Abstract
Human mitochondrial (mt) protein assemblies are vital for neuronal and brain function, and their alteration contributes to many human disorders, e.g., neurodegenerative diseases resulting from abnormal protein–protein interactions (PPIs). Knowledge of the composition of mt protein complexes is, however, still limited. Affinity purification mass spectrometry (MS) and proximity-dependent biotinylation MS have defined protein partners of some mt proteins, but are too technically challenging and laborious to be practical for analyzing large numbers of samples at the proteome level, e.g., for the study of neuronal or brain-specific mt assemblies, as well as altered mtPPIs on a proteome-wide scale for a disease of interest in brain regions, disease tissues or neurons derived from patients. To address this challenge, we adapted a co-fractionation–MS platform to survey native mt assemblies in adult mouse brain and in human NTERA-2 embryonal carcinoma stem cells or differentiated neuronal-like cells. The workflow consists of orthogonal separations of mt extracts isolated from chemically cross-linked samples to stabilize PPIs, data-dependent acquisition MS to identify co-eluted mt protein profiles from collected fractions and a computational scoring pipeline to predict mtPPIs, followed by network partitioning to define complexes linked to mt functions as well as those essential for neuronal and brain physiological homeostasis. We developed an R/CRAN software package, Macromolecular Assemblies from Co-elution Profiles for automated scoring of co-fractionation–MS data to define complexes from mtPPI networks. Presently, the co-fractionation–MS procedure takes 1.5–3.5 d of proteomic sample preparation, 31 d of MS data acquisition and 8.5 d of data analyses to produce meaningful biological insights.
Key points
-
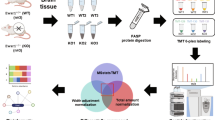
Mitochondrial function is achieved by protein assemblies. Studying the composition and protein–protein interactions (PPIs) of these complexes is essential for identifying and understanding human disorders resulting from abnormal PPIs. This is especially relevant for neuronal cells.
-
Mitochondria are isolated and undergo chemical cross-linking to preserve the PPIs. Mitochondrial extracts are fractionated using orthogonal chromatographic methods. After proteomic mass spectrometry, an R/CRAN software package (Macromolecular Assemblies from Co-elution Profiles) is used to define mitochondrial complexes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The R-package MACP is released as an open-source code under the MIT license that is available on GitHub at https://github.com/BabuLab-UofR/MACP, as well as on CRAN at https://cran.r-project.org/package=MACP. A detailed documentation on the use of MACP package in R that is provided through vignette, along with the demo data, can be downloaded from https://github.com/BabuLab-UofR/MACP/blob/main/vignettes/MACP_tutorial.Rmd. The raw co-fractionation data from this work is available at ProteomeXchange with the identifier PXD039444, in accordance with the data sharing policy. Source data are provided with this paper.
Code availability
The MACP software source code is provided on GitHub at https://github.com/BabuLab-UofR/ MACP. To guide users with MACP functionalities, illustrative datasets and output for testing, as well as documentation and tutorial vignette of MACP describing a detailed protocol on how to perform CF–MS analysis is provided on GitHub and CRAN at https://cran.r-project.org/package=MACP.
References
Cho, N. H. et al. OpenCell: endogenous tagging for the cartography of human cellular organization. Science. 375, eabi6983 (2022).
Hu, L. Z. et al. EPIC: software toolkit for elution profile-based inference of protein complexes. Nat. Methods 16, 737–742 (2019).
Gupta, R., Karczewski, K. J., Howrigan, D., Neale, B. M. & Mootha, V. K. Human genetic analyses of organelles highlight the nucleus in age-related trait heritability. eLife 10, e68610 (2021).
Uher, R. The role of genetic variation in the causation of mental illness: an evolution-informed framework. Mol. Psychiatry 14, 1072–1082 (2009).
Pang, S. Y. et al. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 8, 23 (2019).
Kuzmanov, U. & Emili, A. Protein–protein interaction networks: probing disease mechanisms using model systems. Genome Med. 5, 37 (2013).
Hartwell, L. H., Hopfield, J. J., Leibler, S. & Murray, A. W. From molecular to modular cell biology. Nature 402, C47–C52 (1999).
Walhout, A. J. & Vidal, M. Protein interaction maps for model organisms. Nat. Rev. Mol. Cell Biol. 2, 55–62 (2001).
Malty, R. H. et al. A map of human mitochondrial protein interactions linked to neurodegeneration reveals new mechanisms of redox homeostasis and NF-kappaB signaling. Cell Syst. 5, 1–14 (2017).
Pourhaghighi, R. et al. BraInMap elucidates the macromolecular connectivity landscape of mammalian brain. Cell Syst. 10, 333–350.e14 (2020).
Barabasi, A. L., Gulbahce, N. & Loscalzo, J. Network medicine: a network-based approach to human disease. Nat. Rev. Genet. 12, 56–68 (2011).
Babu, M. et al. Global landscape of cell envelope protein complexes in Escherichia coli. Nat. Biotechnol. 36, 103–112 (2018).
Babu, M. et al. Interaction landscape of membrane-protein complexes in Saccharomyces cerevisiae. Nature 489, 585–589 (2012).
Krogan, N. J. et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 (2006).
Malovannaya, A. et al. Analysis of the human endogenous coregulator complexome. Cell 145, 787–799 (2011).
Huttlin, E. L. et al. Architecture of the human interactome defines protein communities and disease networks. Nature. 545, 505–509 (2017).
Huttlin, E. L. et al. The BioPlex network: a systematic exploration of the human interactome. Cell 162, 425–440 (2015).
Huttlin, E. L. et al. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 184, 3022–3040 (2021).
Guruharsha, K. G. et al. A protein complex network of Drosophila melanogaster. Cell 147, 690–703 (2011).
Cho, K. F. et al. Proximity labeling in mammalian cells with TurboID and split-TurboID. Nat. Protoc. 15, 3971–3999 (2020).
Havugimana, P. C. et al. A census of human soluble protein complexes. Cell 150, 1068–1081 (2012).
Bludau, I. et al. Complex-centric proteome profiling by SEC-SWATH-MS for the parallel detection of hundreds of protein complexes. Nat. Protoc. 15, 2341–2386 (2020).
Liu, X. et al. An AP-MS- and BioID-compatible MAC-tag enables comprehensive mapping of protein interactions and subcellular localizations. Nat. Commun. 9, 1188 (2018).
Hung, V. et al. Proteomic mapping of the human mitochondrial intermembrane space in live cells via ratiometric APEX tagging. Mol. Cell 55, 332–341 (2014).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Go, C. D. et al. A proximity-dependent biotinylation map of a human cell. Nature 595, 120–124 (2021).
Samavarchi-Tehrani, P., Samson, R. & Gingras, A. C. Proximity dependent biotinylation: key enzymes and adaptation to proteomics approaches. Mol. Cell Proteomics 19, 757–773 (2020).
Wan, C. et al. Panorama of ancient metazoan macromolecular complexes. Nature 525, 339–344 (2015).
Skinnider, M. A. et al. An atlas of protein–protein interactions across mouse tissues. Cell 184, 4073–4089.e17 (2021).
Skinnider, M. A. & Foster, L. J. Meta-analysis defines principles for the design and analysis of co-fractionation mass spectrometry experiments. Nat. Methods 18, 806–815 (2021).
Magistretti, P. J. & Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 86, 883–901 (2015).
Picard, M. & McEwen, B. S. Mitochondria impact brain function and cognition. Proc. Natl Acad. Sci. USA 111, 7–8 (2014).
Aly, K. A., Moutaoufik, M. T., Phanse, S., Zhang, Q. & Babu, M. From fuzziness to precision medicine: on the rapidly evolving proteomics with implications in mitochondrial connectivity to rare human disease. iScience 24, 102030 (2021).
Norat, P. et al. Mitochondrial dysfunction in neurological disorders: exploring mitochondrial transplantation. NPJ Regen. Med. 5, 22 (2020).
Zilocchi, M., Broderick, K., Phanse, S., Aly, K. A. & Babu, M. Mitochondria under the spotlight: on the implications of mitochondrial dysfunction and its connectivity to neuropsychiatric disorders. Comput. Struct. Biotechnol. J 18, 2535–2546 (2020).
DiMauro, S. & Schon, E. A. Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 31, 91–123 (2008).
Floyd, B. J. et al. Mitochondrial protein interaction mapping identifies regulators of respiratory chain function. Mol. Cell 63, 621–632 (2016).
Moutaoufik, M. T. et al. Rewiring of the human mitochondrial interactome during neuronal reprogramming reveals regulators of the respirasome and neurogenesis. iScience 19, 1114–1132 (2019).
Antonicka, H. et al. A high-density human mitochondrial proximity interaction network. Cell Metab. 32, 479–497.e9 (2020).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Salas, D., Stacey, R. G., Akinlaja, M. & Foster, L. J. Next-generation interactomics: considerations for the use of co-elution to measure protein interaction networks. Mol. Cell. Proteomics 19, 1–10 (2020).
Scott, N. E. et al. Interactome disassembly during apoptosis occurs independent of caspase cleavage. Mol. Syst. Biol. 13, 906 (2017).
Gonzalez-Franquesa, A. et al. Mass-spectrometry-based proteomics reveals mitochondrial supercomplexome plasticity. Cell Rep. 35, 109180 (2021).
Hevler, J. F. et al. Selective cross-linking of coinciding protein assemblies by in-gel cross-linking mass spectrometry. EMBO J. 40, e106174 (2021).
Heusel, M. et al. Complex-centric proteome profiling by SEC-SWATH-MS. Mol. Syst. Biol. 15, e8438 (2019).
Kristensen, A. R., Gsponer, J. & Foster, L. J. A high-throughput approach for measuring temporal changes in the interactome. Nat. Methods 9, 907–909 (2012).
O’Meara, T. R. et al. Global proteomic analyses define an environmentally contingent Hsp90 interactome and reveal chaperone-dependent regulation of stress granule proteins and the R2TP complex in a fungal pathogen. PLoS Biol. 17, e3000358 (2019).
Stacey, R. G., Skinnider, M. A., Scott, N. E. & Foster, L. J. A rapid and accurate approach for prediction of interactomes from co-elution data (PrInCE). BMC Bioinformatics 18, 457 (2017).
Cogliati, S., Herranz, F., Ruiz-Cabello, J. & Enriquez, J. A. Digitonin concentration is determinant for mitochondrial supercomplexes analysis by BlueNative page. Biochim. Biophys. Acta Bioenerg. 1862, 148332 (2021).
Ford, H. C. et al. Towards a molecular mechanism underlying mitochondrial protein import through the TOM and TIM23 complexes. eLife 11, e75426 (2022).
Lee, Y. C. et al. Impact of detergents on membrane protein complex isolation. J. Proteome Res. 17, 348–358 (2018).
Liao, P. C., Bergamini, C., Fato, R., Pon, L. A. & Pallotti, F. Isolation of mitochondria from cells and tissues. Methods Cell. Biol. 155, 3–31 (2020).
Lanza, I. R. & Nair, K. S. Functional assessment of isolated mitochondria in vitro. Methods Enzymol. 457, 349–372 (2009).
Wettmarshausen, J. & Perocchi, F. Isolation of functional mitochondria from cultured cells and mouse tissues. Methods Mol. Biol. 1567, 15–32 (2017).
Kunji, E. R., Harding, M., Butler, P. J. & Akamine, P. Determination of the molecular mass and dimensions of membrane proteins by size exclusion chromatography. Methods 46, 62–72 (2008).
Havugimana, P. C., Wong, P. & Emili, A. Improved proteomic discovery by sample pre-fractionation using dual-column ion-exchange high performance liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 847, 54–61 (2007).
Li, J. et al. TMTpro-18plex: the expanded and complete set of TMTpro reagents for sample multiplexing. J. Proteome Res. 20, 2964–2972 (2021).
Li, J. et al. TMTpro reagents: a set of isobaric labeling mass tags enables simultaneous proteome-wide measurements across 16 samples. Nat. Methods 17, 399–404 (2020).
McWhite, C. D. et al. A pan-plant protein complex map reveals deep conservation and novel assemblies. Cell 181, 460–474.e14 (2020).
Kislinger, T. et al. PRISM, a generic large scale proteomic investigation strategy for mammals. Mol. Cell. Proteomics 2, 96–106 (2003).
Kwon, T., Choi, H., Vogel, C., Nesvizhskii, A. I. & Marcotte, E. M. Msblender: a probabilistic approach for integrating peptide identifications from multiple database search engines. J. Proteome Res. 10, 2949–2958 (2011).
Choi, H., Glatter, T., Gstaiger, M. & Nesvizhskii, A. I. SAINT-MS1: protein–protein interaction scoring using label-free intensity data in affinity purification–mass spectrometry experiments. J. Proteome Res. 11, 2619–2624 (2012).
Gingras, A. C., Gstaiger, M., Raught, B. & Aebersold, R. Analysis of protein complexes using mass spectrometry. Nat. Rev. Mol. Cell. Biol. 8, 645–654 (2007).
Giurgiu, M. et al. CORUM: the comprehensive resource of mammalian protein complexes-2019. Nucleic Acids Res. 47, D559–D563 (2019).
Zhang, T. & Wong, G. Gene expression data analysis using Hellinger correlation in weighted gene co-expression networks (WGCNA). Comput. Struct. Biotechnol. J 20, 3851–3863 (2022).
Song, L., Langfelder, P. & Horvath, S. Comparison of co-expression measures: mutual information, correlation, and model based indices. BMC Bioinformatics 13, 328 (2012).
Pang, C. N. I. et al. Analytical guidelines for co-fractionation mass spectrometry obtained through global profiling of gold standard saccharomyces cerevisiae protein complexes. Mol. Cell. Proteomics 19, 1876–1895 (2020).
Correia, F. B., Coelho, E. D., Oliveira, J. L. & Arrais, J. P. Handling noise in protein interaction networks. BioMed Res. Int. 2019, 8984248 (2019).
Orre, L. M. et al. SubCellBarCode: Proteome-wide Mapping of Protein Localization and Relocalization. Mol. Cell 73, 166–182.e7 (2019).
Nepusz, T., Yu, H. & Paccanaro, A. Detecting overlapping protein complexes in protein–protein interaction networks. Nat. Methods 9, 471–472 (2012).
Leitner, A. et al. Probing native protein structures by chemical cross-linking, mass spectrometry, and bioinformatics. Mol. Cell. Proteomics 9, 1634–1649 (2010).
McWhite, C. D. et al. Co-fractionation/mass spectrometry to identify protein complexes. STAR Protoc. 2, 100370 (2021).
Hutchinson-Bunch, C. et al. Assessment of TMT labeling efficiency in large-scale quantitative proteomics: the critical effect of sample pH. ACS Omega 6, 12660–12666 (2021).
Skinnider, M. A., Stacey, R. G. & Foster, L. J. Genomic data integration systematically biases interactome mapping. PLoS Comput. Biol. 14, e1006474 (2018).
Li, Z. & Graham, B. H. Measurement of mitochondrial oxygen consumption using a Clark electrode. Methods Mol. Biol. 837, 63–72 (2012).
Li, T. et al. A scored human protein–protein interaction network to catalyze genomic interpretation. Nat. Methods 14, 61–64 (2017).
Pan, J. et al. Interrogation of mammalian protein complex structure, function, and membership using genome-scale fitness screens. Cell Syst. 6, 555–568.e7 (2018).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Le Vasseur, M. et al. Genome-wide CRISPRi screening identifies OCIAD1 as a prohibitin client and regulatory determinant of mitochondrial complex III assembly in human cells. eLife 10, e67624 (2021).
Shetty, D. K., Kalamkar, K. P. & Inamdar, M. S. OCIAD1 controls electron transport chain complex I activity to regulate energy metabolism in human pluripotent stem cells. Stem Cell Rep. 11, 128–141 (2018).
Guarani, V. et al. TIMMDC1/C3orf1 functions as a membrane-embedded mitochondrial complex I assembly factor through association with the MCIA complex. Mol. Cell Biol. 34, 847–861 (2014).
Nazli, A. et al. A mutation in the TMEM65 gene results in mitochondrial myopathy with severe neurological manifestations. Eur. J. Hum. Genet. 25, 744–751 (2017).
Acknowledgements
M.T.M., M.J. and A.G. are supported by the Canadian Institutes of Health Research, Parkinson Society of Canada and/or the Saskatchewan Health Research Foundation postdoctoral fellowships. M.B. is a Chancellors Research Chair in Network Biology. This work was supported by the grants from the Canadian Institutes of Health Research (FDN-154318; PJT-186258), ALS Society of Canada, ARSACS foundation and National Institutes of Health (R01GM106019) to M.B.
Author information
Authors and Affiliations
Contributions
M.B. designed, conceived and supervised the study. M.Z., M.T.M. and K.B. established the iPSC procedure, as well as performed immunoblotting, immunocytochemistry, flow cytometry and mitochondrial assays. M.T.M., M.Z., A.G., M.J. and K.B. developed and optimized mt isolation and HPLC-based separations for the CF–MS procedure. H.A. performed mass spectrometry and S.P performed mass spectra searches. M.R. performed data analyses and developed MACP software, with input from M.B. M.Z., M.T.M., M.R., K.A.A. and M.B. wrote the manuscript with feedback from others. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Moutaoufik, M. T. et al. iScience 19, 1114–1132 (2019): https://doi.org/10.1016/j.isci.2019.08.057
Pourhaghighi, R. et al. Cell Syst. 10, 333–350.e14 (2020): https://doi.org/10.1016/j.cels.2020.03.003
Supplementary information
Supplementary Data 1
High-quality mt protein interactions predicted in mouse brain.
Supplementary Data 2
Mt protein complexes derived from protein interactions in mouse brain.
Supplementary Data 3
Mt subunits of putative protein complexes enriched for GO annotations, human disorders and phenotypic aberrations in human disease.
Source data
Source Data Fig. 1,2,3,4,5,6,7,8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zilocchi, M., Rahmatbakhsh, M., Moutaoufik, M.T. et al. Co-fractionation–mass spectrometry to characterize native mitochondrial protein assemblies in mammalian neurons and brain. Nat Protoc 18, 3918–3973 (2023). https://doi.org/10.1038/s41596-023-00901-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00901-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.