Abstract
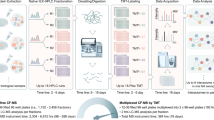
Most catalytic, structural and regulatory functions of the cell are carried out by functional modules, typically complexes containing or consisting of proteins. The composition and abundance of these complexes and the quantitative distribution of specific proteins across different modules are therefore of major significance in basic and translational biology. However, detection and quantification of protein complexes on a proteome-wide scale is technically challenging. We have recently extended the targeted proteomics rationale to the level of native protein complex analysis (complex-centric proteome profiling). The complex-centric workflow described herein consists of size exclusion chromatography (SEC) to fractionate native protein complexes, data-independent acquisition mass spectrometry to precisely quantify the proteins in each SEC fraction based on a set of proteotypic peptides and targeted, complex-centric analysis where prior information from generic protein interaction maps is used to detect and quantify protein complexes with high selectivity and statistical error control via the computational framework CCprofiler (https://github.com/CCprofiler/CCprofiler). Complex-centric proteome profiling captures most proteins in complex-assembled state and reveals their organization into hundreds of complexes and complex variants observable in a given cellular state. The protocol is applicable to cultured cells and can potentially also be adapted to primary tissue and does not require any genetic engineering of the respective sample sources. At present, it requires ~8 d of wet-laboratory work, 15 d of mass spectrometry measurement time and 7 d of computational analysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The MS data for the HEK293 SEC-SWATH-MS experiment16 is available at ProteomeXchange Consortium PXD007038 (http://proteomecentral.proteomexchange.org). The R-package CCprofiler is available on GitHub at https://github.com/CCprofiler/CCprofiler/.
Code availability
A detailed protocol on how to perform peptide-centric SEC-SWATH-MS data analysis is available on GitHub at https://github.com/CCprofiler/SECSWATH_PeptideCentricAnalysis. A detailed protocol on how to perform complex-centric SEC-SWATH-MS data analysis with the CCprofiler package as well as example data of our HEK293 experiment are available on GitHub at https://github.com/CCprofiler/SECSWATH_ComplexCentricAnalysis and in the Supplementary CCprofiler manual.
References
Bludau, I. & Aebersold, R. Proteomic and interactomic insights into the molecular basis of cell functional diversity. Nat. Rev. Mol. Biol. 21, 327–340 (2020).
Huttlin, E. L. et al. The BioPlex network: a systematic exploration of the human interactome. Cell 162, 425–440 (2015).
Huttlin, E. L. et al. Architecture of the human interactome defines protein communities and disease networks. Nature 545, 505 (2017).
Hein, M. Y. et al. A human interactome in three quantitative dimensions organized by stoichiometries and abundances. Cell 163, 712–723 (2015).
Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 196, 801–810 (2012).
Liu, X., Yang, W., Gao, Q. & Regnier, F. Toward chromatographic analysis of interacting protein networks. J. Chromatogr. A 1178, 24–32 (2008).
Dong, M. et al. A “tagless” strategy for identification of stable protein complexes genome-wide by multidimensional orthogonal chromatographic separation and iTRAQ reagent tracking. J. Proteome Res. 7, 1836–1849 (2008).
Kristensen, A. R., Gsponer, J. & Foster, L. J. A high-throughput approach for measuring temporal changes in the interactome. Nat. Methods 9, 907 (2012).
Kristensen, A. R. & Foster, L. J. Protein correlation profiling-SILAC to study protein-protein interactions. in Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC). Methods in Molecular Biology (Methods and Protocols) Vol. 1188 (ed. Warscheid, B.) 263–270 (Humana Press, 2014).
Havugimana, P. C. et al. A census of human soluble protein complexes. Cell 150, 1068–1081 (2012).
Wan, C. et al. Panorama of ancient metazoan macromolecular complexes. Nature 525, 339–344 (2015).
Kirkwood, K. J., Ahmad, Y., Larance, M. & Lamond, A. I. Characterization of native protein complexes and protein isoform variation using size-fractionation-based quantitative proteomics. Mol. Cell. Proteomics 12, 3851–3873 (2013).
Larance, M. et al. Global membrane protein interactome analysis using in vivo crosslinking and mass spectrometry-based protein correlation profiling. Mol. Cell. Proteomics 15, 2476–2490 (2016).
Scott, N. E. et al. Interactome disassembly during apoptosis occurs independent of caspase cleavage. Mol. Syst. Biol. 13, 906 (2017).
Stacey, R. G., Skinnider, M. A., Scott, N. E. & Foster, L. J. A rapid and accurate approach for prediction of interactomes from co-elution data (PrInCE). BMC Bioinformatics 18, 457 (2017).
Heusel, M. et al. Complex-centric proteome profiling by SEC-SWATH-MS. Mol. Syst. Biol. 15, e8438 (2019).
Scott, N. E., Brown, L. M., Kristensen, A. R. & Foster, L. J. Development of a computational framework for the analysis of protein correlation profiling and spatial proteomics experiments. J. Proteomics 118, 112–129 (2015).
Heusel, M. et al. A global screen for assembly state changes of the mitotic proteome by SEC-SWATH-MS. Cell Syst 10, 133–155.e6 (2020).
Pauling, L., Itano, H. A., Singer, S. J. & Wells, I. C. Sickle cell anemia, a molecular disease. Science 110, 543–548 (1949).
Bache, N. et al. A novel LC system embeds analytes in pre-formed gradients for rapid, ultra-robust proteomics. Mol. Cell. Proteomics 17, 2284–2296 (2018).
Wessels, H. J. C. T. et al. LC-MS/MS as an alternative for SDS-PAGE in blue native analysis of protein complexes. Proteomics 9, 4221–4228 (2009).
Ong, S. E. et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1, 376–386 (2002).
Hu, L. Z. et al. EPIC: software toolkit for elution profile-based inference of protein complexes. Nat. Methods 16, 737–742 (2019).
Glatter, T., Wepf, A., Aebersold, R. & Gstaiger, M. An integrated workflow for charting the human interaction proteome: insights into the PP2A system. Mol. Syst. Biol. 5, 237 (2009).
Roncagalli, R. et al. Quantitative proteomics analysis of signalosome dynamics in primary T cells identifies the surface receptor CD6 as a Lat adaptor–independent TCR signaling hub. Nat. Immunol. 15, 384–392 (2014).
Collins, B. C. et al. Quantifying protein interaction dynamics by SWATH mass spectrometry: application to the 14-3-3 system. Nat. Methods 10, 1246–1253 (2013).
Lange, V., Picotti, P., Domon, B. & Aebersold, R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol. Syst. Biol. 4, 222 (2008).
Picotti, P. & Aebersold, R. Selected reaction monitoring–based proteomics: workflows, potential, pitfalls and future directions. Nat. Methods 9, 555–566 (2012).
Schubert, O. T. et al. Building high-quality assay libraries for targeted analysis of SWATH MS data. Nat. Protoc. 10, 426–441 (2015).
Collins, B. C. et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 8, 291 (2017).
Bruderer, R. et al. Optimization of experimental parameters in data-independent mass spectrometry significantly increases depth and reproducibility of results. Mol. Cell. Proteomics 16, 2296–2309 (2017).
Kelstrup, C. D. et al. Performance evaluation of the Q exactive HF-X for shotgun proteomics. J. Proteome Res. 17, 727–738 (2018).
Meier, F. et al. Parallel accumulation—serial fragmentation combined with data-independent acquisition (diaPASEF): bottom-up proteomics with near optimal ion usage. Preprint at https://www.biorxiv.org/content/10.1101/656207v2 (2019).
Rosenberger, G. et al. A repository of assays to quantify 10,000 human proteins by SWATH-MS. Sci. Data 1, 140031 (2014).
Picotti, P. et al. A complete mass-spectrometric map of the yeast proteome applied to quantitative trait analysis. Nature 494, 266–270 (2013).
Blattmann, P. et al. Generation of a zebrafish SWATH-MS spectral library to quantify 10,000 proteins. Sci. Data 6, 190011 (2019).
Heusel, M. Complex-Centric Proteome Profiling by SEC-SWATH Mass Spectrometry. Dissertation, ETH Zurich (2017). https://www.research-collection.ethz.ch/handle/20.500.11850/220300
Gillet, L. C. et al. Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics 11, O111.016717 (2012).
Röst, H. L. et al. OpenSWATH enables automated, targeted analysis of data-independent acquisition MS data. Nat. Biotechnol. 32, 219 (2014).
Reiter, L. et al. mProphet: automated data processing and statistical validation for large-scale SRM experiments. Nat. Methods 8, 430–435 (2011).
Teleman, J. et al. DIANA—algorithmic improvements for analysis of data-independent acquisition MS data. Bioinformatics 31, 555–562 (2015).
Rosenberger, G. et al. Statistical control of peptide and protein error rates in large-scale targeted data-independent acquisition analyses. Nat. Methods 14, 921–927 (2017).
Röst, H. L. et al. TRIC: an automated alignment strategy for reproducible protein quantification in targeted proteomics. Nat. Methods 13, 777 (2016).
Ruepp, A. et al. CORUM: the comprehensive resource of mammalian protein complexes–2009. Nucleic Acids Res. 38, D497–D501 (2009).
Franceschini, A. et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41, D808–D815 (2012).
Szklarczyk, D. et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015).
Rolland, T. et al. A proteome-scale map of the human interactome network. Cell 159, 1212–1226 (2014).
Choi, S. G., Richardson, A., Lambourne, L., Hill, D. E. & Vidal, M. Protein interactomics by two-hybrid methods. Methods Mol. Biol. 1794, 1–14 (2018).
Gavin, A.-C. et al. Proteome survey reveals modularity of the yeast cell machinery. Nature 440, 631–636 (2006).
Krogan, N. J. et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 (2006).
Guruharsha, K. G. et al. A protein complex network of Drosophila melanogaster. Cell 147, 690–703 (2011).
Storey, J. D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl Acad. Sci. USA 100, 9440–9445 (2003).
Burger, T. Gentle introduction to the statistical foundations of false discovery rate in quantitative proteomics. J. Proteome Res. 17, 12–22 (2018).
Breheny, P., Stromberg, A. & Lambert, J. p-value histograms: inference and diagnostics. High Throughput 7, E23 (2018).
Adusumilli, R. & Mallick, P. Data conversion with ProteoWizard msConvert. in Proteomics. Methods in Molecular Biology Vol. 1550 (eds. Comai, L., Katz, J. & Mallick, P.) 339–368 (Humana Press, 2017).
Giurgiu, M. et al. CORUM: the comprehensive resource of mammalian protein complexes-2019. Nucleic Acids Res. 47, D559–D563 (2019).
Hirano, Y. et al. A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes. Nature 437, 1381–1385 (2005).
Hirano, Y. et al. Dissecting β-ring assembly pathway of the mammalian 20S proteasome. EMBO J. 27, 2204–2213 (2008).
Acknowledgements
The project was supported by the SystemsX.ch projects PhosphoNetX PPM and project TbX (to R.A.) and the European Research Council (ERC-20140AdG 670821 to R.A.). M.H. was supported by a grant from Institut Mérieux. I.B. was supported by the Swiss National Science Foundation (grant no. 31003A_166435). B.C.C. was supported by a Swiss National Science Foundation Ambizione grant (PZ00P3_161435). A.B.-E. was supported by the National Institutes of Health project Omics4TB Disease Progression (U19 AI106761).
Author information
Authors and Affiliations
Contributions
I.B., M.H. and R.A. wrote the manuscript with input from all authors. I.B. and M.H. developed the presented workflow, implemented the analysis scripts and performed all analyses. M.H. developed and optimized the experimental protocol for SEC-SWATH-MS. G.R. and M.H. optimized the peptide-centric analysis for SEC-SWATH-MS applications. I.B., M.H., M.F., G.R., R.H. and A.B.-E. developed the CCprofiler software. A.V.D. performed validation experiments. R.A., M.G., B.C.C. and M.H. conceptualized the primary study. B.C.C., M.G. and R.A. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Bruno Manadas, Leonard Foster and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Heusel, M. et al. Mol. Syst. Biol. 15, e8438 (2019): https://doi.org/10.15252/msb.20188438
Heusel, M. et al. Cell Syst. 10, 133–155.e6 (2020): https://doi.org/10.1016/j.cels.2020.01.001
Key data used in this protocol
Heusel, M. et al. Mol. Syst. Biol. 15, e8438 (2019): https://doi.org/10.15252/msb.20188438
Extended data
Extended Data Fig. 1 Overlap of proteins and protein complexes across the SEC fractionation dimension.
a, Heat-map representation of the percentage of detected proteins that are shared between each pair of SEC fractions (top). The percentage overlap is calculated as the number of shared proteins relative to the total set of proteins detected in any pair of SEC fractions, as a percentage. The bottom panel illustrates the average percentage of overlapping proteins at different distance thresholds between SEC fractions. b, Heat-map representation of the percentage of detected protein complexes that are shared between each pair of SEC fractions (top). The percentage overlap is calculated as the number of shared protein complexes relative to the total set of protein complexes detected in any pair of SEC fractions, as a percentage. The bottom panel illustrates the average percentage of overlapping protein complexes at different distance thresholds between SEC fractions.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary CCprofiler Manual.
Rights and permissions
About this article
Cite this article
Bludau, I., Heusel, M., Frank, M. et al. Complex-centric proteome profiling by SEC-SWATH-MS for the parallel detection of hundreds of protein complexes. Nat Protoc 15, 2341–2386 (2020). https://doi.org/10.1038/s41596-020-0332-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-0332-6
This article is cited by
-
Co-fractionation–mass spectrometry to characterize native mitochondrial protein assemblies in mammalian neurons and brain
Nature Protocols (2023)
-
A computational framework for the inference of protein complex remodeling from whole-proteome measurements
Nature Methods (2023)
-
Improved in situ characterization of protein complex dynamics at scale with thermal proximity co-aggregation
Nature Communications (2023)
-
Meta-analysis defines principles for the design and analysis of co-fractionation mass spectrometry experiments
Nature Methods (2021)
-
PCprophet: a framework for protein complex prediction and differential analysis using proteomic data
Nature Methods (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.