypertrophy and fluid overload statuses.
Results
The characteristics and laboratory data of the study patients undergoing hemodialysis
The mean age of our study dialysis patients was approximately 59 ± 14 years old (Table 1). The prevalence of DM was approximately 38%, and the average dialysis duration was approximately 7 ± 25 years. The nutritional status of the patients was adequate, with an albumin level of approximately 3.8 ± 0.4 g/dL and a normalized protein catabolic rate (nPCR) of approximately 1.2 ± 0.3 g/kg/day (Table 2). The CTR of the study patients was approximately 50 ± 7% (Fig. 1). The mean serum level of 25 (OH) D was approximately 27 ± 15 ng/mL (Fig. 2). The prevalence of vitamin D deficiency was approximately 37%.
Differences in characteristic and laboratory data between dialysis patients with and without vitamin D deficiency
Vitamin D deficiency patients had a significantly higher prevalence of DM (48% vs. 33%, p = 0.04) and stroke (18% vs. 6%, p = 0.01) than patients without vitamin D deficiency. The age, sex, smoking status and prevalence of congestive heart failure (CHF), coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), peripheral arterial occlusive disease (PAD), peptic ulcer disease, and cancer were similar between study patients with and without vitamin D deficiency. However, the CTR was greater in patients with vitamin D deficiency than in those without vitamin D deficiency (52 ± 7 vs. 49 ± 6, p = 0.01). The serum vitamin D levels were negatively associated with CTR (r = −0.2, p = 0.01) (Fig. 3).
Regarding levels of serum, vitamin D deficiency patients had significantly lower levels of creatinine (10 ± 3 vs. 11 ± 3 mg/dL, p = 0.045). Nutritional status was similar between study patients with and without vitamin D deficiency based on serum levels of albumin and nPCR. In addition, the calcium and phosphate products, the serum levels of calcium and phosphate were also not different in these two groups of patients. The serum levels of intact parathyroid hormone (iPTH) were higher in vitamin D deficiency patients than those without vitamin D deficiency 450 ± 860 vs. 330 ± 330 pg/mL, p = 0.16), but the difference was not significant. The levels of hemoglobin were also similar between these two groups of patients. The dialysis efficiency presented by the urea reduction rate and Kt/V were also similar between these two groups of patients. The inflammation markers, including interleukin (IL)−1 beta, IL-6, tumor necrosis factor (TNF)-alpha, and, high-sensitivity C-reactive protein (hs-CRP) were also not significantly different between the study patients with and without vitamin D deficiency. The serum levels of 25(OH)D were not unexpectedly lower in patients with vitamin D deficiency than in those without vitamin D deficiency (14 ± 4 vs. 35 ± 14, p < 0.001).
Factors associated with 25 (OH) D deficiency
To identify the factors associated with vitamin D deficiency, we used univariate logistic regression and found that patients with poor fluid status or cardiac function who presented with high CTR, lower serum creatinine, and higher prevalence of DM and stroke were significantly more possible to develop vitamin D deficiency (Table 3). Then, we used multivariate logistic regression and found that the cardiothoracic ratio was independently significantly connected with vitamin D deficiency [odds ratio: 1.07, 95% confidence internal (CI): 1.01–1.13, p = 0.02].
Long-term prognosis between patients with and without vitamin D deficiency
In this 5-year follow-up study, we found that vitamin D deficiency hemodialysis patients had a trend of higher all-cause mortality than those without vitamin D deficiency (Fig. 4), although the difference was not significant (log-rank: χ2: 3.65, p = 0.06).
Because CTR was independently significantly associated with vitamin D deficiency, our study patients were sub-grouped by a CTR cutoff of 50%. Patients with a CTR higher than 50% were considered to have poor cardiac function and fluid status. In contrast, patients with a CTR less than 50% were considered to have good cardiac function and fluid status.
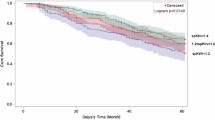
In patients with poor fluid status and cardiac function, patients with vitamin D deficiency had significantly higher all-cause mortality than those without vitamin D deficiency (Fig. 5) (log-rank χ2: 5.07, p = 0.02). However, in patients with good fluid status and cardiac function, the all-cause mortality was similar between patients with and without vitamin D deficiency (Fig. 6) (log-rank χ2: 0.03, p = 0.87).
We used Cox regression to determine the prognostic value of vitamin D deficiency in patients with poor fluid status and cardiac function. Vitamin D deficiency was persistently significantly related with all-cause mortality in Model 1 (adjusted for age and sex) [Hazard ratio (HR): 2.5, 95% CI: 1.0–6.3, p = 0.048], Model 2 (HR: 2.6, 95% CI: 1.0–6.7, p = 0.04) (adjusted for comorbidities of DM, CHF, CAD), Model 3 (HR: 2.8, 95% CI: 1.1–7.1, p = 0.03) (adjusted for CTR), Model 4 (HR: 2.6, 95% CI: 1.0–6.5, p = 0.047) (adjusted for serum albumin), and Model 5 (HR: 3.1, 95% CI: 1.1–9.3, p = 0.04) (adjusted for hemoglobin, serum creatinine, hs-CRP, calcium, phosphate, and cholesterol) (Table 4) (Fig. 7).
Discussion
In this study, we found that hemodialysis patients with comorbidities of DM and stroke were significantly more possible to develop vitamin D deficiency. In addition, those patients with fluid overload or poor cardiac function evidenced by CTR were also significantly more likely to have vitamin D deficiency. In addition, vitamin D deficiency patients had significantly lesser serum creatinine levels than those without vitamin D deficiency. Vitamin D deficiency patients had a worse prognosis during this 5-year follow-up period, but the difference was not significant. However, vitamin D deficiency was significantly related with greater all-cause mortality in patients with higher CTR.
In this study, CTR was independently linked with vitamin D deficiency. CTR derived from chest X-ray is conventionally used to assess left ventricle (LV) size29,30 and is also associated with LV systolic dysfunction31,32 (Fig. 8). Furthermore, CTR is also used to determine fluid status in dialysis patients28. Vitamin D deficiency was related to various cardiovascular diseases, including hypertension33, increased carotid artery intima-medial thickness34, and cardiac hypertrophy23. The finding that vitamin D deficiency was significantly related with the CTR may be explained by the impaired cardiac function and remodeling linked with vitamin D deficiency. In additional, experimental studies similarly indicated that vitamin D deficiency was associated with cardiac inflammation, oxidative stress, energetic metabolic changes, fibrosis, apoptosis, cardiac hypertrophy, left chamber alterations, and systolic dysfunction23. Accordingly, this finding may further confirm the importance of vitamin D in the maintenance of normal cardiac function and repairing after stress or remodeling.
In this study by a follow-up duration of 5 years, a higher CTR was significantly related with poor prognosis. However, the prognostic value was less significant in all study patients. There are only few studies showing the predicative value of CTR for the long-term prognosis of hemodialysis patients. Chen et al. found that CTR was prognostic for all-cause mortality in 468 non-DM hemodialysis patients35, but Gao et al. showed that mortality risk was not associated by CTR36. In addition, Yujiro et al. also showed that CTR was not significantly connected with all-cause mortality except in those patients in the highest CTR quartile (0.535–0.569)37. Thus, the connection between CTR and all-cause mortality in hemodialysis patients has not been consistent between studies. Our study found that vitamin D deficiency was significantly associated with all-cause mortality in patients with a CTR higher than 0.5. Similar to the findings of the report by Yujiro et al., CTR and vitamin D deficiency were related with all-cause mortality. Heart dysfunction with hypertrophy, systolic dysfunction, and left chamber alterations were more prominent in patients with higher CTR. Vitamin D is likely essential for the maintenance of normal cardiac function and repair after stress or remodeling. Patients with higher CTR were considered to be the group of patients with poor fluid status and cardiac hypertrophy. Consequently, it is reasonable that vitamin D deficiency was connected with all-cause mortality in patients with higher CTR who suffering from more cardiac stress and fluid overload.
Comorbidity of DM was significantly associated with vitamin D deficiency in hemodialysis patients. Epidemiological and association study data clearly showed a correlation between vitamin D deficiency and a higher prevalence of DM38. Vitamin D deficiency provides to both the initial insulin resistance and the following onset of diabetes caused by beta-cell death. Vitamin D undertakes to reduce inflammation, which is a main component in the induction of insulin resistance39. Comorbidity of stroke was also significantly connected with vitamin D deficiency in hemodialysis patients. Due to the numerous non-calcemic autocrine and paracrine actions of vitamin D, an relationship between stroke and vitamin D deficiency has been stated, too. Judd et al. found that vitamin D deficiency was associated with incident stroke and the potency of this relationship does not show to differ by race40. Because vitamin D deficiency was also correlated with atrial fibrillation41, a higher prevalence of stroke in individuals with vitamin D deficiency may also excuse for atrial fibrillation. In addition, vitamin D levels are more strongly related to sun exposure. Patients with stroke might go outdoors less frequently due to their inability and have less sun exposure; therefore, it is not surprising that they are high risk for vitamin D deficiency.
We found that the inflammatory markers, comprising hs-CRP and inflammatory cytokines (IL-1 beta, IL-6, and TNF-alpha), were similar between hemodialysis patients with and without vitamin D deficiency. Numerous studies have reported that vitamin D is also essential for immune modulation through the immune cells vitamin D receptor42, and vitamin D deficiency was linked with death in critically ill patients16,43,44. Tiwari et al. reported significantly higher serum levels of IL-1 beta and IL-6 in vitamin D-deficient DM patients45. Kalkwarf et al. found that serum 25 (OH) D levels were inversely related with CRP and IL-6 in children and adolescents with CKD4. However, information on the relationship between inflammatory markers and vitamin D deficiency is requiring in CKD patients with hemodialysis. The results showed that the levels of TNF-alpha, IL-1 beta, IL-6, and hs-CRP were not connected with vitamin D deficiency. Furthermore, vitamin D deficiency was not linked with infection-related mortality (data not shown). These findings may provide information about vitamin D deficiency, inflammation, and infection-related death in hemodialysis patients.
There are several limitations to this study. First, we do not get records on the left ventricular mass from echocardiography, the gold standard for the evaluation of cardiac hypertrophy. Therefore, we cannot discuss whether CTR indicates fluid overload or cardiac hypertrophy. Second, the study results were built on one measurements of the variables, including the CTR and serum 25(OH) D levels. Therefore, the results might overestimate or underestimate the true association. In addition, seasonal variation in 25 (OH) D levels in hemodialysis patients has also been observed, with lower levels in winter and higher levels in summer. However, we checked the serum levels of vitamin D from dialysis patients within almost the same month (no more than 2 months). Therefore, the seasonal variation in 25 (OH) D levels might not be prominent in our study. Fourth, the results of this observational study do not certainly show causation. Therefore, the study does not provide the goal range of CTR in clinical practice. Fifth, because this is a cohort study that enrolled approximately 186 patients in a dialysis center with approximately 435 dialysis patients in total in Taiwan, our results need to be confirmed by more studies with a large sample size. In addition, other types of vitamin D, such as 1,25-di-OH-D or vitamin D binding protein, were not measured. However, our results revealed an independent significant connection between CTR and vitamin D deficiency and revealed the prognostic value of vitamin D deficiency in higher CTR patients. These findings may emphasize the importance of vitamin D in maintaining cardiac function and morphology and improving long-term outcomes in excess fluid overload or cardiac hypertrophy patients. In addition, this finding may raise further questions concerning vitamin D supplementation in hemodialysis patients with higher CTR to reduce all-cause mortality.
Conclusion
We showed that vitamin D deficiency was independently significantly related with higher CTR. We additionally revealed that vitamin D deficiency was an independent predicator of all-cause mortality in hemodialysis patients with higher CTR. Further studies are needed to approve these consequences.
Materials and Methods
Study population and design
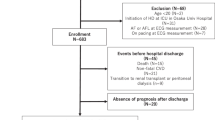
This was a prospective cohort study in single dialysis center that was accomplished to analyze the factors associated with vitamin D deficiency and the prognostic value of vitamin D on the mortality of hemodialysis patients with different statuses of cardiac hypertrophy and fluid overload. The total of 186 patients who had been undertaking regular dialysis for more than 6 months were joined. Those patients were excluded from the study if they were under 20 years old, were hospitalized for acute illnesses, or had not proposed their informed consent for participation. The patients were separated into 2 groups according to their levels of plasma 25 (OH) D by increments of 20 ng/mL46,47,48,49. Patients with plasma levels of 25 (OH) D below 20 ng/mL were thought to be vitamin D deficient. The study period was from August 1, 2006 to July 31, 2011. Patients who were absent from our dialysis program, shifted to peritoneal dialysis, or experienced transplantation were censored. Overall mortality was analyzed and compared between groups with vitamin D deficiency and without vitamin D deficiency. This study was approved by Chang Gung Memorial Hospital the Institutional Review Board (IRB) of the (99–2112B). Besides, all processes were performed in accordance with the related regulations and guidelines.
We collected basic demographic data for each patient, including age, sex, nutritional status (assessed by body mass index and nPCR), dialysis duration, and the presence/absence of DM, CAD, CHF, PAD, COPD, peptic ulcer disease, stroke, and cancer. We additionally collected the baseline biochemical, hematological, and dialysis-related parameters of these patients. DM was defined by the American Diabetes Association criteria in 1997. The utilization of more than 10 cigarettes per day for at least 1 year was considered as smoking. CHF was diagnosed by the Framingham criteria for heart failure. CAD was diagnosed by coronary angiography, PAD was diagnosed by peripheral arterial Doppler examination or amputation history, COPD was identified by pulmonary function tests and chest radiography, peptic ulcer disease was diagnosed by panendoscopy result, stroke was diagnosed by imaging studies and clinical symptoms, and cancer was diagnosed by pathological studies.
Cardiothoracic ratio evaluation
Posterior-anterior chest radiograph was taken to measure the CTR just after hemodialysis sessions for all study patients at our center. CTR was expressed as the ratio of the width of the heart to the thoracic width by a posterior to anterior view chest X-ray about 120 kV acquired in a standing position with inspiration entirely. The thoracic width was calculated as the greatest diameter of the inner borders of the ribs and the cardiac width was calculated as the sum of the right and left greatest diameters from the midline. The CTR was analyzed by dividing the cardiac width by the thoracic width. Therefore, a CTR > 0.5 was defined as cardiomegaly, and higher CTR levels reveal a greater severity of cardiomegaly or fluid overload.
Laboratory studies
We got whole laboratory data and dialysis-related parameters for patients in both groups. The laboratory profiles included the hemoglobin, plasma levels of albumin, blood urea nitrogen (BUN), creatinine, hs-CRP, calcium, phosphate, i-PTH, and cholesterol. Besides, parameters reflecting urea clearance, including Kt/V value (Daugirdas) and URR were evaluated to determine the dialysis treatment adequacy. Blood samples were collected and performed according to the recommended procedures by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines. Plasma levels of hemoglobin, BUN, creatinine, hs-CRP, albumin, cholesterol, calcium, and phosphate were determined by spectrophotometric analysis by using the modified kinetic Jaffe reaction. Plasma i-PTH levels were evaluated by a commercially available radioimmunoassay kit (Scantibodies Laboratory; Santee, CA, USA). 25-OH vitamin D was measured at the initial assessment by electrochemiluminescence immunoassay (Cobas® Vitamin D3 assay by Roche Diagnostics GmbH, Mannheim, Germany) with an inter-assay coefficient of variation of 2.2–13.6% from blood samples drawn before the beginning of dialysis session.
Statistical analysis
Group difference in categorical variables was determined via either the chi-square test or Fisher’s exact test. Continuous variables are presented as the means ± standard deviations. A two-tailed Student’s unpaired t test was employed to evaluate the differences between the means for those normally distributed continuous variables. To evaluate the factors linked with vitamin D deficiency in the hemodialysis patients, univariate followed by multivariate logistic regression models were applied. Mortality was treated as the end point in the survival analysis for all-cause mortality. Besides, patient survival was evaluated using the Cox proportional hazards model to control for covariates. We used Cox proportional hazards regression models to compare survival between groups with and without vitamin D deficiency. Hierarchical regression models were engaged to construct a potent covariate set for adjustment in the major hypothesized variable of vitamin D deficiency. A p value <0.05 was cogitated statistically significant. All analyses were made by means of commercially available statistics software SPSS software version 23.0 (SPSS; Chicago, IL, USA) for Mac.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of the Chang Gung Memorial Hospital (99–2112B), and informed consent was obtained from all participants of the study.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Jabbar, Z. et al. High prevalence of vitamin D deficiency in north Indian adults is exacerbated in those with chronic kidney disease. Nephrology 14, 345–349, https://doi.org/10.1111/j.1440-1797.2008.01082.x (2009).
Patel, N. M. et al. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int. 77, 715–720, https://doi.org/10.1038/ki.2009.551 (2010).
Echida, Y., Mochizuki, T., Uchida, K., Tsuchiya, K. & Nitta, K. Risk factors for vitamin D deficiency in patients with chronic kidney disease. Intern. Med. 51, 845–850 (2012).
Kalkwarf, H. J. et al. Vitamin D deficiency is common in children and adolescents with chronic kidney disease. Kidney Int. 81, 690–697, https://doi.org/10.1038/ki.2011.431 (2012).
Bhan, I., Burnett-Bowie, S. A., Ye, J., Tonelli, M. & Thadhani, R. Clinical measures identify vitamin D deficiency in dialysis. Clin. J. Am. Soc. Nephrol. 5, 460–467, https://doi.org/10.2215/CJN.06440909 (2010).
Wolf, M. et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 72, 1004–1013, https://doi.org/10.1038/sj.ki.5002451 (2007).
Drechsler, C. et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol. Dial. Transpl. 26, 1024–1032, https://doi.org/10.1093/ndt/gfq606 (2011).
Anand, S. et al. Vitamin D deficiency, self-reported physical activity and health-related quality of life: the Comprehensive Dialysis Study. Nephrol. Dial. Transpl. 26, 3683–3688, https://doi.org/10.1093/ndt/gfr098 (2011).
Jacob, A. I., Sallman, A., Santiz, Z. & Hollis, B. W. Defective photoproduction of cholecalciferol in normal and uremic humans. J. Nutr. 114, 1313–1319 (1984).
Lee, C. C., Sun, C. Y. & Wu, M. S. Long-term modality-related mortality analysis in incident dialysis patients. Perit. Dial. Int. 29, 182–190 (2009).
Collins, A. J. et al. US Renal Data System 2013 Annual Data Report. Am. J. Kidney Dis. 63, A7, https://doi.org/10.1053/j.ajkd.2013.11.001 (2014).
Hill, C. J. & Fogarty, D. G. Changing trends in end-stage renal disease due to diabetes in the United kingdom. J. Ren. Care 38(Suppl 1), 12–22, https://doi.org/10.1111/j.1755-6686.2012.00273.x (2012).
Zittermann, A. et al. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 95, 91–100, https://doi.org/10.3945/ajcn.111.014779 (2012).
Arnson, Y., Gringauz, I., Itzhaky, D. & Amital, H. Vitamin D deficiency is associated with poor outcomes and increased mortality in severely ill patients. QJM 105, 633–639, https://doi.org/10.1093/qjmed/hcs014 (2012).
Venkatram, S. et al. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit. Care 15, R292, https://doi.org/10.1186/cc10585 (2011).
de Haan, K., Groeneveld, A. B., de Geus, H. R., Egal, M. & Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit. Care 18, 660, https://doi.org/10.1186/s13054-014-0660-4 (2014).
Drechsler, C. et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur. Heart J. 31, 2253–2261, https://doi.org/10.1093/eurheartj/ehq246 (2010).
Walker, J. P. et al. Vitamin D deficiency is associated with mortality and adverse vascular access outcomes in patients with end-stage renal disease. J. Vasc. Surg. 60, 176–183, https://doi.org/10.1016/j.jvs.2014.01.037 (2014).
Anand, S. et al. Vitamin D deficiency and mortality in patients receiving dialysis: the Comprehensive Dialysis Study. J. Ren. Nutr. 23, 422–427, https://doi.org/10.1053/j.jrn.2013.05.003 (2013).
Bouillon, R. et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr. Rev. 29, 726–776, https://doi.org/10.1210/er.2008-0004 (2008).
O’Kelly, J. et al. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J. Clin. Invest. 109, 1091–1099, https://doi.org/10.1172/JCI12392 (2002).
Zeitz, U. et al. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 17, 509–511, https://doi.org/10.1096/fj.02-0424fje (2003).
Assalin, H. B. et al. Impact of the length of vitamin D deficiency on cardiac remodeling. Circ. Heart Fail. 6, 809–816, https://doi.org/10.1161/CIRCHEARTFAILURE.112.000298 (2013).
Gupta, G. K., Agrawal, T., DelCore, M. G., Mohiuddin, S. M. & Agrawal, D. K. Vitamin D deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swine. Exp. Mol. Pathol. 93, 82–90, https://doi.org/10.1016/j.yexmp.2012.04.006 (2012).
Gezmish, O. et al. Maternal vitamin D deficiency leads to cardiac hypertrophy in rat offspring. Reprod. Sci. 17, 168–176, https://doi.org/10.1177/1933719109349536 (2010).
Patange, A. R., Valentini, R. P., Gothe, M. P., Du, W. & Pettersen, M. D. Vitamin D deficiency is associated with increased left ventricular mass and diastolic dysfunction in children with chronic kidney disease. Pediatr. Cardiol. 34, 536–542, https://doi.org/10.1007/s00246-012-0489-z (2013).
Patange, A. R., Valentini, R. P., Du, W. & Pettersen, M. D. Vitamin D deficiency and arterial wall stiffness in children with chronic kidney disease. Pediatr. Cardiol. 33, 122–128, https://doi.org/10.1007/s00246-011-0101-y (2012).
Harasawa, H., Yamazaki, C., Itoh, A. & Masuko, K. [Cardiothoracic ratio and roentgenologic heart size as the indices of body fluid retention in uremics under hemodialysis]. Nihon Igaku Hoshasen Gakkai Zasshi 49, 191–198 (1989).
Laczkovics, A. et al. Noninvasive assessment of acute rejection after orthotopic heart transplantation: value of changes in cardiac volume and cardiothoracic ratio. J. Cardiovasc. Surg. 29, 582–586 (1988).
Hammermeister, K. E., Chikos, P. M., Fisher, L. & Dodge, H. T. Relationship of cardiothoracic ratio and plain film heart volume to late survival. Circulation 59, 89–95 (1979).
Cohn, J. N. et al. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation 87, VI5–16 (1993).
Bertolet, B. D. et al. Unrecognized left ventricular dysfunction in an apparently healthy alcohol abuse population. Drug. Alcohol. Depend. 28, 113–119 (1991).
Martins, D. et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 167, 1159–1165, https://doi.org/10.1001/archinte.167.11.1159 (2007).
Targher, G. et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin. Endocrinol. 65, 593–597, https://doi.org/10.1111/j.1365-2265.2006.02633.x (2006).
Chen, K. H. et al. Cardiothoracic ratio, malnutrition, inflammation, and two-year mortality in non-diabetic patients on maintenance hemodialysis. Kidney Blood Press. Res. 31, 143–151, https://doi.org/10.1159/000127388 (2008).
Gao, N. et al. Measurements on the routine chest radiograph as prognostic markers in Chinese peritoneal dialysis patients. Clin. Nephrol. 76, 16–22 (2011).
Okute, Y. et al. Cardiothoracic Ratio as a Predictor of Cardiovascular Events in a Cohort of Hemodialysis Patients. J. Atheroscler. Thromb. 24, 412–421, https://doi.org/10.5551/jat.36426 (2017).
Mathieu, C. Vitamin D and diabetes: Where do we stand? Diabetes Res. Clin. Pract. 108, 201–209, https://doi.org/10.1016/j.diabres.2015.01.036 (2015).
Berridge, M. J. Vitamin D deficiency and diabetes. Biochem. J. 474, 1321–1332, https://doi.org/10.1042/BCJ20170042 (2017).
Judd, S. E. et al. Vitamin D deficiency and incident stroke risk in community-living black and white adults. Int. J. Stroke 11, 93–102, https://doi.org/10.1177/1747493015607515 (2016).
Thompson, J., Nitiahpapand, R., Bhatti, P. & Kourliouros, A. Vitamin D deficiency and atrial fibrillation. Int. J. Cardiol. 184, 159–162, https://doi.org/10.1016/j.ijcard.2015.02.012 (2015).
Schwalfenberg, G. K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 55, 96–108, https://doi.org/10.1002/mnfr.201000174 (2011).
Zhang, Y. P., Wan, Y. D., Sun, T. W., Kan, Q. C. & Wang, L. X. Association between vitamin D deficiency and mortality in critically ill adult patients: a meta-analysis of cohort studies. Crit. Care 18, 684, https://doi.org/10.1186/s13054-014-0684-9 (2014).
Zajic, P. & Amrein, K. Vitamin D deficiency in the ICU: a systematic review. Minerva Endocrinol. 39, 275–287 (2014).
Tiwari, S., Pratyush, D. D., Gupta, S. K. & Singh, S. K. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br. J. Nutr. 112, 1938–1943, https://doi.org/10.1017/S0007114514003018 (2014).
Forrest, K. Y. & Stuhldreher, W. L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 31, 48–54, https://doi.org/10.1016/j.nutres.2010.12.001 (2011).
Holick, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1911–1930, https://doi.org/10.1210/jc.2011-0385 (2011).
Oh, T. R. et al. Association between vitamin D deficiency and health-related quality of life in patients with chronic kidney disease from the KNOW-CKD study. PLoS One 12, e0174282, https://doi.org/10.1371/journal.pone.0174282 (2017).
Kara, A. V., Aldemir, M. N., Soylu, Y. E. & Arslan, Y. K. Relationship between Serum Vitamin D Levels and Health-Related Quality of Life in Maintenance Hemodialysis Patients. Blood Purif, 1-9, https://doi.org/10.1159/000497242 (2019).
Acknowledgements
This investigation was partially supported by a grant from Chang Gung Medical Foundation Chang Gung Memorial Hospital, Keelung (CMRPG260362-3, CMRPG2A0451-3, CMRPG2B0151-3 and CMRPG2E0251-3). The authors wish to express their deepest gratitude to all the patients who participated in this study.
Author information
Authors and Affiliations
Contributions
H.J.H. performed the study and wrote the manuscript, I.W.W. and C.Y.S. managed the patients, C.Y.C. and K.H.H. analyzed the data, and C.C.L. designed and supervised the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsu, HJ., Wu, IW., Hsu, KH. et al. Vitamin D deficiency, cardiothoracic ratio, and long-term mortality in hemodialysis patients. Sci Rep 10, 7533 (2020). https://doi.org/10.1038/s41598-020-64359-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64359-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.