Abstract
Atropisomers are molecules whose stereogenicity arises from restricted rotation about a single bond. They are of current importance because of their applications in catalysis, medicine and materials science. The defining feature of atropisomeric molecules is that their stereoisomers are related to one another by bond rotation: as a result, evaluating their configurational stability (i.e., the rate at which their stereoisomers interconvert) is central to any work in this area. Important atropisomeric scaffolds include C–C linked biaryls, such as the ligand BINAP and the drug vancomycin, and C–N linked amine derivatives such as the drug telenzepine. This article focuses on the three most widely used experimental methods that are available to measure the rate of racemization in atropisomers, namely: (i) kinetic analysis of the racemization of an enantioenriched sample, (ii) dynamic HPLC and (iii) variable-temperature NMR. For each technique, an explanation of the theory is set out, followed by a detailed experimental procedure. A discussion is also included of which technique to try when confronted with a new molecular structure whose properties are not yet known. None of the three procedures require complex experimental techniques, and all can be performed by using standard analytical equipment (NMR and HPLC). The time taken to determine a racemization rate depends on which experimental method is required, but for a new compound it is generally possible to measure a racemization rate in <1 d.
Key points
-
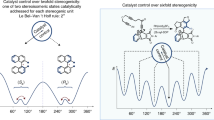
Stereoisomers of atropisomeric molecules interconvert by rotation of a single bond. If the interconversion rate is slow enough, the atropisomers can be separated by HPLC. After enrichment of one isomer, the kinetics of racemization can be determined.
-
At increasing rates of interconversion, analytical HPLC shows two peaks, a ‘Batman’ profile (dynamic HPLC) or a single peak.
-
For molecules with faster interconversion, variable-temperature NMR can be performed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


















Similar content being viewed by others
References
Oki, M. Topics in Stereochemistry. Vol. 1 Atropisomerism (Wiley Interscience, 1983).
Noyori, R. Asymmetric catalysis: science and opportunities (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 41, 2008–2022 (2002).
Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 44, 3418–3430 (2015).
Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev. 121, 4805–4902 (2021).
Sweet, J. S. & Knipe, P. C. Catalytic enantioselective synthesis of C–N atropisomeric heterobiaryls. Synthesis 54, 2119–2132 (2022).
Kumarasamy, E., Raghunathan, R., Sibi, M. P. & Sivaguru, J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 115, 11239–11300 (2015).
Mei, G.-J., Koay, W. L., Guan, C.-Y. & Lu, Y. Atropisomers beyond the C–C axial chirality: advances in catalytic asymmetric synthesis. Chem 8, 1855–1893 (2022).
Rodríguez-Salamanca, P., Fernández, R., Hornillos, V. & Lassaletta, J. M. Asymmetric synthesis of axially chiral C−N atropisomers. Chemistry 28, e202104442 (2022).
Wu, Y.-J., Liao, G. & Shi, B.-F. Stereoselective construction of atropisomers featuring a C–N chiral axis. Green. Synth. Catal. 3, 117–136 (2022).
Clayden, J. Atropisomers and near-atropisomers: achieving stereoselectivity by exploiting the conformational preferences of aromatic amides. Chem. Commun. (Camb.) 2004, 127–135 (2004).
Costil, R., Sterling, A. J., Duarte, F. & Clayden, J. Atropisomerism in diarylamines: structural requirements and mechanisms of conformational interconversion. Angew. Chem. Int. Ed. Engl. 59, 18670–18678 (2020).
Vaidya, S. D., Toenjes, S. T., Yamamoto, N., Maddox, S. M. & Gustafson, J. L. Catalytic atroposelective synthesis of N-aryl quinoid compounds. J. Am. Chem. Soc. 142, 2198–2203 (2020).
Toenjes, S. T. & Gustafson, J. L. Atropisomerism in medicinal chemistry: challenges and opportunities. Future Med. Chem. 10, 409–422 (2018).
Rickhaus, M., Jundt, L. & Mayor, M. Determining inversion barriers in atropisomers—a tutorial for organic chemists. Chimia 70, 192–192 (2016).
Reist, M., Testa, B., Carrupt, P.-A., Jung, M. & Schurig, V. Racemization, enantiomerization, diastereomerization, and epimerization: their meaning and pharmacological significance. Chirality 7, 396–400 (1995).
LaPlante, S. R., Edwards, P. J., Fader, L. D., Jakalian, A. & Hucke, O. Revealing atropisomer axial chirality in drug discovery. ChemMedChem 6, 505–513 (2011).
Bragg, R. A., Clayden, J., Morris, G. A. & Pink, J. H. Stereodynamics of bond rotation in tertiary aromatic amides. Chemistry 8, 1279–1289 (2002).
Jolliffe, J. D., Armstrong, R. J. & Smith, M. D. Catalytic enantioselective synthesis of atropisomeric biaryls by a cation-directed O-alkylation. Nat. Chem. 9, 558–562 (2017).
Trapp, O., Schoetz, G. & Schurig, V. Determination of enantiomerization barriers by dynamic and stopped-flow chromatographic methods. Chirality 13, 403–414 (2001).
Trapp, O. Unified equation for access to rate constants of first-order reactions in dynamic and on-column reaction chromatography. Anal. Chem. 78, 189–198 (2006).
Trapp, O. Fast and precise access to enantiomerization rate constants in dynamic chromatography. Chirality 18, 489–497 (2006).
Trapp, O. The unified equation for the evaluation of degenerated first-order reactions in dynamic electrophoresis. Electrophoresis 27, 2999–3006 (2006).
Trapp, O. Interconversion of stereochemically labile enantiomers (enantiomerization). Top. Curr. Chem. 341, 231–270 (2013).
DCXplorer MCXVII download link: https://www.cup.lmu.de/oc/trapp/tools.html (2023).
Lanman, B. A., Parsons, A. T. & Zech, S. G. Addressing atropisomerism in the development of sotorasib, a covalent inhibitor of KRAS G12C: structural, analytical, and synthetic considerations. Acc. Chem. Res. 55, 2892–2903 (2022).
Hirsch, D. R. et al. Troponoid atropisomerism: studies on the configurational stability of tropone-amide chiral axes. Org. Lett. 21, 2412–2415 (2019).
Clark, A. J. et al. Axially chiral enamides: substituent effects, rotation barriers, and implications for their cyclization reactions. J. Org. Chem. 81, 5547–5565 (2016).
Claridge, T. D. W. Introducing high-resolution NMR. In High-Resolution NMR Techniques in Organic Chemistry Edn. 3 (Elsevier, 2016).
Kost, D., Carlson, E. H. & Raban, M. The validity of approximate equations for kc in dynamic nuclear resonance. J. Chem. Soc. D. 1971, 656–657 (1971).
Download link: https://home.cc.umanitoba.ca/~wolowiec/spinworks/index.html
TopSpin download link: https://www.bruker.com/en/products-and-solutions/mr/nmr-software/topspin.html (2023).
Loening, N. M. & Keeler, J. Temperature accuracy and temperature gradients in solution-state NMR spectrometers. J. Magn. Reson. 159, 55–61 (2002).
Van Geet, A. L. Calibration of methanol nuclear magnetic resonance thermometer at low temperature. Anal. Chem. 42, 679–680 (1970).
Bruker User Manual. (Section 1.3: Temperature Calibration). Available at https://www.bruker.com/protected/en/services/user-manuals/nmr/technical-manuals.html (2023).
Acknowledgements
We gratefully acknowledge the EPSRC (EP/S024107/1, EP/R005826/1, EP/L015838/1), ERC (DOGMATRON AdG 883786) and Royal Society (RGS\R1\221162) for financial support.
Author information
Authors and Affiliations
Contributions
The manuscript was jointly conceived and written through the contributions of all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Zhenhua Gu, Osamu Kitagawa and Bingfeng Shi for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Costil, R. et al. J. Angew. Chem. Int. Ed. Engl. 59, 18670–18678 (2020): https://doi.org/10.1002/anie.202007595
Staniland, S. et al. Angew. Chem. Int. Ed. Engl. 55, 10755–10759 (2016): https://doi.org/10.1002/anie.201605486
Jolliffe, J. D. et al. Nat. Chem. 9, 558–562 (2017): https://doi.org/10.1038/nchem.2710
Armstrong, R. J. & Smith, M. D. Angew. Chem. Int. Ed. Engl. 53, 12822–12826 (2014): https://doi.org/10.1002/anie.201408205
Surgenor, R. R. et al. Nat. Chem. 15, 357–365 (2023): https://doi.org/10.1038/s41557-022-01095-9
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heeb, JP., Clayden, J., Smith, M.D. et al. Interrogating the configurational stability of atropisomers. Nat Protoc 18, 2745–2771 (2023). https://doi.org/10.1038/s41596-023-00859-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00859-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.