Abstract
Recently, celastrol has shown great potential for inducing apoptosis in acute myeloid leukemia cells, especially acute promyelocytic leukaemia cells. However, the mechanism is poorly understood. Metabolomics provides an overall understanding of metabolic mechanisms to illustrate celastrol's mechanism of action. We treated both nude mice bearing HL-60 cell xenografts in vivo and HL-60 cells as well as NB-4 cells in vitro with celastrol. Ultra-performance liquid chromatography coupled with mass spectrometry was used for metabolomics analysis of HL-60 cells in vivo and for targeted L-cysteine analysis in HL-60 and NB-4 cells in vitro. Flow cytometric analysis was performed to assess mitochondrial membrane potential, reactive oxygen species and apoptosis. Western blotting was conducted to detect the p53, Bax, cleaved caspase 9 and cleaved caspase 3 proteins. Celastrol inhibited tumour growth, induced apoptosis, and upregulated pro-apoptotic proteins in the xenograft tumour mouse model. Metabolomics showed that cysteine metabolism was the key metabolic alteration after celastrol treatment in HL-60 cells in vivo. Celastrol decreased L-cysteine in HL-60 cells. Acetylcysteine supplementation reversed reactive oxygen species accumulation and apoptosis induced by celastrol and reversed the dramatic decrease in the mitochondrial membrane potential and upregulation of pro-apoptotic proteins in HL-60 cells. In NB-4 cells, celastrol decreased L-cysteine, and acetylcysteine reversed celastrol-induced reactive oxygen species accumulation and apoptosis. We are the first to identify the involvement of a cysteine metabolism/reactive oxygen species/p53/Bax/caspase 9/caspase 3 pathway in celastrol-triggered mitochondrial apoptosis in HL-60 and NB-4 cells, providing a novel underlying mechanism through which celastrol could be used to treat acute myeloid leukaemia, especially acute promyelocytic leukaemia.
Similar content being viewed by others
Introduction
Acute leukemia is the most common malignant hematological malignancy, of which acute myeloid leukemia (AML) accounts for over 80% of all acute leukemias in individuals aged >18 years1. Chemotherapy is the main treatment of AML. There are still problems such as drug resistance and relapse of leukemia after drug withdrawal, which seriously affect the quality of life and long-term survival of patients. Therefore, it is of great significance to find effective new antineoplastic drugs and explore new therapeutic schemes.
Acute promyelocytic leukaemia (APL), a unique subtype of acute myeloid leukaemia (AML), is a devastating disease characterized by neoplastic proliferation of cells in the bone marrow that have a promyelocytic phenotype and fail to terminally differentiate2. Unlike other forms of AML, APL can cause diffuse intravascular coagulopathy and early haemorrhagic death if not promptly diagnosed3,4. With the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), APL treatment has achieved good outcomes. However, ATRA and ATO can lead to the development of differentiation syndrome and multiorgan toxicity, and a few patients do not respond favourably to the drugs5,6. Thus, the, development of less toxic and more effective agents to treat APL is urgent7
Traditional Chinese medicine, as an important source of therapeutically effective drugs, has attracted increasing attention in cancer therapy8. Recently, celastrol an active component of Tripterygium wilfordii Hook f. (Supplementary Material: Fig. S1), is receiving increasing attention. Celastrol has shown great potential for the treatment of inflammation9, Gaucher disease8, obesity10 and multiple cancers, such as prostate cancer11, breast cancer12, and leukaemia13.
The investigation of the effect of celastrol on AML and APL is an interesting topic, and has been widely conducted. Celastrol could significantly prolong the survival of mice in HoxA9/Meis1-induced AML model and APL model13,14. In fresh cells from patients of various types of AML, celastrol showed effect for the treatment of leukemia13,15. In addition, celastrol could eradicate leukemia stem cell which is the key cause of relapse16,17. Importantly, the previous study has demonstrated that celastrol showed stronger anti-tumour effect than ATRA in leukemia cells13. Celastrol is also found as a promising and unique agent for managing the sid e effects of ATRA application on APL18. Interestingly, the anti-tumour effects of celastrol have been consistently attributed to its ability to induce apoptosis in AML and APL NB-4 cells15,19,20,21, but the mechanism is poorly understood.
HL-60 cells is a widely used model system for studying the molecular events of AML, which lack the t(15;17) translocation characteristic of most cases of APL13,22,23. However, HL-60 can respond to ATRA22, which is widely used as a cell line in the APL studies24,25,26,27. In our previous study28, consistent to previous reports15,19,20,21, we also found celastrol caused apoptosis in HL-60 cells, indicating the key role of apoptosis in the effect of celastol in the treatment of acute leukemia.
Metabolomics, the systematic measurement and biological interpretation of metabolites within a biological sample, is used to study small molecules and is an integral technology for understanding the function of biological systems. Surveying these small molecules provides an overall understanding of biological mechanisms, thereby creating a more complete picture of the phenotype (the observable characteristics of a living system). In our previous study, we used metabolomics to study the underlying mechanism in HL-60 cells in vitro, and identified uridine was the most notable changed metabolite after celastrol treatment28. However, the metabolome changes in vivo are unknown. As we know, pathogenesis and therapeutic target of leukemia may be not limited in one pathway. Different and complementary conclusions may be reached by using omics analyses of in vitro and in vivo samples. The hypothesis of this study was that key metabolism changes extracted from metabolome of animal model could reveal mechanism underlying celastrol-induced apoptosis in AML, especially APL. Therefore, in the present study, we treated xenograft HL-60 cell-bearing nude mice with celastrol and used metabolomics to identify the key metabolic changes in tumour tissues in vivo. To validate the key findings with the focus on APL, we also applied NB-4 cell according to previous report26. NB-4 is a APL cell line, which has the t(15;17) translocation and also has response to ATRA26. The role of key metabolic pathway in apoptosis was verified by disrupting the key metabolic changes in both cell lines, revealing that cysteine metabolism plays a key role in celastrol-induced ROS-dependent mitochondrial apoptosis in HL-60 and NB-4 cells.
Results
Celastrol inhibited tumour growth and increased apoptotic gene expression in HL-60 cells in vivo
In the nude mouse xenograft tumour model, 2 mg/kg celastrol decreased tumour volume and weight (Fig. 1a–c). The TUNEL assay results showed that celastrol induced apoptosis in tumours (Fig. 1d). In addition, we found increased protein levels of p53, Bax, cleaved caspase 9 and cleaved caspase 3 in the celastrol treatment group in vivo (Figs. 1e, S2–S5).
The effects of celastrol on tumour growth and apoptosis in vivo. (a) The effect of celastrol on tumour volume growth. (b) The effect of celastrol on tumour weight. (c) The representative graph of the effect of celastrol on tumour volume growth. (d) TUNEL assay detecting the effect of celastrol on tumour cell apoptosis. (e) The effect of celastrol on the protein levels of apoptosis-related genes in HL-60 cells in vivo. *p < 0.05 when compared with control.
Metabolomics profiles showed that cysteine metabolism was the key metabolic alteration after celastrol treatment in HL-60 cells in vivo
A total of 129 metabolites were found in tumour tissues (Supplementary Material: Fig. S6). After false discovery rate (FDR) correction, 121 metabolites were found to be significantly changed (q<0.05) (Supplementary Material: Table S1). A total of 43 metabolites significantly increased, while 78 significantly decreased. Principal component analysis (PCA) is an unbiased and unsupervised model that can reveal global celastrol-induced metabolic changes in tumour tissue. We established a 3D PCA model and found good separation between the treatment and control groups (Fig. 2a). The above results indicate that along with the significant growth-inhibitory and pro-apoptotic effect of celastrol on tumour tissue, the metabolome was also dramatically disrupted in tumour tissue, a quite important finding in the search for the underlying mechanism. Among the metabolites, L-cysteine and adenine showed the most marked change. These metabolites were significantly decreased—indeed, totally undetectable—after celastrol treatment in tumour tissues, indicating the importance of L-cysteine metabolism and adenine metabolism in celastrol-induced apoptosis in tumour tissues. To further identify key metabolic changes, the changed metabolites were imported into the “enrichment analysis” module (www.metaboanalyst.ca/), and protein biosynthesis and glutathione metabolism were identified as the key changed pathways (Fig. 2b, Supplementary Material: Table S2). Importantly, glutathione metabolism was directly linked to L-cysteine metabolism; in addition, acetylcysteine and homocysteine, the upstream metabolites of L-cysteine metabolism, were also significantly decreased after celastrol treatment (Fig. 2c,d). These results indicated the importance of cysteine metabolism in HL-60 cells in vivo after celastrol treatment. Raw metabolomic data can be found in the Supplementary Material: Table S3.
Cysteine metabolism was the key changed metabolism after celastrol treatment. (a) 3D PCA model showed good separation between control and celastrol-treated group in vivo. (b) Pathway enrichment analysis of the metabolomics data in vivo. The red indicated significant enriched pathways. (c) Heatmap of the metabolomics data in vivo. The metabolites in the red boxes were related to cysteine metabolism. (d) Cysteine metabolism alteration after celastrol treatment in HL-60 cells in vivo. Green arrows indicates decrease in vivo. The bar chart shows the statistical results from in vivo study. *p < 0.05 when compared with control.
Celastrol decreased L-cysteine levels and induced significant accumulation of ROS in HL-60 cells, which could be reversed by acetylcysteine
To verify the change in cysteine metabolism in vivo, we analysed L-cysteine in HL-60 cells in vitro and found that it was decreased after celastrol treatment in a dose-related manner, as assessed by Spearman correlation analysis (rs = −0.4522, p = 0.0265) (Fig. 3a). As cysteine and its related glutathione metabolism were enriched in vivo and the accumulation of intracellular ROS is one of the most important upstream stimuli of p53 activation in apoptosis29, the above metabolomics findings prompted us to focus on the intracellular ROS level after celastrol treatment, as ROS might be the intermediate linking the observed deficiency in oxidized glutathione and its upstream metabolites with the decreased anti-oxidative capacity and increased apoptosis in HL-60 cells after celastrol treatment. As shown in Fig. 3b, ROS was detected in control HL-60 cells, and the ROS positive control reagent Rosup led to a dramatic increase in the ROS level in the treated cells, indicating the efficiency of the ROS detection method. The intracellular ROS level was significantly increased in a dose-dependent manner after celastrol treatment (Fig. 3b). Acetylcysteine is an upstream metabolite of cysteine metabolism and was a significantly decreased metabolite after celastrol treatment (Fig. 2). Acetylcysteine exerts an anti-oxidant effect related to its role as a metabolic precursor of glutathione30. Based on the metabolomics findings, we used acetylcysteine to disrupt cysteine metabolism in order to verify the connection among cysteine metabolism, ROS and apoptosis. The ROS level in HL-60 cells treated with both celastrol and acetylcysteine was drastically decreased to the level in control cells, indicating that cysteine metabolism plays a key role in the induction of ROS by celastrol (Fig. 3c).
The effects of celastrol on L-cysteine levels and ROS and the effects of acetylcysteine on ROS, apoptosis, mitochondrial membrane potential, and apoptosis-related proteins expression disrupted by celastrol in HL-60 cells. (a) L-cysteine levels were decreased after celastrol treatment in HL-60 cells. Bar chart shows the statistical results from six independent experiments (*p < 0.05). (b) Celastrol induced the accumulation of ROS in HL-60 cells. Bar chart shows the statistical results from three independent experiments. *p < 0.05 when compared with control. (c) Acetylcysteine decreased HL-60 cells ROS induced by celastrol. Bar chart shows the statistical results from three independent experiments. Asterisks indicated statistical significance (***p < 0.001). (d) Acetylcysteine rescued HL-60 cells from increased apoptosis induced by celastrol. Bar chart shows the statistical results from three independent experiments. Asterisks indicated statistical significance (***p < 0.001). (e) Acetylcysteine rescued the decrease of mitochondrial membrane potential induced by celastrol. Bar chart shows the statistical results from three independent experiments. Asterisks indicated statistical significance (***p < 0.001). (f) Acetylcysteine inhibited the up-regulation of apoptosis-related proteins induced by celastrol in HL-60 cells. The quantification results of relative protein levels from three independent experiments are shown. Asterisks indicated statistical significance (*p < 0.05).
Acetylcysteine reversed celastrol-induced apoptosis of HL-60 cells
As shown in Fig. 3d, celastrol treatment induced significant apoptosis of HL-60 cells, and acetylcysteine treatment exerted no effect on apoptosis. The apoptosis rate of cells treated with both celastrol and acetylcysteine was drastically decreased to the level of control cells, indicating that cysteine metabolism plays a key role in celastrol-induced apoptosis of HL-60 cells.
Acetylcysteine reversed the dramatic decrease in the mitochondrial membrane potential and upregulation of apoptosis-related proteins
ROS are mainly produced by mitochondria31. To further identify the intermediate linking cysteine metabolism, ROS and apoptosis, we measured the mitochondrial membrane potential. The mitochondrial membrane potential was significantly decreased after celastrol treatment, and this decrease was dramatically reversed by the addition of acetylcysteine, indicating that cysteine metabolism-initiated, ROS-triggered apoptosis might occur through the mitochondrial pathway after celastrol treatment (Fig. 3e). We next assessed the effect of celastrol on the protein levels of p53, Bax, cleaved caspase 9 and cleaved caspase 3. As shown in Figs. 3f, S7–S10, the protein levels of p53, Bax, cleaved caspase 9 and cleaved caspase 3 were significantly increased after celastrol treatment, and this increase was dramatically inhibited in the presence of acetylcysteine, indicating that cysteine metabolism plays a role in celastrol-induced mitochondrial apoptosis in HL-60 cells.
Cysteine metabolism played a role in celastrol-induced apoptosis of NB-4 cells
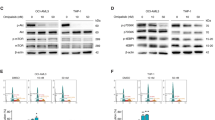
To determine whether celastrol operated through the same metabolic pathway to induce apoptosis in different model systems, we used human APL NB-4 cells. Similar to its effects on HL-60 cells, celastrol induced ROS accumulation and apoptosis in NB-4 cells; effects were reversed by acetylcysteine (Fig. 4a,b). In addition, L-cysteine, the key metabolite of cysteine metabolism, was significantly decreased after celastrol treatment, validating the changes in cysteine metabolism in different cell models (Fig. 4c).
The effects of celastrol on L-cysteine levels and the effect of acetylcysteine on ROS, apoptosis disrupted by celastrol in NB-4 cells. (a) Acetylcysteine decreased NB-4 cells ROS induced by celastrol. Bar chart shows the statistical results from three independent experiments. Asterisks indicated statistical significance (**p < 0.01, ***p < 0.001). (b) Acetylcysteine rescued NB-4 cells from increased apoptosis induced by celastrol. Bar chart shows the statistical results from three independent experiments. Asterisks indicated statistical significance (***p < 0.001). (c) L-cysteine levels were decreased after 0.5 μM celastrol treatment in NB-4 cells. Bar chart shows the statistical results from three independent experiments. Asterisks indicated statistical significance (*p < 0.05).
Discussion
In the xenograft tumour mouse model, celastrol inhibited tumour growth, induced apoptosis, and upregulated pro-apoptotic proteins (Fig. 1). Unbiased metabolomics profiling revealed that a decrease in the metabolites involved in cysteine metabolism was the key metabolic change after celastrol treatment (Fig. 2). Cysteine metabolism and its derived metabolites are related to cells’ anti-oxidative stress capacity32, and the final product of cysteine metabolism is glutathione. Glutathione is an important biological anti-oxidant and can protect important cellular components from ROS-induced damage33. ROS are formed by the transfer of one electron from a redox donor to molecular oxygen (O2)31. Emerging evidence suggests that ROS are critically involved in cancer cell functions34. Cancer cells, which have higher levels of basal ROS than normal cells, are likely to be more vulnerable to damage by further ROS insults35. Hypothesis-free screening indicated that the decrease in the anti-oxidative capacity and accumulation of intracellular ROS in HL-60 cells were key events after celastrol treatment. The increased ROS level was then verified in vitro (Fig. 3).
In this study, we found that the mitochondrial membrane potential significantly decreased after celastrol treatment (Fig. 3). Mitochondria, one of the most important cellular organelles, can produce energy, generate ROS, regulate cell signalling and participate in biosynthetic metabolism36. Mitochondria can impart considerable flexibility to tumour cell survival and growth under harsh environmental conditions, such as nutrient depletion, hypoxia, and cancer treatment exposure, and many classical hallmarks of cancer result in altered mitochondrial function. Therefore, mitochondria are key factors in tumourigenesis and treatment.
The tumour suppressor p53 plays a pivotal role in regulating the cell cycle, genomic integrity and apoptosis37. Among signalling components, p53 has one of the highest sensitivities to the cellular redox state38. p53 mutations often occur in most cancers. However, in the vast majority of AML cells, wild-type p53 is retained39. ROS may attack proteins and nucleic acids, and this event is followed by upregulation of wild-type p53. Upregulation of p53 was also verified in this study after celastrol-induced elevation of intracellular ROS levels (Fig. 3). Notably, as a sensor of cellular stress, p53 is a critical initiator of the intrinsic apoptotic pathway under robust or sustained stress40.
Apoptosis plays a critical role during various physiological (vertebrate development and postnatal tissue homeostasis) and pathological processes, including tumourigenesis. Resistance to apoptosis is a hallmark of cancer cells41,42. Therefore, targeting apoptosis for anticancer therapy is promising. The mitochondrial pathway of apoptosis is activated following cellular stress (DNA damage, nutrient deprivation, endoplasmic reticulum stress, and so on)43. The mitochondrial pathway of apoptosis is essential for metazoan development, tissue homeostasis, and cellular responses to therapeutics43. In human cancers, signalling via the mitochondrial (intrinsic) pathway of apoptosis is frequently impaired44. BCL-2 family proteins, especially Bax, are the central regulators of the intrinsic apoptotic pathway via the control of mitochondrial outer membrane permeabilization (MOMP)45, which is frequently the decisive event preceding cell death46. In the present study, we verified the pro-apoptotic effect of celastrol on HL-60 cells via the mitochondrial pathway (Fig. 3). We further found the clearance of ROS reversed the upregulation of p53 and the increased apoptosis induced by celastrol (Fig. 3). The elevated ROS is an activating signal for p53-mediated apoptosis in leukemia35,47. Therefore, the elevation of ROS induced p53-mediated mitochondrial apoptosis in leukemia cells after celastrol treatment.
Acetylcysteine is an upstream metabolite of cysteine metabolism and was significantly decreased after celastrol treatment (Fig. 2). Acetylcysteine can support cysteine metabolism to increase glutathione production in order to increase the anti-oxidative stress capacity30. In this study, when acetylcysteine was added, the mitochondrial membrane potential was dramatically recovered, and the increases in intracellular ROS, p53 expression and apoptosis were inhibited (Fig. 3), verify ing the involvement of a cysteine metabolism/ROS/p53/Bax/caspase 9/caspase 3 pathway in the apoptosis of HL-60 cells after celastrol treatment.
Studying the effect and underlying mechanism in NB-4 cell lines could enhance the significance of the current findings in APL26. In this study, the effect of celastrol on cysteine metabolism and the rescue effect of acetylcysteine on celastrol-induced ROS and apoptosis were also verified in NB-4 cells, indicating that celastrol operates through the same metabolic pathway to induce apoptosis in HL-60 and NB-4 cells (Fig. 4).
Conclusions
In conclusion, by combining an in vivo unbiased metabolomics analysis with follow-up in vitro experiments, we found consistent results indicating that cysteine metabolism was the key metabolic change in HL-60 cells and NB-4 cells after celastrol treatment. This finding linked the depleted intracellular anti-oxidative capacity, increased ROS accumulation, and increased mitochondrial apoptosis in human HL-60 and NB-4 cells (Fig. 5), thus providing a novel underlying mechanism through which celastrol might be used to treat AML, especially APL.
Materials and Methods
Cell culture
Human HL-60 cells and NB-4 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and grown in Iscove's modified Dulbecco's medium supplemented with 20% foetal bovine serum, 100 IU/ml penicillin and 100 μg/ml streptomycin. Purified celastrol (purity ≥98%, HPLC-grade) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetylcysteine (purity >99%) was purchased from Beyotime (Shanghai, China).
Nude mouse xenograft tumour assay
According to protocols in previous reports48,49,50, after one week of acclimation, male BALB/c nude mice (five weeks old and weighing 20–22 g, obtained from NLARSH (Shanghai, China)) were injected subcutaneously with HL-60 cells (5×106 in 100 μl of PBS) under the shoulder. Tumours were allowed to grow to a volume of 100 to 200 mm3. Then, mice were randomized into two groups (9 mice per group), and injected intraperitoneally with vehicle control (5% DMSO in 0.9% saline) or celastrol (2 mg/kg/day). The dosage of celastrol was determined according to previous reports11,51. Tumour volume (0.5 × larger diameter × smaller diameter) and body weight were measured. Mice were maintained on a 12:12-h light-dark cycle under specific pathogen-free conditions. Mice were fasted for 12 h before sacrifice on day 21 after celastrol treatment. Tumour weight and volume were measured. TUNEL was performed to identify the apoptotic cells in tumours using an ApopTag® Peroxidase In Situ Oligo Ligation Kit (Chemicon International, Inc., Cat. #S7200) according to the manufacturer's instructions. Mice were sacrificed after anaesthetization by intraperitoneal injection of ketamine and diazepam. All animal experiments were performed in accordance with the protocol approved by the Nanjing Medical University Institutional Animal Care and Use Comm ittee.
Metabolomics analysis
Sample preparation was performed according to our previous report52. Homogenized tumour tissues (50 mg) (n = 9 per group) were mixed with 150 μl of water and 600 μl of methanol. Tissues were ultrasonicated for 5 min (power: 60%, pulses: 3/3), and the supernatant was obtained after centrifugation at 20,000 × g for 15 min. After drying, the residue was reconstituted for metabolomics analysis. The extracted samples were subjected to unbiased metabolomics analysis according to protocols in our previous studies52,53. Metabolomics analysis was performed on a UPLC Ultimate 3000 system (Dionex, Germering, Germany) coupled to a Q-Exactive Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). The UPLC Ultimate 3000 system was used with a C18 column (Hypersil Gold, 1.9 μm, 100 mm × 2.1 mm; Thermo Fisher Scientific), which was maintained at 40 °C. Mobile phase A was 0.1% formic acid in ultra-pure water, and mobile phase B was acetonitrile acidified with 0.1% formic acid. Chromatographic separation was conducted using a multistep gradient with a flow rate of 0.4 ml/min over 15 min. The UPLC autosampler temperature was set at 4 °C, and the injection volume for each sample was 10 μl. All samples were analysed in a randomized fashion to avoid complications related to the injection order. The Orbitrap mass spectrometer was equipped with a heated electrospray source using full scan analysis (70 to 1050 amu) in both positive and negative ion models simultaneously. For both the positive and negative ion modes, the mass spectrometer parameters were set as follows: spray voltage of 3.5 kV for positive ion model and 2.5 kV for negative ion model, capillary temperature of 300 °C, sheath gas flow of 50 arbitrary units, auxiliary gas flow of 13 arbitrary units, sweep gas flow of 0 arbitrary units, S-Lens RF level of 60, and resolution of 70,000 with an automatic gain control (AGC) target of 3 × 106 charges. The mass spectrometer system was calibrated according to the manufacturer's instructions. Chemical identification was based on the retention time and accurate mass of 270 commercial standards using the author-constructed library. The standard compounds were purchased from SigmaAldrich (St. Louis, MO), Damasbeta Co., Ltd. (Basel, Switzerland), Adamas Reagent Co., Ltd. (Shanghai, China), and Aladdin Reagent Company (Shanghai, China). Quality control (QC) sample was prepared by mixing each original sample according to a previous metabolomics study54, and was analysed in parallel with original samples.
Targeted L-cysteine analysis
HL-60 cells and NB-4 cells were plated at a density of 2 × 105 cells/well in six-well plates and exposed to celastrol for 24 h. Cells were harvested and washed three times with ice-cold PBS. Then, 1 ml of ice-cold 50% methanol was added, and all cells were lysed by ultrasonication for 1 min (power: 60%, pulses: 3/3). Following centrifugation (16,000 × g, 10 min, 4 °C), the supernatant was used for detection. L-cysteine was detected using the UPLC Ultimate 3000 system coupled to the Orbitrap mass spectrometer. The conditions of the chromatograph and mass spectrometer were the same as those described in the “Metabolomics analysis” section. The [M+H]+ ions of L-cysteine at m/z 122.02703 were monitored.
Measurement of intracellular ROS
Intracellular ROS generation was measured by a ROS assay with DCFH-DA (Beyotime, Shanghai, China) according to a previous report55. Rosup was used as the positive control reagent according to the manufacturer's instructions. In brief, HL-60 cells were plated at a density of 2 × 105 cells/well in six-well plates and exposed to celastrol (0.125, 0.25 and 0.5 μM) for 24 h. Then, cells were incubated with DCFH-DA (10 μM) for 30 min at 37 °C. DCFH-DA is hydrolysed by intracellular esterase to produce a nonfluorescent DCFH product, that can then be oxidized by ROS to produce a highly fluorescent DCF product. For the follow-up study, HL-60 cells and NB-4 cells were treated with 0.1% DMSO, celastrol (0.5 μM) and celastrol (0.5 μM)+acetylcysteine (5 mM) for 24 h. The level of ROS was measured by flow cytometry (BD Biosciences, NJ, USA). All experiments were repeated at least three times.
Determination of apoptosis by flow cytometric analysis
HL-60 and NB-4 cells were treated with 0.1% DMSO, acetylcysteine (5 mM), celastrol (0.5 μM) and celastrol (0.5 μM)+acetylcysteine (5 mM) for 24 h. The concentration of acetylcysteine was determined according to a previous report56. The percentage of apoptotic cells was assessed using a FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, NJ, USA) as described in the manufacturer's instructions.
Measurement of the mitochondrial membrane potential
The mitochondrial membrane potential was measured with a JC-1 Mitochondrial Membrane Potential Assay Kit (Yeasen, Shanghai, China) according to the manufacturer's instructions, as described previously57. In brief, HL-60 cells were treated with 0.1% DMSO, acetylcysteine (5 mM), celastrol (0.5 μM) and celastrol (0.5 μM) + acetylcysteine (5 mM) for 24 h. Then, cells were incubated with JC-1 for 20 min in the dark at 37 °C. After being washed twice with staining buffer, cells were analysed by flow cytometry (BD Biosciences, NJ, USA). JC-1 forms aggregates and emits red fluorescence to indicate a normal mitochondrial membrane potential. This red fluorescence was collected as the PE signal, while the accumulation of green fluorescent monomers due to depolarization of the mitochondrial membrane potential was indicated by an increase in FITC fluorescence.
Western blot analysis
Western blot analysis was performed as described previously58 using the following antibodies: primary rabbit polyclonal antibodies against cleaved caspase 9 (Cat. #9505T), cleaved caspase 3 (Cat. #9664T), Bax (Cat. #2772), and p53 (Cat. #2524) (Cell Signaling Technology, Beverly, MA, USA); a mouse monoclonal antibody against β-actin (Cat. #AA128, Beyotime, Shanghai, China); and an HRP-conjugated secondary antibody (Cat. #A0208, Cat #A0216, Beyotime, Shanghai, China). Western blot data were quantified with Image-Pro Plus software. All experiments were repeated at least three times.
Statistical analyses
Statistical analysis of the data was performed using the Stata statistical package (Version 9.2, Stata Corp, LP); “R” (http://cran.r-project.org/), a freely available open source software package; and SIMCA-P software (Umetrics, Sweden). The metabolomics data were imported into SIMCA-P software for multivariate analysis. All data were UV-scaled and automatically log transformed by the software. PCA was applied to identify the metabolic changes between groups. The differences in metabolomics data between the treatment group and control group were identified with t-tests. To improve the statistical robustness, we used the FDR correction for multiple testing. We imported the changed metabolites into the “enrichment analysis” module to analyse the key metabolic changes (www.metaboanalyst.ca/). Statistically significant differences between two groups and among more than two groups were determined using a two-sided, unpaired t-test and one-way ANOVA with Dunnett's post hoc test, respectively. Values are expressed as the means ± standard errors. A p value of <0.05 was considered statistically significant.
Data availability
The raw metabolomic data are available in the electronic Supplementary Material.
References
Thein, M. S., Ershler, W. B., Jemal, A., Yates, J. W. & Baer, M. R. Outcome of older patients with acute myeloid leukemia: an analysis of SEER data over 3 decades. Cancer. 119, 2720–2727 (2013).
Adams, J. & Nassiri, M. Acute promyelocytic leukemia: a review and discussion of variant translocations. Arch. Pathol. Lab. Med. 139, 1308–1313 (2015).
Avvisati, G. Coagulopathy in APL: a step forward? Blood 120, 4–6 (2012).
Ades, L. et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood 115, 1690–1696 (2010).
Shen, Y. et al. Mutations of epigenetic modifier genes as a poor prognostic factor in acute promyelocytic leukemia under treatment with all-trans retinoic acid and arsenic trioxide. EBioMedicine 2, 563–571 (2015).
Alfonso, V. et al. Early and sensitive detection of PML-A216V mutation by droplet digital PCR in ATO-resistant acute promyelocytic leukemia. Leukemia 33, 1527–1530 (2019).
Wang, H. et al. Arsenic trioxide and mannitol for the treatment of acute promyelocytic leukemia relapse in the central nervous system. Blood. 124, 1998–2000 (2014).
Millimouno, F. M., Dong, J., Yang, L., Li, J. & Li, X. Targeting apoptosis pathways in cancer and perspectives with natural compounds from mother nature. Cancer Prev Res (Phila). 7, 1081–1107 (2014).
Hu, M. et al. Celastrol-induced Nur77 interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol. Cell. 66, 41–153 e146 (2017).
Liu, J., Lee, J., Salazar Hernandez, M. A., Mazitschek, R. & Ozcan, U. Treatment of obesity with celastrol. Cell 161, 999–1011 (2015).
Yang, H., Chen, D., Cui, Q. C., Yuan, X. & Dou, Q. P. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 66, 4758–4765 (2006).
Dandawate, P. R., Subramaniam, D., Jensen, R. A. & Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: novel approach for breast cancer therapy. Semin Cancer Biol. 40–41, 192–208 (2016).
Uttarkar, S. et al. Targeting acute myeloid leukemia with a small molecule inhibitor of the Myb/p300 interaction. Blood 127, 1173–1182 (2016).
Wang, L., Qin, G. F., Ke, H. & Wei, G. M. Effect of tripterine on coagulation function in human acute promyelocytic leukemia SCID mice xenograft model. J. Emerg in Tradit Chin. Med. 28, 232–235 (2019).
Cheng, W., Zhang, Y., Zhai, Y. & Ma, B. G. Effects of tripterine on proliferation and apoptosis of acute myeloid leukemia primary cells. J. Chin. Pract. Diag. Ther. 25, 1171–1173 (2011).
Hassane, D. C. et al. Discovery of agents that eradicate leukemia stem cells using an in silico screen of public gene expression data. Blood 111, 5654–5662 (2008).
Hartwell, K. A. et al. Niche-based screening identifies small-molecule inhibitors of leukemia stem cells. Nat. Chem. Biol. 9, 840–848 (2013).
Xu, L. M. et al. Celastrol inhibits lung infiltration in differential syndrome animal models by reducing TNF-α and ICAM-1 levels while preserving differentiation in ATRA-induced acute promyelocytic leukemia cells. PloS One 9, e105131 (2014).
Wang, D., Zhao, W. H., Lei, M. & Zheng, Y. H. Effects of Celastrol on proliferation and apoptosis of acute promyelocyticl euke-mia cell lines NB4. Modern Oncol. 21, 478–482 (2013).
Yu, X., Ruan, X., Zhang, J. & Zhao, Q. Celastrol induces cell apoptosis and inhibits the expression of the AML1-ETO/C-KIT oncoprotein in t(8;21) leukemia. Molecules. 21, pii: E574 (2016).
Nagase, M. et al. Apoptosis induction in HL-60 cells and inhibition of topoisomerase II by triterpene celastrol. Biosci Biotechnol Biochem. 67, 1883–1887 (2003).
Gallagher, R. et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979; 54(3):713–733. Blood 128, 2871 (2016).
Gallagher, R. et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 54, 713–733 (1979).
Zhong, P. Q. et al. ACTL6A interacts with p53 in acute promyelocytic leukemia cell lines to affect differentiation via the Sox2/Notch1 signaling pathway. Cell Signal. 53, 390–399 (2019).
Xu, J. H. et al. PU.1 can regulate the ZNF300 promoter in APL-derived promyelocytes HL-60. Leukemia Res. 34, 1636–1646 (2010).
Lee, K. H. et al. Differential gene expression in retinoic acid-induced differentiation of acute promyelocytic leukemia cells, NB4 and HL-60 cells. Biochem Biophys Res. Commun. 296, 1125–1133 (2002).
Robertson, K. A., Emami, B. & Collins, S. J. Retinoic acid-resistant HL-60R cells harbor a point mutation in the retinoic acid receptor ligand-binding domain that confers dominant negative activity. Blood 80, 1885–1889 (1992).
Zhang, X. L. et al. Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells. Oncotarget 7, 46557–46572 (2016).
Bose, D., Banerjee, S., Das, S., Chatterjee, N. & Saha, K. D. Heat killed attenuated leishmania induces apoptosis of hepG2 cells through ROS mediated p53 dependent mitochondrial pathway. Cell. Physiol. Biochem. 38, 1303–1318 (2016).
Dekhuijzen, P. N. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur. Respir. J. 23, 629–636 (2004).
Shadel, G. S. & Horvath, T. L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 163, 560–569 (2015).
Turnbull, A. L. & Surette, M. G. Cysteine biosynthesis, oxidative stress and antibiotic resistance in salmonella typhimurium. Res. Microbiol. 161, 643–650 (2010).
Cui, Q. et al. Modulating ROS to overcome multidrug resistance in cancer. Drug. Resist. Updat. 41, 1–25 (2018).
Cui, X. Reactive oxygen species: the achilles’ heel of cancer cells? Antioxid. Redox. Signal. 16, 1212–1214 (2012).
Shi, Y. et al. ROS-dependent activation of JNK converts p53 into an efficient inhibitor of oncogenes leading to robust apoptosis. Cell Death Differ. 21, 612–623 (2014).
Vyas, S., Zaganjor, E. & Haigis, M. C. Mitochondria and Cancer. Cell. 166, 555–566 (2016).
Vousden, K. H. & Lu, X. Live or let die: the cell's response to p53. Nat. Rev. Cancer. 2, 594–604 (2002).
Maryanovich, M. & Gross, A. A ROS rheostat for cell fate regulation. Trends Cell. Biol. 23, 129–134 (2013).
Croce, C. M. & Reed, J. C. Finally, An apoptosis-targeting therapeutic for cancer. Cancer. Res. 76, 5914–5920 (2016).
Carroll, R. G., Hollville, E. & Martin, S. J. Parkin sensitizes toward apoptosis induced by mitochondrial depolarization through promoting degradation of Mcl-1. Cell. Rep. 9, 1538–1553 (2014).
Giampazolias, E. & Tait, S. W. Mitochondria and the hallmarks of cancer. FEBS. J. 283, 803–814 (2016).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell. 100, 57–70 (2000).
Luna-Vargas, M. P. & Chipuk, J. E. Physiological and pharmacological control of BAK, BAX, and Beyond. Trends Cell. Biol. 26, 906–917 (2016).
Fulda, S. Targeting apoptosis for anticancer therapy. Semin. Cancer. Biol. 31, 84–88 (2015).
Green, D. R. & Llambi, F. Cell death signaling. Cold. Spring. Harb. Perspect. Biol. 7, https://doi.org/10.1101/cshperspect.a006080 (2015).
Volkmann, N., Marassi, F. M., Newmeyer, D. D. & Hanein, D. The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ. 21, 206–215 (2014).
Karawajew, L., Rhein, P., Czerwony, G. & Ludwig, W. D. Stress-induced activation of the p53 tumor suppressor in leukemia cells and normal lymphocytes requires mitochondrial activity and reactive oxygen species. Blood. 105, 4767–4775 (2005).
Rahmani, M. et al. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood 119, 6089–6098 (2012).
Weng, H. et al. Inhibition of miR-17 and miR-20a by oridonin triggers apoptosis and reverses chemoresistance by derepressing BIM-S. Cancer Res. 74, 4409–4419 (2014).
Nishioka, C., Ikezoe, T., Yang, J., Koeffler, H. P. & Yokoyama, A. Blockade of mTOR signaling potentiates the ability of histone deacetylase inhibitor to induce growth arrest and differentiation of acute myelogenous leukemia cells. Leukemia 22, 2159–2168 (2008).
Li, H. Y. et al. Celastrol induces apoptosis and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells: an in vitro and in vivo study. Cell. Death. Dis. 6, e1604 (2015).
Zhou, K. et al. Metabolomics reveals metabolic changes caused by low-dose 4-tert-octylphenol in mice liver. Int. J. Environ. Res. Public. Health. 15, https://doi.org/10.3390/ijerph15122686 (2018).
Hu, W. Y. et al. Obesity aggravates toxic effect of BPA on spermatogenesis. Environ. Int. 105, 56–65 (2017).
Gika, H. G., Macpherson, E., Theodoridis, G. A. & Wilson, I. D. Evaluation of the repeatability of ultra-performance liquid chromatography-TOF-MS for global metabolic profiling of human urine samples. J. Chromatogr B. Analyt Technol Biomed Life Sci. 871, 299–305 (2008).
Kang, M. Y. et al. The critical role of catalase in prooxidant and antioxidant function of p53. Cell Death Differ. 20, 117–129 (2013).
Amen, F. et al. N-acetylcysteine improves the quality of red blood cells stored for transfusion. Arch. Biochem. Biophys. 621, 31–37 (2017).
Cheng, W. Y. et al. An integrated imaging approach to the study of oxidative stress generation by mitochondrial dysfunction in living cells. Environ. Health. Perspect. 118, 902–908 (2010).
Yu, X. et al. Celastrol induces apoptosis of human osteosarcoma cells via the mitochondrial apoptotic pathway. Oncol Rep. 34, 1129–1136 (2015).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81503134, 81872650, 81573182); the Key Natural Science Foundation of the Jiangsu Higher Education Institutions of China (18KJA320003); the Key Project of the Science and Technology Development Foundation of Nanjing Medical University (2014NJMUZD005); the Key Research & Development Plan of Jiangsu Province (BE2017628); the Southeast University & Nanjing Medical University Collaborative Research Project (2242018K3DN25, 2018DN0025); the Innovation Fund Project of the State Key Laboratory of Reproductive Medicine (SKLRM-GC201901); the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
C.M.J. and Z.X.L. conceived and designed the study. Y.J.and L.X.M. performed the experiments. C.M.J. and Y.J. performed the statistical analysis and wrote the manuscript. L.L., H.Y.H., S.R.L., W.Y.B. and W.X.R. contributed reagents, materials, and analysis tools. C.M.J. revised manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, M., Yang, J., Li, L. et al. Metabolomics Reveals that Cysteine Metabolism Plays a Role in Celastrol-Induced Mitochondrial Apoptosis in HL-60 and NB-4 Cells. Sci Rep 10, 471 (2020). https://doi.org/10.1038/s41598-019-57312-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-57312-y
This article is cited by
-
ERO1α promotes the proliferation and inhibits apoptosis of colorectal cancer cells by regulating the PI3K/AKT pathway
Journal of Molecular Histology (2023)
-
Immunometabolic and potential tumor-promoting changes in 3D cervical cell models infected with bacterial vaginosis-associated bacteria
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.