Abstract
A lack of generic and effective genetic manipulation methods for Pseudomonas has restricted fundamental research and utilization of this genus for biotechnology applications. Phage-encoded homologous recombination (PEHR) is an efficient tool for bacterial genome engineering. This PEHR system is based on a lambda Red-like operon (BAS) from Pseudomonas aeruginosa phage Ab31 and a Rac bacteriophage RecET-like operon (Rec-TEPsy) from P. syringae pv. syringae B728a and also contains exogenous elements, including the RecBCD inhibitor (Redγ or Pluγ) or single-stranded DNA-binding protein (SSB), that were added to enhance the PEHR recombineering efficiency. To solve the problem of false positives in Pseudomonas editing with the PEHR system, the processive enzyme Cas3 with a minimal Type I-C Cascade-based system was combined with PEHR. This protocol describes the utilization of a Pseudomonas-specific PEHR–Cas3 system that was designed to universally and proficiently modify the genomes of Pseudomonas species. The pipeline uses standardized cassettes combined with the concerted use of SacB counterselection and Cre site-specific recombinase for markerless or seamless genome modification, in association with vectors that possess the selectively replicating template R6K to minimize recombineering background. Compared with the traditional allelic exchange editing method, the PEHR–Cas3 system does not need to construct suicide plasmids carrying long homologous arms, thus simplifying the experimental procedure and shortening the traceless editing period. Compared with general editing systems based on phage recombinases, the PEHR–Cas3 system can effectively improve the screening efficiency of mutants using the cutting ability of Cas3 protein. The entire procedure requires ~12 days.
Key points
-
This protocol uses phage-encoded homologous recombination combined with Cascade–Cas3 for two- or three-step seamless genome modification in Pseudomonas, creating deletions, insertions or single-nucleotide substitutions. The authors also describe how to optimize the procedure for further Pseudomonas strains.
-
Compared with the traditional allelic exchange approach, the phage-encoded homologous recombination–Cas3 system provides a simpler and faster editing procedure, and the inclusion of Cas3 also improves recombineering accuracy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
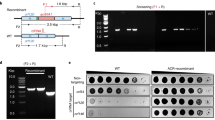
Data shown in Figs. 2–4 as examples or anticipated results are available in the original papers7,28. Supplementary Figs. 1–5 are unpublished data, and the raw data behind the graphs are provided as Supplementary Data 1. Other supporting data are available upon reasonable request to the corresponding author.
References
Palleroni, N. J. in Bergey’s Manual of Systematics of Archaea and Bacteria (eds. W. B. Whitman et al.) (2015).
Hmelo, L. R. et al. Precision-engineering the Pseudomonas aeruginosa genome with two-step allelic exchange. Nat. Protoc. 10, 1820–1841 (2015).
Xin, X.-F., Kvitko, B. & He, S. Y. Pseudomonas syringae: what it takes to be a pathogen. Nat. Rev. Microbiol. 16, 316 (2018).
Choi, K. R., Cho, J. S., Cho, I. J., Park, D. & Lee, S. Y. Markerless gene knockout and integration to express heterologous biosynthetic gene clusters in Pseudomonas putida. Metab. Eng. 47, 463–474 (2018).
Paulsen, I. T. et al. Complete genome sequence of the plant commensal Pseudomonas fluorescens Pf-5. Nat. Biotechnol. 23, 873–878 (2005).
Winsor, G. L. et al. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 44, D646–D653 (2016).
Yin, J. et al. Single-stranded DNA-binding protein and exogenous RecBCD inhibitors enhance phage-derived homologous recombination in Pseudomonas. iScience 14, 1–14 (2019).
Yu, F. et al. Recombineering Pseudomonas protegens CHA0: an innovative approach that improves nitrogen fixation with impressive bactericidal potency. Microbiol. Res. 218, 58–65 (2019).
Jing, X. et al. Engineering Pseudomonas protegens Pf-5 to improve its antifungal activity and nitrogen fixation. Microb. Biotechnol. 13, 118–133 (2020).
Swingle, B., Bao, Z., Markel, E., Chambers, A. & Cartinhour, S. Recombineering using RecTE from Pseudomonas syringae. Appl. Environ. Microbiol. 76, 4960–4968 (2010).
Bao, Z., Cartinhour, S. & Swingle, B. Substrate and target sequence length influence RecTEPsy recombineering efficiency in Pseudomonas syringae. PLoS One 7, e50617 (2012).
Quenee, L., Lamotte, D. & Polack, B. Combined sacB-based negative selection and cre-lox antibiotic marker recycling for efficient gene deletion in Pseudomonas aeruginosa. Biotechniques 38, 63–67 (2005).
Gay, P., Le Coq, D., Steinmetz, M., Berkelman, T. & Kado, C. I. Positive selection procedure for entrapment of insertion sequence elements in Gram-negative bacteria. J. Bacteriol. 164, 918–921 (1985).
Li, R. et al. Development and application of an efficient recombineering system for Burkholderia glumae and Burkholderia plantarii. Microb. Biotechnol. 14, 1809–1826 (2021).
Aparicio, T., de Lorenzo, V. & Martinez-Garcia, E. CRISPR/Cas9-enhanced ssDNA recombineering for Pseudomonas putida. Microb. Biotechnol. 12, 1076–1089 (2019).
Czajka, J. J. et al. Tuning a high performing multiplexed-CRISPRi Pseudomonas putida strain to further enhance indigoidine production. Metab. Eng. Commun. 15, e00206 (2022).
Vareechon, C., Zmina, S. E., Karmakar, M., Pearlman, E. & Rietsch, A. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe 21, 611–618 (2017).
Rangel, S. M., Diaz, M. H., Knoten, C. A., Zhang, A. & Hauser, A. R. The role of ExoS in dissemination of Pseudomonas aeruginosa during pneumonia. PLoS Pathog. 11, 681 (2015).
Cimermancic, P. et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 158, 412–421 (2014).
Zheng, W. et al. Recombineering facilitates the discovery of natural product biosynthetic pathways in Pseudomonas parafulva. Biotechnol. J. 16, e2000575 (2021).
Wang, X. et al. Improved dsDNA recombineering enables versatile multiplex genome engineering of kilobase-scale sequences in diverse bacteria. Nucleic Acids Res. 50, e15 (2022).
Oliver, A., Mulet, X., Lopez-Causape, C. & Juan, C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updat. 21–22, 41–59 (2015).
Santajit, S. & Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016, 2475067 (2016).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Hille, F. et al. The biology of CRISPR–Cas: backward and forward. Cell 172, 1239–1259 (2018).
van Belkum, A. et al. Phylogenetic distribution of CRISPR–Cas systems in antibiotic-resistant Pseudomonas aeruginosa. mBio 6, e01796–01715 (2015).
Csorgo, B. et al. A compact Cascade–Cas3 system for targeted genome engineering. Nat. Methods 17, 1183–1190 (2020).
W, Z. et al. Cascade–Cas3 facilitates high accuracy of genome engineering in Pseudomonas using phage-encoded homologous recombination. Eng. Microbiol. 2, 10046 (2022).
Zhang, Y., Buchholz, F., Muyrers, J. P. P. & Stewart, A. F. A new logic for DNA engineering using recombination in Escherichia coli. Nat. Genet. 20, 123–128 (1998).
Zhang, Y., Muyrers, J. P. P., Testa, G. & Stewart, A. F. DNA cloning by homologous recombination in Escherichia coli. Nat. Biotechnol. 18, 1314–1317 (2000).
Fu, J. et al. Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 30, 440–446 (2012).
Yin, J. et al. A new recombineering system for Photorhabdus and Xenorhabdus. Nucleic Acids Res. 43, e36 (2015).
Fels, U., Gevaert, K. & Van Damme, P. Bacterial genetic engineering by means of recombineering for reverse genetics. Front. Microbiol. 11, 548410 (2020).
Bunny, K., Liu, J. & Roth, J. Phenotypes of lexA Mutations in Salmonella enterica: evidence for a lethal lexa null phenotype due to the Fels-2 prophage. J. Bacteriol. 184, 6235–6249 (2002).
Beloin, C., Deighan, P., Doyle, M. & Dorman, C. J. Shigella flexneri 2a strain 2457T expresses three members of the H-NS-like protein family: characterization of the Sfh protein. Mol. Genet. Genom. 270, 66–77 (2003).
Derbise, A., Lesic, B., Dacheux, D., Ghigo, J. M. & Carniel, E. A rapid and simple method for inactivating chromosomal genes in Yersinia. FEMS Immunol. Med. Microbiol. 38, 113–116 (2003).
Rossi, M.-S., Paquelin, A., Ghigo, J. M. & Wandersman, C. Haemophore-mediated signal transduction across the bacterial cell envelope in Serratia marcescens: the inducer and the transported substrate are different molecules. Mol. Microbiol. 48, 1467–1480 (2003).
Hu, S. et al. Genome engineering of Agrobacterium tumefaciens using the lambda Red recombination system. Appl. Environ. Microbiol. 98, 2165–2172 (2014).
Egan, M., Ramirez, J., Xander, C., Upreti, C. & Bhatt, S. Lambda Red-mediated recombineering in the attaching and effacing pathogen Escherichia albertii. Biol. Proc. Online 18, 3 (2016).
Wannier, T. M. et al. Improved bacterial recombineering by parallelized protein discovery. Proc. Natl Acad. Sci. USA 117, 13689–13698 (2020).
Van Kessel, J. C. & Hatfull, G. F. Recombineering in Mycobacterium tuberculosis. Nat. Methods 4, 147–152 (2006).
Pijkeren, J. P., Neoh, K. M., Sirias, D., Findley, A. S. & Britton, R. A. Exploring optimization parameters to increase ssDNA recombineering in Lactococcus lactis and Lactobacillus reuteri. Bioengineered 3, 209–217 (2012).
Pijkeren, J. P. & Britton, R. A. High efficiency recombineering in lactic acid bacteria. Nucleic Acids Res. 40, e76 (2012).
Dong, H., Tao, W., Gong, F., Li, Y. & Zhang, Y. A functional recT gene for recombineering of Clostridium. J. Biotechnol. 173, 65–67 (2013).
Sun, Z. et al. A high-efficiency recombineering system with PCR-based ssDNA in Bacillus subtilis mediated by the native phage recombinase GP35. Appl. Environ. Microbiol. 99, 5151–5162 (2015).
Yang, P., Wang, J. & Qi, Q. Prophage recombinases-mediated genome engineering in Lactobacillus plantarum. Microb. Cell Fact. 14, 154 (2015).
Bian, Z. et al. Development of a new recombineering system for Agrobacterium species. Appl. Environ. Microbiol. 88, e0249921 (2022).
Cook, T. B. et al. Genetic tools for reliable gene expression and recombineering in Pseudomonas putida. J. Ind. Microbiol. Biotechnol. 45, 517–527 (2018).
Chen, Z., Ling, W. & Shang, G. Recombineering and I-SceI-mediated Pseudomonas putida KT2440 scarless gene deletion. FEMS Microbiol. Lett. 363, fnw231 (2016).
Luo, X. et al. Pseudomonas putida KT2440 markerless gene deletion using a combination of lambda Red recombineering and Cre/loxP site-specific recombination. FEMS Microbiol. Lett. 363, fnw014 (2016).
Liang, R. & Liu, J. Scarless and sequential gene modification in Pseudomonas using PCR product flanked by short homology regions. BMC Microbiol. 10, 209–209 (2010).
Aparicio, T., Jensen, S. I., Nielsen, A. T., de Lorenzo, V. & Martínez-García, E. The Ssr protein (T1E_1405) from Pseudomonas putida DOT-T1E enables oligonucleotide-based recombineering in platform strain P. putida EM42. Biotechnol. J. 11, 1309–EM1319 (2016).
Aparicio, T., de Lorenzo, V. & Martínez-García, E. CRISPR/Cas9-based counterselection boosts recombineering efficiency in Pseudomonas putida. Biotechnol. J. 13, 1700161 (2018).
Ricaurte, D. E. et al. A standardized workflow for surveying recombinases expands bacterial genome-editing capabilities. Microb. Biotechnol. 11, 176–188 (2018).
Velazquez, E., Al-Ramahi, Y. & de Lorenzo, V. CRISPR/Cas9-enhanced targetron insertion for delivery of heterologous sequences into the genome of Gram-negative bacteria. Curr. Protoc. 2, e532 (2022).
Martinez-Garcia, E. & de Lorenzo, V. Engineering multiple genomic deletions in Gram-negative bacteria: analysis of the multi-resistant antibiotic profile of Pseudomonas putida KT2440. Environ. Microbiol. 13, 2702–2716 (2011).
Volke, D. C., Martino, R. A., Kozaeva, E., Smania, A. M. & Nikel, P. I. Modular (de)construction of complex bacterial phenotypes by CRISPR/nCas9-assisted, multiplex cytidine base-editing. Nat. Commun. 13, 3026 (2022).
Yue, S. J. et al. Developing a CRISPR-assisted base-editing system for genome engineering of Pseudomonas chlororaphis. Microb. Biotechnol. 15, 2324–2336 (2022).
Volke, D. C., Friis, L., Wirth, N. T., Turlin, J. & Nikel, P. I. Synthetic control of plasmid replication enables target- and self-curing of vectors and expedites genome engineering of Pseudomonas putida. Metab. Eng. Commun. 10, e00126 (2020).
Wirth, N. T., Kozaeva, E. & Nikel, P. I. Accelerated genome engineering of Pseudomonas putida by I-SceI-mediated recombination and CRISPR–Cas9 counterselection. Microb. Biotechnol. 13, 233–249 (2020).
Jeske, A., Arce-Rodriguez, A., Thoming, J. G., Tomasch, J. & Haussler, S. Evolution of biofilm-adapted gene expression profiles in lasR-deficient clinical Pseudomonas aeruginosa isolates. NPJ Biofilms Microbiomes 8, 6 (2022).
Qiu, D., Damron, F. H., Mima, T., Schweizer, H. P. & Yu, H. D. PBAD-based shuttle vectors for functional analysis of toxic and highly regulated genes in Pseudomonas and Burkholderia spp. and other bacteria. Appl. Environ. Microbiol. 74, 7422–7426 (2008).
Wang, F. et al. BrlR from Pseudomonas aeruginosa is a receptor for both cyclic di-GMP and pyocyanin. Nat. Commun. 9, 2563 (2018).
Jacobs, M. A. et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 100, 14339–14344 (2003).
Lee, S. A. et al. General and condition-specific essential functions of Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 112, 5189–5194 (2015).
Andersen, J. B. et al. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 64, 2240–2246 (1998).
Sharan, S. K., Thomason, L. C., Kuznetsov, S. G. & Court, D. L. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 4, 206–223 (2009).
Fu, J., Teucher, M., Anastassiadis, K., Skarnes, W. & Stewart, A.F. in Methods in Enzymology, 477. (eds. M. W. Paul & M. S. Philippe) 125–144 (Academic Press, 2010).
Egan, S. M. & Schleif, R. F. DNA-dependent renaturation of an insoluble DNA binding protein. J. Mol. Biol. 243, 821–829 (1994).
Egan, S. M. & Schleif, R. F. A regulatory cascade in the induction of rhaBAD. J. Mol. Biol. 234, 87–98 (1993).
Filutowicz, M., McEachern, M. J. & Helinski, D. R. Positive and negative roles of an initiator protein at an origin of replication. Proc. Natl Acad. Sci. USA 83, 9645–9649 (1986).
Yang, S. H. et al. The SOSS1 single‐stranded DNA binding complex promotes DNA end resection in concert with Exo1. EMBO J. 32, 126–139 (2013).
Pelicic, V., Reyrat, J. M. & Gicquel, B. Expression of the Bacillus subtilis sacB gene confers sucrose sensitivity on mycobacteria. J. Bacteriol. 178, 1197–1199 (1996).
Zhang, Z. & Lutz, B. Cre recombinase-mediated inversion using lox66 and lox71: method to introduce conditional point mutations into the CREB-binding protein. Nucleic Acids Res. 30, e90–e90 (2002).
Mei, J., Benashski, S. & Firshein, W. Interactions of the origin of replication (oriV) and initiation proteins (TrfA) of plasmid RK2 with submembrane domains of Escherichia coli. J. Bacteriol. 177, 6766–6772 (1995).
Wang, H. L. et al. RecET direct cloning and Red alpha beta recombineering of biosynthetic gene clusters, large operons or single genes for heterologous expression. Nat. Protoc. 11, 1175–1190 (2016).
Iwasaki, K. et al. Transformation of Pseudomonas putida by electroporation. Biosci. Biotechnol. Biochem. 58, 851–854 (1994).
Wang, Q. et al. Quick and efficient method for genetic transformation of biopolymer-producing bacteria. J. Chem. Technol. Biot. 85, 775–778 (2010).
Kim, J. & Park, W. Oxidative stress response in Pseudomonas putida. Appl. Microbiol. Biotechnol. 98, 6933–6946 (2014).
Sriwiriyarat, T., Jangkorn, S., Charoenpanich, J., Chinwetkitvanich, S. & Fongsatitkul, P. Occurrence of aerobic denitrifying bacteria in integrated fixed film activated sludge system. Chemosphere 285, 131504 (2021).
Wang, H. et al. ExoCET: exonuclease in vitro assembly combined with RecET recombination for highly efficient direct DNA cloning from complex genomes. Nucleic Acids Res. 46, e28 (2018).
Acknowledgements
This study was supported by grants from the National Key R&D Program of China (2019YFA0904000), the National Natural Science Foundation of China (31570094, 31670097 and 81502962); the 111 Project (B16030), the China Postdoctoral Science Foundation (2022M711925) to W.Z., the Shandong Provincial Natural Science Foundation of China (ZR2020MC015) to R.L. and (ZR2022QC107) to W.Z., the Guangdong Basic and Applied Basic Research Foundation (2022A1515110795) to W.Z., the Natural Science Foundation of Changsha (kq2208167) to J.Y., the Natural Science Foundation for Distinguished Young Scholars of Hunan Province (2023JJ10029) to J.Y. and the Taishan Scholar Program of Shandong Province to J.F.
Author information
Authors and Affiliations
Contributions
W.Z., Y.X., Y.Y., Y.Z., J.F., R.L. and J.Y. designed the experiments. W.Z., Y.X., X.W., S.G., D.Z., C.C., V.R., C.J. and Q.T. performed the experiments. W.Z., Y.X., J.F., R.L. and J.Y. wrote the manuscript with help from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Thomas Eng, Kenan Murphy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Yin, J. et al. iScience 14, 1–14 (2019): https://doi.org/10.1016/j.isci.2019.03.007
Zheng, W. et al. Biotechnol. J. 16, e2000575 (2021): https://doi.org/10.1002/biot.202000575
Zheng, W. et al. Eng. Microbiol. 2, 10046 (2022): https://doi.org/10.1016/j.engmic.2022.100046
Supplementary information
Supplementary Information
Supplementary Tables 1–4, Figs. 1–5 and Notes 1–27.
Supplementary Data 1
Statistical source data for Supplementary Figs. 1, 2 and 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, W., Xia, Y., Wang, X. et al. Precise genome engineering in Pseudomonas using phage-encoded homologous recombination and the Cascade–Cas3 system. Nat Protoc 18, 2642–2670 (2023). https://doi.org/10.1038/s41596-023-00856-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00856-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.