Abstract
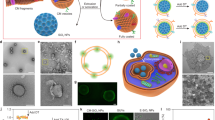
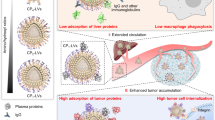
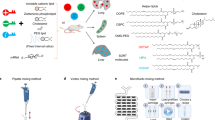
Polymeric nanoparticles (NPs) are a promising platform for medical applications in drug delivery. However, their use as drug carriers is limited by biological (e.g., immunological) barriers after intravenous administration. Ionic liquids (ILs), formed from bulky asymmetric cations and anions, have a wide variety of physical internal and external interfacing properties. When assembled on polymeric NPs as biomaterial coatings, these external-interfacing properties can be tuned to extend their circulation half-life when intravenously injected, as well as drive biodistribution to sites of interest for selective organ accumulation. In our work, we are particularly interested in optimizing IL coatings to enable red blood cell hitchhiking in whole blood. In this protocol, we describe the preparation and physicochemical and biological characterization of choline carboxylate IL-coated polymeric NPs. The procedure is divided into five stages: (1) synthesis and characterization of choline-based ILs (1 week); (2) bare poly(lactic-co-glycolic acid) (50:50, acid terminated) Resomer 504H (PLGA) NP assembly, modified from previously established protocols, with dye encapsulation (7 h); (3) modification of the bare particles with IL coating (3 h); (4) physicochemical characterization of both PLGA and IL-PLGA NPs by dynamic light scattering, 1H nuclear magnetic resonance spectroscopy, and transmission electron microscopy (1 week); (5) ex vivo evaluation of intravenous biocompatibility (including serum-protein resistance and hemolysis) and red blood cell hitchhiking in whole BALB/c mouse blood via fluorescence-activated cell sorting (1 week). With practice and technique refinement, this protocol is accessible to late-stage graduate students and early-stage postdoctoral scientists.
Key points
-
Ionic liquids (ILs) are organic salts that stay in liquid form below 100 °C. Examples have been found that promote drug solubility, permeability, and stability. Choline-based ILs are prepared and used to coat polymeric nanoparticles to improve their circulation half-life and biodistribution
-
The biological mechanism is red blood cell hitchiking. The composition of the IL coating can be screened for optimal red blood cell binding of the particles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
Data availability
The datasets generated during the study have been uploaded to FigShare (https://doi.org/10.6084/m9.figshare.c.6279060.v2).
References
Lü, J. M. et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 9, 325–341 (2009).
Park, J. et al. Enhancement of surface ligand display on PLGA nanoparticles with amphiphilic ligand conjugates. J. Control. Release 156, 109–115 (2011).
Manoochehri, S. et al. Surface modification of PLGA nanoparticles via human serum albumin conjugation for controlled delivery of docetaxel. Daru. https://doi.org/10.1186/2008-2231-21-58 (2013).
Amo, L. et al. Surface functionalization of PLGA nanoparticles to increase transport across the BBB for Alzheimer’s disease. Appl. Sci. 11, 4305 (2021).
Rezvantalab, S. et al. PLGA-based nanoparticles in cancer treatment. Front. Pharmacol. 9, 1260 (2018).
Hashemi, M., Shamshiri, A., Saeedi, M., Tayebi, L. & Yazdian-Robati, R. Aptamer-conjugated PLGA nanoparticles for delivery and imaging of cancer therapeutic drugs. Arch. Biochem. Biophys. 691, 108485 (2020).
Dang, Y. & Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 1, 10–19 (2020).
Chung, T. W., Tsai, Y. L., Hsieh, J. H. & Tsai, W. J. Different ratios of lactide and glycolide in PLGA affect the surface property and protein delivery characteristics of the PLGA microspheres with hydrophobic additives. J. Microencapsul. 23, 15–27 (2006).
Keles, H., Naylor, A., Clegg, F. & Sammon, C. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polym. Degrad. Stab. 119, 228–241 (2015).
Amjadi, I., Rabiee, M. & Hosseini, M. S. Anticancer activity of nanoparticles based on PLGA and its co-polymer: in-vitro evaluation. Iran. J. Pharm. Res. 12, 623–634 (2013).
Gentile, P., Chiono, V., Carmagnola, I. & Hatton, P. V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 15, 3640–3659 (2014).
Suk, J. S., Xu, Q., Kim, N., Hanes, J. & Ensign, L. M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. https://doi.org/10.1016/j.addr.2015.09.012 (2016).
Nie, S. Editorial: understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 5, 523–528 (2010).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Ferrari, R., Sponchioni, M., Morbidelli, M. & Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: the checkpoints on the road from the synthesis to clinical translation. Nanoscale 10, 22701–22719 (2018).
Barui, A. K., Oh, J. Y., Jana, B., Kim, C. & Ryu, J. Cancer‐targeted nanomedicine: overcoming the barrier of the protein corona. Adv. Ther. 3, 1900124 (2020).
Foroozandeh, P. & Aziz, A. A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. https://doi.org/10.1186/S11671-018-2728-6 (2018).
Hoang Thi, T. T. et al. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 12, 298 (2020).
Zhang, P., Sun, F., Liu, S. & Jiang, S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J. Control. Release 244, 184–193 (2016).
Garay, R. P., El-Gewely, R., Armstrong, J. K., Garratty, G. & Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents, Expert Opin. Drug Deliv. https://doi.org/10.1517/17425247.2012.720969 (2012).
López-Royo, T. et al. Encapsulation of large-size plasmids in PLGA nanoparticles for gene editing: comparison of three different synthesis methods. Nanomater 11, 2723 (2021).
Caparica, R. et al. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 10, 233 (2020).
Tahara, Y., Morita, K., Wakabayashi, R., Kamiya, N. & Goto, M. Biocompatible ionic liquid enhances transdermal antigen peptide delivery and preventive vaccination effect. Mol. Pharm. 17, 3845–3856 (2020).
Tanner, E. E. L. et al. Design principles of ionic liquids for transdermal drug delivery. Adv. Mater. 31, 1901103 (2019).
Shi, Y. et al. Oral delivery of sorafenib through spontaneous formation of ionic liquid nanocomplexes. J. Control. Release 322, 602–609 (2020).
Banerjee, A. et al. Ionic liquids for oral insulin delivery. Proc. Natl Acad. Sci. USA 115, 7296–7301 (2018).
Halayqa, M., Pobudkowska, A., Domańska, U. & Zawadzki, M. Studying of drug solubility in water and alcohols using drug-ammonium ionic liquid-compounds. Eur. J. Pharm. Sci. 111, 270–277 (2018).
Hamadani, C. M., Goetz, M. J., Mitragotri, S. & Tanner, E. E. L. Protein-avoidant ionic liquid (PAIL)–coated nanoparticles to increase bloodstream circulation and drive biodistribution. Sci. Adv. 6, eabd7563 (2020).
Hamadani, C. M. et al. Improved nanoformulation and biofunctionalization of linear-dendritic block copolymers with biocompatible ionic liquids. Nanoscale https://doi.org/10.1039/D2NR00538G (2022).
Hu, C. M. J., et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1106634108 (2011).
Makadia, H. K. & Siegel, S. J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011).
Govender, T., Stolnik, S., Garnett, M. C., Illum, L. & Davis, S. S. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J. Control. Release 57, 171–185 (1999).
Haque, S. et al. Suggested procedures for the reproducible synthesis of poly(d,l-lactideco-glycolide) nanoparticles using the emulsification solvent diffusion platform. Curr. Nanosci. 14, 448–453 (2018).
Astete, C. E. & Sabliov, C. M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 17, 247–289 (2006).
Hernández-Giottonini, K. Y. et al. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: effects of formulation parameters. RSC Adv. 10, 4218–4231 (2020).
Kizilbey, K. Optimization of rutin-loaded PLGA nanoparticles synthesized by single-emulsion solvent evaporation method. ACS Omega 4, 555–562 (2019).
McCall, R. L. & Sirianni, R. W. PLGA nanoparticles formed by single- or double-emulsion with vitamin E-TPGS. J. Vis. Exp. https://doi.org/10.3791/51015 (2013).
Alshamsan, A. Nanoprecipitation is more efficient than emulsion solvent evaporation method to encapsulate cucurbitacin I in PLGA nanoparticles. Saudi Pharm. J. 22, 219–222 (2014).
Bilati, U., Allémann, E. & Doelker, E. Nanoprecipitation versus emulsion-based techniques or the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech https://doi.org/10.1208/pt060474 (2005)
Shkodra-Pula, B. et al. Encapsulation of the dual FLAP/mPEGS-1 inhibitor BRP-187 into acetalated dextran and PLGA nanoparticles improves its cellular bioactivity. J. Nanobiotechnol. 18, 73 (2020).
Huang, W. & Zhang, C. Tuning the size of poly(lactic-co-glycolic acid) (PLGA) nanoparticles fabricated by nanoprecipitation. Biotechnol. J. 13, 1700203 (2018).
Whittington, N. C. & Wray, S. Suppression of red blood cell autofluorescence for immunocytochemistry on fixed embryonic mouse tissue. Curr. Protoc. Neurosci. 81, 2.28.1–2.28.12 (2017).
Zhang, Y. et al. Near-infrared fluorescent thienothiadiazole dyes with large Stokes shifts and high photostability. J. Org. Chem. 82, 5597–5606 (2017).
Hamadani, C. M. A Novel Chemotherapeutic Nanoparticle Drug Delivery System: Surface Functionalization of Polymeric Nanoparticles with Protein-Phobic Ionic Liquid to Enhance Drug Bioavailability in Systemic Circulation. MSc thesis, Harvard Univ. (2020).
Brenner, J. S. et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 9, 2684 (2018).
Evans, B. C. et al. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. https://doi.org/10.3791/50166 (2013).
Gindri, I. M. et al. Preparation of TiO2 nanoparticles coated with ionic liquids: a supramolecular approach. ACS Appl. Mater. Interfaces 6, 11536–11543 (2014).
Pourjavadi, A., Hosseini, S. H., Doulabi, M., Fakoorpoor, S. M. & Seidi, F. Multi-layer functionalized poly(ionic liquid) coated magnetic nanoparticles: highly recoverable and magnetically separable brønsted acid catalyst. ACS Catal. 2, 1259–1266 (2012).
Adumitrăchioaie, A., Tertis, M., Cernat, A., Sandulescu, R. & Cristea, C. Electrochemical methods based on molecularly imprinted polymers for drug detection: a review. Int. J. Electrochem. Sci. 13, 2556–2576 (2018).
Zhang, R., Gao, R., Gou, Q., Lai, J. & Li, X. Precipitation polymerization: a powerful tool for preparation of uniform polymer particles. Polymers 14, 1851 (2022).
Takahashi, C. et al. Optimization of ionic liquid-incorporated PLGA nanoparticles for treatment of biofilm infections. Mater. Sci. Eng. C. 97, 78–83 (2019).
Abid, Z. et al. Investigation of mucoadhesion and degradation of PCL and PLGA microcontainers for oral drug delivery. Polymers 11, 1828 (2019).
Acknowledgements
Procedures developed and optimized in this protocol are supported by a PhRMA Foundation grant and Sigma Xi Student grant G0315202198510166.
Author information
Authors and Affiliations
Contributions
Under the supervision of E.E.L.T., C.M.H developed the original experimental procedure and data, with updated troubleshooting, data and optimization/experimental advice for all sections, and wrote the paper with contributions from all coauthors. G.S.D., M.E.G., C.M.C. and W.M. helped develop and optimize the separation procedure of all blood components when isolating RBCs in whole blood (Box 3). G.S.D. contributed experimental development, procedure, text, data and troubleshooting advice for the alternative sonication synthesis. M.E.G. contributed optimization of experimental development, text and troubleshooting data, and advice for all aspects of NP synthesis with encapsulation of DiD dye, NMR quantitative characterization, optimization of RBC biocompatibility and optimization of RBC hitchhiking procedure/FACS gating analysis. M.C.P contributed optimization of experimental development and experimental setup for the ‘whip-in’ (displayed in Fig. 1c) method of stirring IL-PLGA DiD NP synthesis. W.M. and R.H. provided critical feedback on experimental method procedures and supplied data used for EE, DiD NP characterization and experimental setup figures (Figs. 1 and 3). W.M. provided troubleshooting supplementary data and created the supplementary video with assistance from G.S. G.S. provided critical feedback on NMR characterization of ILs and quantitative NMR characterization and provided further optimization of the sonication NP synthesis method. C.M.C. and E.J. synthesized and provided some of the ILs displayed in Fig. 2 from Table 1. C.M.C. provided the ‘Timing’ section in the manuscript. S.X.E. provided critical text formatting, procedure organization and troubleshooting section numbering, troubleshooting and critical callouts. E.E.L.T. provided resources, supervision, funding, draft editing and project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Antonio Benedetto and Jianping Qi for their contribution to their peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Hamadani, C. M. et al. Sci Adv. 6, eabd7563 (2020): https://doi.org/10.1126/sciadv.abd7563
Hamadani, C. M. et al. Nanoscale 14, 6021–6036 (2022): https://doi.org/10.1039/d2nr00538g
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hamadani, C.M., Dasanayake, G.S., Gorniak, M.E. et al. Development of ionic liquid-coated PLGA nanoparticles for applications in intravenous drug delivery. Nat Protoc 18, 2509–2557 (2023). https://doi.org/10.1038/s41596-023-00843-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00843-6
This article is cited by
-
Bibliometric and visualized analysis of cancer nanomedicine from 2013 to 2023
Drug Delivery and Translational Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.