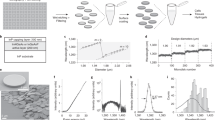
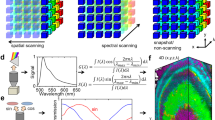
Abstract
Integrating micro- and nanolasers into live cells, tissue cultures and small animals is an emerging and rapidly evolving technique that offers noninvasive interrogation and labeling with unprecedented information density. The bright and distinct spectra of such lasers make this approach particularly attractive for high-throughput applications requiring single-cell specificity, such as multiplexed cell tracking and intracellular biosensing. The implementation of these applications requires high-resolution, high-speed spectral readout and advanced analysis routines, which leads to unique technical challenges. Here, we present a modular approach consisting of two separate procedures. The first procedure instructs users on how to efficiently integrate different types of lasers into living cells, and the second procedure presents a workflow for obtaining intracellular lasing spectra with high spectral resolution and up to 125-kHz readout rate and starts from the construction of a custom hyperspectral confocal microscope. We provide guidance on running hyperspectral imaging routines for various experimental designs and recommend specific workflows for processing the resulting large data sets along with an open-source Python library of functions covering the analysis pipeline. We illustrate three applications including the rapid, large-volume mapping of absolute refractive index by using polystyrene microbead lasers, the intracellular sensing of cardiac contractility with polystyrene microbead lasers and long-term cell tracking by using semiconductor nanodisk lasers. Our sample preparation and imaging procedures require 2 days, and setting up the hyperspectral confocal microscope for microlaser characterization requires <2 weeks to complete for users with limited experience in optical and software engineering.
Key points
-
The protocol describes the integration of microlasers into living cells and a workflow for obtaining lasing spectra with high spectral resolution using a customized hyperspectral confocal microscope.
-
Bio-integrated lasers enable high-throughput and highly multiplexed cell-tracking and biosensing, offering higher penetration depth and information density than alternative approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The research data supporting this publication can be accessed at https://doi.org/10.17630/bec9180a-86e6-4157-822f-fa84249452dd56.
Code availability
A repository containing the supplementary code files can be found at https://github.com/GatherLab/sphyncs55. Custom hardware control software is available upon request.
References
Schubert, M. et al. Lasing within live cells containing intracellular optical microresonators for barcode-type cell tagging and tracking. Nano Lett. 15, 5647–5652 (2015).
Humar, M. & Yun, S. H. Intracellular microlasers. Nat. Photonics 9, 572–576 (2015).
Schubert, M. et al. Lasing in live mitotic and non-phagocytic cells by efficient delivery of microresonators. Sci. Rep. 7, 40877 (2017).
Li, X. et al. In vivo tracking of individual stem cells labeled with nanowire lasers using multimodality imaging. Biomed. Opt. Express 13, 4706–4717 (2022).
Martino, N. et al. Wavelength-encoded laser particles for massively multiplexed cell tagging. Nat. Photonics 13, 720–727 (2019).
Foreman, M. R., Swaim, J. D. & Vollmer, F. Whispering gallery mode sensors. Adv. Opt. Photonics 7, 168–240 (2015).
Su, J. Label-free biological and chemical sensing using whispering gallery mode optical resonators: past, present, and future. Sens. (Basel) 17, 540 (2017).
Schubert, M. et al. Monitoring contractility in cardiac tissue with cellular resolution using biointegrated microlasers. Nat. Photonics 14, 452–458 (2020).
Kavčič, A. et al. Deep tissue localization and sensing using optical microcavity probes. Nat. Commun. 13, 1269 (2022).
Titze, V. M., Caixeiro, S., Di Falco, A., Schubert, M. & Gather, M. C. Red-shifted excitation and two-photon pumping of biointegrated GaInP/AlGaInP quantum well microlasers. ACS Photonics 9, 952–960 (2022).
Gather, M. C. & Yun, S. H. Single-cell biological lasers. Nat. Photonics 5, 406–410 (2011).
Wei, Y. et al. Starch-based biological microlasers. ACS Nano 11, 597–602 (2017).
Humar, M. & Yun, S. H. Whispering-gallery-mode emission from biological luminescent protein microcavity assemblies. Optica 4, 222–228 (2017).
Lee, S. S., Kim, J. B., Kim, Y. H. & Kim, S.-H. Wavelength-tunable and shape-reconfigurable photonic capsule resonators containing cholesteric liquid crystals. Sci. Adv. 4, 8276–8298 (2018).
Hales, J. E., Matmon, G., Dalby, P. A., Ward, J. M. & Aeppli, G. Virus lasers for biological detection. Nat. Commun. 10, 3594 (2019).
Xu, Z. et al. Random lasing from label-free living cells for rapid cytometry of apoptosis. Nano Lett. 22, 172–178 (2022).
Fikouras, A. H. et al. Non-obstructive intracellular nanolasers. Nat. Commun. 9, 4817 (2018).
Lv, Z. et al. Intracellular near-infrared microlaser probes based on organic microsphere-SiO2 core-shell structures for cell tagging and tracking. ACS Appl. Mater. Interfaces 10, 32981–32987 (2018).
Tang, S.-J. et al. Laser particles with omnidirectional emission for cell tracking. Light Sci. Appl. 10, 23 (2021).
Tang, S. K. Y. et al. A multi-color fast-switching microfluidic droplet dye laser. Lab Chip 9, 2767–2771 (2009).
Richter, D., Marinčič, M. & Humar, M. Optical-resonance-assisted generation of super monodisperse microdroplets and microbeads with nanometer precision. Lab Chip 20, 734–740 (2020).
Humar, M., Muševič, I., Sullivan, K. G. & Hall, D. G. 3D microlasers from self-assembled cholesteric liquid-crystal microdroplets. Opt. Express 18, 26995–27003 (2010).
Chen, Y. C. et al. Monitoring neuron activities and interactions with laser emissions. ACS Photonics 7, 2182–2189 (2020).
Wu, X. et al. Nanowire lasers as intracellular probes. Nanoscale 10, 9729–9735 (2018).
Hua, B., Motohisa, J., Kobayashi, Y., Hara, S. & Fukui, T. Single GaAs/GaAsP coaxial core−shell nanowire lasers. Nano Lett. 9, 112–116 (2009).
Feng, C. et al. Organic-nanowire-SiO2 core-shell microlasers with highly polarized and narrow emissions for biological imaging. ACS Appl. Mater. Interfaces 9, 7385–7391 (2017).
Fernandez-Bravo, A. et al. Continuous-wave upconverting nanoparticle microlasers. Nat. Nanotechnol. 13, 572–577 (2018).
Wang, S. et al. High-yield plasmonic nanolasers with superior stability for sensing in aqueous solution. ACS Photonics 4, 1355–1360 (2017).
Caixeiro, S., Gaio, M., Marelli, B., Omenetto, F. G. & Sapienza, R. Silk-based biocompatible random lasing. Adv. Opt. Mater. 4, 998–1003 (2016).
Mysliwiec, J., Cyprych, K., Sznitko, L. & Miniewicz, A. Biomaterials in light amplification. J. Opt. 19, 033003 (2017).
Cho, S., Yang, Y., Soljačić, M. & Yun, S. H. Submicrometer perovskite plasmonic lasers at room temperature. Sci. Adv. 7, 3362–3387 (2021).
Liu, P. Y. et al. Cell refractive index for cell biology and disease diagnosis: past, present and future. Lab Chip 16, 634–644 (2016).
Chan, K. K. et al. Monitoring amyloidogenesis with a 3D deep-learning-guided biolaser imaging array. Nano Lett. 22, 8949–8956 (2022).
Chen, Q. et al. Highly sensitive fluorescent protein FRET detection using optofluidic lasers. Lab Chip 13, 2679–2681 (2013).
Oki, O. et al. FRET-mediated near infrared whispering gallery modes: studies on the relevance of intracavity energy transfer with Q-factors. Mater. Chem. Front. 2, 270–274 (2018).
Yuan, Z., Wang, Z., Guan, P., Wu, X. & Chen, Y. C. Lasing-encoded microsensor driven by interfacial cavity resonance energy transfer. Adv. Opt. Mater. 8, 1–9 (2020).
Wang, Y. et al. Demonstration of intracellular real-time molecular quantification via FRET-enhanced optical microcavity. Nat. Commun. 13, 6685 (2022).
Li, X. et al. Optical coherence tomography and fluorescence microscopy dual-modality imaging for in vivo single-cell tracking with nanowire lasers. Biomed. Opt. Express 11, 3659–3672 (2020).
Champion, J. A. & Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl Acad. Sci. USA 103, 4930–4934 (2006).
Gratton, S. E. A. et al. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. USA 105, 11613–11618 (2008).
Stewart, M. P. et al. In vitro and ex vivo strategies for intracellular delivery. Nature 538, 183–192 (2016).
Stewart, M. P., Langer, R. & Jensen, K. F. Intracellular delivery by membrane disruption: mechanisms, strategies, and concepts. Chem. Rev. 118, 7409–7531 (2018).
Wu, Y. C. et al. Massively parallel delivery of large cargo into mammalian cells with light pulses. Nat. Methods 12, 439–444 (2015).
Cho, S., Humar, M., Martino, N. & Yun, S. H. Laser particle stimulated emission microscopy. Phys. Rev. Lett. 117, 193902 (2016).
Humar, M., Upadhya, A. & Yun, S. H. Spectral reading of optical resonance-encoded cells in microfluidics. Lab Chip 17, 2777–2784 (2017).
Dannenberg, P. H. et al. Laser particle activated cell sorting in microfluidics. Lab Chip 22, 2343–2351 (2022).
Bednarkiewicz, A., Chan, E. M., Kotulska, A., Marciniak, L. & Prorok, K. Photon avalanche in lanthanide doped nanoparticles for biomedical applications: super-resolution imaging. Nanoscale Horiz. 4, 881–889 (2019).
Hill, M. T. & Gather, M. C. Advances in small lasers. Nat. Photonics 8, 908–918 (2014).
Dannenberg, P. H. et al. Droplet microfluidic generation of a million optical microparticle barcodes. Opt. Express 29, 38109–38118 (2021).
Liapis, A. C. et al. Conformal coating of freestanding particles by vapor-phase infiltration. Adv. Mater. Interfaces 7, 2001323 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Ferrand, P. GPScan.VI: a general-purpose LabVIEW program for scanning imaging or any application requiring synchronous analog voltage generation and data acquisition. Comput. Phys. Commun. 192, 342–347 (2015).
Sofroniew, N. et al. napari: a multi-dimensional image viewer for python. Zenodo https://doi.org/10.5281/zenodo.8115575 (2022).
Allan, D. B., Caswell, T., Keim, N. C., van der Wel, C. M. & Verweij, R. W. soft-matter/trackpy: Trackpy v0.6.1. Zenodo https://doi.org/10.5281/zenodo.7670439 (2021).
Titze, V. M., Caixeiro, S., Schubert, M. & Gather, M. C. GatherLab/sphyncs. Zenodo https://doi.org/10.5281/zenodo.8121099 (2023).
Titze, V. M. et al. Hyperspectral confocal imaging for high-throughput readout and analysis of bio-integrated microlasers (dataset). Available at https://doi.org/10.17630/bec9180a-86e6-4157-822f-fa84249452dd (2023).
Caixeiro, S. et al. Micro and nano lasers from III-V semiconductors for intracellular sensing. In Enhanced Spectroscopies and Nanoimaging 2020 Vol. 11468, 1146811 (eds. Verma, P. & Suh, Y. D.) (SPIE, 2020).
Rübsam, M. et al. E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat. Commun. 8, 1250 (2017).
Dannenberg, P. H. et al. Facile layer-by-layer fabrication of semiconductor microdisk laser particles. APL Photonics 8, 021301 (2023).
Yu, D. et al. Whispering-gallery-mode sensors for biological and physical sensing. Nat. Rev. Methods Prim. 1, 83 (2021).
Acknowledgements
We thank Klara Voelckert and Manuel Neubauer for their contributions to lasing data acquisition, and Viktor Klippert and Thomas Michaelis for assistance with the design of custom adapters. This work received financial support from the Leverhulme Trust (RPG-2017-231), the European Union’s Horizon 2020 Framework Programme (FP/2014-2020)/ERC grant agreement no. 640012 (ABLASE), EPSRC (EP/P030017/1), the Humboldt Foundation (Alexander von Humboldt professorship) and the RS Macdonald Charitable Trust (St Andrews Seedcorn Fund for Neurological Research). M.S. acknowledges funding from the European Commission (Marie Skłodowska-Curie Individual Fellowship, 659213) and the Royal Society (Dorothy Hodgkin Fellowship, DH160102; Research Grant, RGF\R1\180070; Enhancement Award, RGF\EA\180051).
Author information
Authors and Affiliations
Contributions
V.M.T., M.S. and M.C.G. designed and planned the microscope. V.M.T. and V.S.D. constructed and automated the microscope. S.C., M.K., N.P. and M.S. prepared laser particles and developed the cell uptake assay. M.K., A.-L.S., M.G. and C.K. carried out cell and laser particle sorting experiments. C.M.N. and M.C.G. conceptualized the cell-tracking experiment, which was conducted by V.M.T., S.C. and M.R. V.M.T. and S.C. performed the high-throughput sensing experiment. V.M.T., S.C. and M.S. contributed to the analysis software. M.S. and M.C.G. supervised the project. V.M.T., M.S. and M.C.G. wrote the manuscript, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Paul Dannenberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Schubert, M. et al. Sci. Rep. 7, 40877 (2017): https://doi.org/10.1038/srep40877
Schubert, M. et al. Nat. Photonics 14, 452–458 (2020): https://doi.org/10.1038/s41566-020-0631-z
Titze, V. M. et al. ACS Photonics 9, 952–960 (2022): https://doi.org/10.1021/acsphotonics.1c01807
Supplementary information
Supplementary Information
Supplementary Notes 1–3, Figs. 1–15 and Tables 1–3
Supplementary Video
Dynamic refractive index sensing of glucose diffusion. x-y maximum intensity projection (MIP) (left) and x-z MIP (center) of the hyperspectral confocal z-stack, color-coded by fitted refractive index, with exemplary raw lasing spectra and calculated external refractive index changes (right). All panels are synchronized.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Titze, V.M., Caixeiro, S., Dinh, V.S. et al. Hyperspectral confocal imaging for high-throughput readout and analysis of bio-integrated microlasers. Nat Protoc 19, 928–959 (2024). https://doi.org/10.1038/s41596-023-00924-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00924-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.