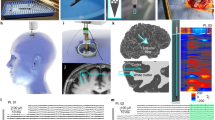
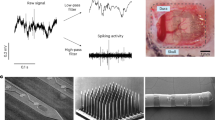
Abstract
Neuropixels are silicon-based electrophysiology-recording probes with high channel count and recording-site density. These probes offer a turnkey platform for measuring neural activity with single-cell resolution and at a scale that is beyond the capabilities of current clinically approved devices. Our team demonstrated the first-in-human use of these probes during resection surgery for epilepsy or tumors and deep brain stimulation electrode placement in patients with Parkinson’s disease. Here, we provide a better understanding of the capabilities and challenges of using Neuropixels as a research tool to study human neurophysiology, with the hope that this information may inform future efforts toward regulatory approval of Neuropixels probes as research devices. In perioperative procedures, the major concerns are the initial sterility of the device, maintaining a sterile field during surgery, having multiple referencing and grounding schemes available to de-noise recordings (if necessary), protecting the silicon probe from accidental contact before insertion and obtaining high-quality action potential and local field potential recordings. The research team ensures that the device is fully operational while coordinating with the surgical team to remove sources of electrical noise that could otherwise substantially affect the signals recorded by the sensitive hardware. Prior preparation using the equipment and training in human clinical research and working in operating rooms maximize effective communication within and between the teams, ensuring high recording quality and minimizing the time added to the surgery. The perioperative procedure requires ~4 h, and the entire protocol requires multiple weeks.
Key points
-
This Protocol describes the structural reinforcement of Neuropixels 1.0-S, ensuring their correct insertion into the human cortex; the extensive perioperative procedures required to maintain sterile conditions; and coordination with the surgical team.
-
Neuropixels 1.0-S enables the recording of spiking activity from up to hundreds of cortical neurons, a feat currently not possible with single-neuron resolution devices such as microwire bundles, laminar microelectrode arrays, microelectrode contacts and the Utah array.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The main data discussed in this protocol are available for download at Dryad (https://doi.org/10.5061/dryad.d2547d840). Further datasets that were used in the study were neural reconstructions of human neurons from NeuroMorpho.Org 61–65 relative to the Neuropixels array and 1.5-mm Utah array. Neural reconstructions were from Neuromorpho.Org ID: NMO_86955, NMO_86997, NMO_109433, NMO_61420 and NMO_61421.
Code availability
Custom code described in this paper has been made available at https://github.com/Center-For-Neurotechnology/HumanNeuropixelsPipeline (currently without a license), which includes links to other useful repositories not maintained by the authors of this paper, with the exceptions of https://github.com/evarol/dredge (available under the Creative Commons Zero v1.0 Universal license) and https://github.com/williamunoz/InterpolationAfterDREDge (available under the MIT license).
References
Jun, J. J. et al. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236 (2017).
Steinmetz, N. A. et al. Neuropixels 2.0: a miniaturized high-density probe for stable, long-term brain recordings. Science 372, eabf4588 (2021).
Dutta, B. et al. The Neuropixels probe: a CMOS based integrated microsystems platform for neuroscience and brain-computer interfaces. In 2019 IEEE International Electron Devices Meeting (IEDM) 10.1.1–10.1.4 https://doi.org/10.1109/IEDM19573.2019.8993611 (2019).
Steinmetz, N. A., Zatka-Haas, P., Carandini, M. & Harris, K. D. Distributed coding of choice, action and engagement across the mouse brain. Nature 576, 266–273 (2019).
Siegle, J. H. et al. Survey of spiking in the mouse visual system reveals functional hierarchy. Nature 592, 86–92 (2021).
Jia, X. et al. High-density extracellular probes reveal dendritic backpropagation and facilitate neuron classification. J. Neurophysiol. 121, 1831–1847 (2019).
Trautmann, E. M. et al. Large-scale brain-wide neural recording in nonhuman primates. Preprint at bioRxiv https://doi.org/10.1101/2023.02.01.526664 (2023).
Durand, S. et al. Acute head-fixed recordings in awake mice with multiple Neuropixels probes. Nat. Protoc. 18, 424–457 (2023).
Trepka, E. B., Zhu, S., Xia, R., Chen, X. & Moore, T. Functional interactions among neurons within single columns of macaque V1. eLife 11, e79322 (2022).
Pachitariu, M., Steinmetz, N., Kadir, S., Carandini, M. & Harris, K. Fast and accurate spike sorting of high-channel count probes with KiloSort. Adv. Neural Inf. Process. Syst. 2016, 1–9 (2016).
Siegle, J. H. et al. Open Ephys: an open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 14, 045003 (2017).
Pachitariu, M., Sridhar, S. & Stringer, C. Solving the spike sorting problem with Kilosort. Preprint at bioRxiv https://doi.org/10.1101/2023.01.07.523036 (2023).
Paulk, A. C. et al. Large-scale neural recordings with single neuron resolution using Neuropixels probes in human cortex. Nat. Neurosci. 25, 252–263 (2022).
Chung, J. E. et al. High-density single-unit human cortical recordings using the Neuropixels probe. Neuron 110, 2409–2421.e3 (2022).
Chari, A., Thornton, R. C., Tisdall, M. M. & Scott, R. C. Microelectrode recordings in human epilepsy: a case for clinical translation. Brain Commun. 2, fcaa082 (2020).
Cash, S. S. & Hochberg, L. R. The emergence of single neurons in clinical neurology. Neuron 86, 79–91 (2015).
Amirnovin, R., Williams, Z. M., Cosgrove, G. R. & Eskandar, E. N. Experience with microelectrode guided subthalamic nucleus deep brain stimulation. Neurosurgery 58, ONS96–ONS102 (2006).
Jamali, M. et al. Dorsolateral prefrontal neurons mediate subjective decisions and their variation in humans. Nat. Neurosci. 22, 1010–1020 (2019).
Sheth, S. A. et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488, 218–221 (2012).
Feinsinger, A. et al. Ethical commitments, principles, and practices guiding intracranial neuroscientific research in humans. Neuron 110, 188–194 (2022).
Greely, H. T. et al. Neuroethics Guiding Principles for the NIH BRAIN Initiative. J. Neurosci. 38, 10586–10588 (2018).
Young, M. J. & Bernat, J. L. Emerging subspecialties in neurology: neuroethics: an emerging career path in neurology. Neurology 98, 505–508 (2022).
Goering, S. et al. Recommendations for responsible development and application of neurotechnologies. Neuroethics 14, 365–386 (2021).
Windolf, C. et al. Robust online multiband drift estimation in electrophysiology data. Preprint at bioRxiv https://doi.org/10.1101/2022.12.04.519043 (2022).
Windolf, C. et al. Robust online multiband drift estimation in electrophysiology data. In ICASSP 2023 IEEE International Conference on Acoustics, Speech and Signal Processing https://doi.org/10.1109/ICASSP49357.2023.10095487 (2023).
Allen, W. E. et al. Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 364, 0–10 (2019).
Trautmann, E. M. et al. Accurate estimation of neural population dynamics without spike sorting. Neuron 103, 292–308.e4 (2019).
Juavinett, A. L., Bekheet, G. & Churchland, A. K. Chronically implanted Neuropixels probes enable high-yield recordings in freely moving mice. eLife 8, e47188 (2019).
Aguillon-Rodriguez, V. et al. Standardized and reproducible measurement of decision-making in mice. eLife 10, 1–28 (2021).
Barack, D. & Krakauer, J. W. Two views on the cognitive brain. Nat. Rev. Neurosci. 22, 359–371 (2021).
Yuste, R. From the neuron doctrine to neural networks. Nat. Rev. Neurosci. 16, 487–497 (2015).
Hesse, J. K. & Tsao, D. Y. A new no-report paradigm reveals that face cells encode both consciously perceived and suppressed stimuli. eLife 9, e58360 (2020).
van Daal, R. J. J. et al. Implantation of Neuropixels probes for chronic recording of neuronal activity in freely behaving mice and rats. Nat. Protoc. 16, 3322–3347 (2021).
Siegle, J. H. et al. Reconciling functional differences in populations of neurons recorded with two-photon imaging and electrophysiology. eLife 10, 1–35 (2021).
Zatka-Haas, P., Steinmetz, N. A., Carandini, M. & Harris, K. D. Sensory coding and the causal impact of mouse cortex in a visual decision. eLife 10, 1–25 (2021).
Truccolo, W. et al. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 14, 635–641 (2011).
Truccolo, W. et al. Neuronal ensemble synchrony during human focal seizures. J. Neurosci. 34, 9927–9944 (2014).
Jamali, M. et al. Single-neuronal predictions of others’ beliefs in humans. Nature 591, 610–614 (2021).
Mian, M. K. et al. Encoding of rules by neurons in the human dorsolateral prefrontal cortex. Cereb. Cortex 24, 807–816 (2014).
Patel, S. R. et al. Studying task-related activity of individual neurons in the human brain. Nat. Protoc. 8, 949–957 (2013).
Aabedi, A. A. et al. Functional alterations in cortical processing of speech in glioma-infiltrated cortex. Proc. Natl Acad. Sci. USA. 118, e2108959118 (2021).
Ulbert, I., Halgren, E., Heit, G. & Karmos, G. Multiple microelectrode-recording system for human intracortical applications. J. Neurosci. Methods 106, 69–79 (2001).
Maynard, E. M., Nordhausen, C. T. & Normann, R. A. The Utah Intracortical Electrode Array: a recording structure for potential brain-computer interfaces. Electroencephalogr. Clin. Neurophysiol. 102, 228–239 (1997).
Nordhausen, C. T., Rousche, P. J. & Normann, R. A. Optimizing recording capabilities of the Utah Intracortical Electrode Array. Brain Res. 637, 27–36 (1994).
Nordhausen, C. T., Maynard, E. M. & Normann, R. A. Single unit recording capabilities of a 100 microelectrode array. Brain Res. 726, 129–140 (1996).
Hochberg, L. R. et al. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature 485, 372–375 (2012).
Hochberg, L. R. et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006).
Willett, F. R. et al. Hand knob area of premotor cortex represents the whole body in a compositional way. Cell 181, 1–14 (2020).
Slutzky, M. W. Brain–machine interfaces: powerful tools for clinical treatment and neuroscientific investigations. Neuroscientist 25, 139–154 (2019).
Lebedev, M. A. & Nicolelis, M. A. L. Brain-machine interfaces: past, present and future. Trends Neurosci. 29, 536–546 (2006).
Saha, S. et al. Progress in brain computer interface: challenges and opportunities. Front. Syst. Neurosci. 15, 1–20 (2021).
Tchoe, Y. et al. Human brain mapping with multi-thousand channel PtNRGrids resolves novel spatiotemporal dynamics. Sci. Transl. Med. 14, eabj1441 (2022).
Paulk, A. C. et al. Microscale physiological events on the human cortical surface. Cereb. Cortex 31, 3678–3700 (2021).
Yang, J. C. et al. Microscale dynamics of electrophysiological markers of epilepsy. Clin. Neurophysiol. 32, 2916–2931 (2021).
Khodagholy, D. et al. NeuroGrid: recording action potentials from the surface of the brain. Nat. Neurosci. 18, 310–315 (2015).
Viventi, J. et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 14, 1599–1605 (2011).
Sun, J. et al. Intraoperative microseizure detection using a high-density micro-electrocorticography electrode array. Brain Commun. 4, fcac122 (2022).
Khodagholy, D. et al. Organic electronics for high-resolution electrocorticography of the human brain. Sci. Adv. 2, e1601027 (2016).
Hassan, A. R. et al. Translational organic neural interface devices at single neuron resolution. Adv. Sci. (Weinh.) 9, e2202306 (2022).
Ward, A. A. & Thomas, L. B. The electrical activity of single units in the cerebral cortex of man. Electroencephalogr. Clin. Neurophysiol. 7, 135–136 (1955).
Verzeano, M., Crandall, P. H. & Dymond, A. Neuronal activity of the amygdala in patients with psychomotor epilepsy. Neuropsychologia 9, 331–344 (1971).
Fried, I. et al. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients: technical note. J. Neurosurg. 91, 697–705 (1999).
Fried, I., Cameron, K. A., Yashar, S., Fong, R. & Morrow, J. W. Inhibitory and excitatory responses of single neurons in the human medial temporal lobe during recognition of faces and objects. Cereb. Cortex 12, 575–584 (2002).
Worrell, G. A. et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain 131, 928–937 (2008).
Kleen, J. K. et al. Bidirectional propagation of low frequency oscillations over the human hippocampal surface. Nat. Commun. 12, 2764 (2021).
Musk, E. An integrated brain-machine interface platform with thousands of channels. Preprint at bioRxiv https://doi.org/10.1101/703801 (2019).
Sahasrabuddhe, K. et al. The Argo: a high channel count recording system for neural recording in vivo. J. Neural Eng. 18, 015002 (2021).
Fiáth, R. et al. Slow insertion of silicon probes improves the quality of acute neuronal recordings. Sci. Rep. 9, 1–17 (2019).
Wang, Q., Yin, J. & Cui, H. Reinforcement of Neuropixels probes for high-density neural recording in non-human primates. In 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER) 128–131 (IEEE, 2021).
Wilkinson, M. D. et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
Mercier, M. R. et al. Advances in human intracranial electroencephalography research, guidelines and good practices. Neuroimage 260, 119438 (2022).
Duncan, D. et al. Data Archive for the BRAIN Initiative (DABI). Sci. Data 10, 83 (2023).
Oostenveld, R., Fries, P., Maris, E. & Schoffelen, J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 156869 (2011).
Bischoff-Grethe, A. et al. A technique for the deidentification of structural brain MR images. Hum. Brain Mapp. 28, 892–903 (2007).
Buimer, E. E. L. et al. De-identification procedures for magnetic resonance images and the impact on structural brain measures at different ages. Hum. Brain Mapp. 42, 3643–3655 (2021).
Holdgraf, C. et al. iEEG-BIDS, extending the Brain Imaging Data Structure specification to human intracranial electrophysiology. Sci. Data 6, 102 (2019).
Investigational Device Exemptions. 21 CFR § 812. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=812 (2023).
U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health (CDRH). Information Sheet Guidance for IRBs, Clinical Investigators, and Sponsors. Significant Risk and Nonsignificant Risk Medical Device Studies https://www.fda.gov/regulatory-information/search-fda-guidance-documents/significant-risk-and-nonsignificant-risk-medical-device-studies (2006).
Kalb, S. IDE Basics. Investigational Device Exemption (IDE) Program Office of Device Evaluation, Center for Devices and Radiological Health U.S. Food and Drug Administration. https://www.fda.gov/media/127955/download (2014).
Kleiner, M., Brainard, D. H. & Pelli, D. What’s new in Psychtoolbox-3? Perception 36, 1–16 (ECVP Abstract Supplement) (2007).
Buccino, A. P. et al. SpikeInterface, a unified framework for spike sorting. eLife 9, e61834 (2020).
Williams, Z. M., Bush, G., Rauch, S. L., Cosgrove, G. R. & Eskandar, E. N. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat. Neurosci. 7, 1370–1375 (2004).
Felsenstein, O. & Peled, N. MMVT—multi-modality visualization tool. GitHub https://doi.org/10.5281/zenodo.438343 (2017).
Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181–1196 (2010).
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 (2012).
Holmes, C. J. et al. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 22, 324–333 (1998).
Community, B. O. Blender—a 3D Modelling and Rendering Package (Blender Foundation, 2018).
Chan, A. M. et al. Speech-specific tuning of neurons in human superior temporal gyrus. Cereb. Cortex 24, 2679–2693 (2014).
Schevon, C. A., Goodman, R. R., McKhann, G. & Emerson, R. G. Propagation of epileptiform activity on a submillimeter scale. J. Clin. Neurophysiol. 27, 406–411 (2010).
Koch, C. & Jones, A. Big science, team science, and open science for neuroscience. Neuron 92, 612–616 (2016).
Ascoli, G. A. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat. Rev. Neurosci. 7, 318–324 (2006).
Ascoli, G. A., Donohue, D. E. & Halavi, M. NeuroMorpho.Org: a central resource for neuronal morphologies. J. Neurosci. 27, 9247–9251 (2007).
Tóth, K. et al. Hyperexcitability of the network contributes to synchronization processes in the human epileptic neocortex. J. Physiol. 596, 317–342 (2018).
Lancaster, J. L. et al. Automated analysis of fundamental features of brain structures. Neuroinformatics 9, 371–380 (2011).
Lancaster, J. L. et al. Anatomical global spatial normalization. Neuroinformatics 8, 171–182 (2010).
Lancaster, J. L. et al. Automated regional behavioral analysis for human brain images. Front. Neuroinform. 6, 1–12 (2012).
Acknowledgements
We thank Y. Chou, A. Tripp, F. Minidio, A. Zhang and A. O’Donnell for help in data collection, and we thank S. Shah and S. Sheth for manuscript input. We especially thank the patients for their willingness to participate in this research. This research was supported by the ECOR and K24-NS088568 (to S.S.C.), the Tiny Blue Dot Foundation (to S.S.C. and A.C.P.) and NIH grant U01NS121616 (to Z.M.W.). This research was also supported by NIH NINDS BRAIN R01NS11662301 (to K.V.S.), NIH NIDCD R01DC01403406 (to K.V.S.), the Simons Foundation (543045 to K.V.S. and 872146SPI to S.D.S.) the Howard Hughes Medical Institute at Stanford University (to K.V.S. and E.M.T.) and NIH/NINDS Neuroscience Resident Research Program R25NS065743 (to W.M.). S.D.S. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. E.M.T. is additionally funded by the Brain and Behavior Research Foundation and the Grossman Institute. E.V. is funded by NIH NIMH 1K99MH128772-01A1. The views and conclusions contained in this document are those of the authors and do not represent the official policies, either expressed or implied, of the funding sources. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The experiment was conceived by S.S.C., Y.K., A.C.P., Z.M.W., K.V.S., L.R.H., E.M.T., W.M., I.C., M.J., D.M., B.C. and S.D.S. Z.M.W. and R.M.R. performed the surgeries and placed the arrays, while A.C.P., D.J.S., B.C., M.L.M., A.K., W.M., I.C., M.J., D.M., C.W., E.V. and Y.K. collected the data or did a first-pass analysis. A.C.P., B.C. and S.S.C. prepared the first manuscript draft. A.C.P., W.M., R.H., C.W. and Y.K. analyzed all the data for the final results, and A.C.P. and B.C. prepared all the figures. E.V. and C.W. developed software for analysis. All authors edited and revised the manuscript. B.D., M.W. and E.M.T. conceived of and advanced the production of the thicker custom Neuropixels probes with sharpened tips used in the study. The imec employees (B.D. and M.W.) are authors because of their technological development of the probe hardware and software and were not involved in these human pilot studies.
Corresponding authors
Ethics declarations
Competing interests
K.V.S. is a consultant to Neuralink Corp. and CTRL-Labs Inc. (now a part of the Meta Reality Labs division of Meta) and is on the Scientific Advisory Boards of Mind-X Inc., Inscopix Inc. and Heal Inc. The MGH Translational Research Center has clinical research support agreements with Neuralink, Paradromics and Synchron, for which S.S.C. and L.R.H. provide consultative input. None of these entities listed are involved with this research or the Neuropixels device. B.D. and M.W. are employees of imec, a non-profit semiconductor research and development organization that manufactures, sells and distributes the Neuropixels probes, at cost, to the research community. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Anton Arkhipov, Anne Churchland, Daniel Denman, Christoph Koch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Paulk, A. C. et al. Nat. Neurosci. 25, 252–263 (2022): https://doi.org/10.1038/s41593-021-00997-0
Windolf, C. et al. Presented at ICASSP 2023–2023 IEEE International Conference on Acoustics, Speech and Signal Processing (2023): https://doi.org/10.1109/ICASSP49357.2023.10095487
Windolf, C. et al. Preprint (2022): https://doi.org/10.1101/2022.12.04.519043
Supplementary information
Supplementary Information
Supplementary Discussion, Table 1 and Figs. 1–5
Supplementary Video 1
Ongoing human brain neural activity recorded via Neuropixels and SpikeGLX and OpenEphys recording software, as recorded in the OR.
Supplementary Video 2
Demonstration of the additional electrical noise from the wall-powered anesthesia pump through a saline tube while recording in saline and gelatine.
Supplementary Video 3
Setting up the Neuropixels probe in the sterile field to be connected to the recording system.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coughlin, B., Muñoz, W., Kfir, Y. et al. Modified Neuropixels probes for recording human neurophysiology in the operating room. Nat Protoc 18, 2927–2953 (2023). https://doi.org/10.1038/s41596-023-00871-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00871-2
This article is cited by
-
Single-neuronal elements of speech production in humans
Nature (2024)
-
Advocating for neurodata privacy and neurotechnology regulation
Nature Protocols (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.