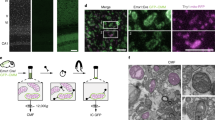
Abstract
Mitochondria are key bioenergetic organelles involved in many biosynthetic and signaling pathways. However, their differential contribution to specific functions of cells within complex tissues is difficult to dissect with current methods. The present protocol addresses this need by enabling the ex vivo immunocapture of cell-type-specific mitochondria directly from their tissue context through a MitoTag reporter mouse. While other available methods were developed for bulk mitochondria isolation or more abundant cell-type-specific mitochondria, this protocol was optimized for the selective isolation of functional mitochondria from medium-to-low-abundant cell types in a heterogeneous tissue, such as the central nervous system. The protocol has three major parts: First, mitochondria of a cell type of interest are tagged via an outer mitochondrial membrane eGFP by crossing MitoTag mice to a cell-type-specific Cre-driver line or by delivery of viral vectors for Cre expression. Second, homogenates are prepared from relevant tissues by nitrogen cavitation, from which tagged organelles are immunocaptured using magnetic microbeads. Third, immunocaptured mitochondria are used for downstream assays, e.g., to probe respiratory capacity or calcium handling, revealing cell-type-specific mitochondrial diversity in molecular composition and function. The MitoTag approach enables the identification of marker proteins to label cell-type-specific organelle populations in situ, elucidates cell-type-enriched mitochondrial metabolic and signaling pathways, and reveals functional mitochondrial diversity between adjacent cell types in complex tissues, such as the brain. Apart from establishing the mouse colony (6–8 weeks without import), the immunocapture protocol takes 2 h and functional assays require 1–2 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data presented in this article have been previously published and associated raw data are provided in the related article6.
References
Pagliarini, D. J. & Rutter, J. Hallmarks of a new era in mitochondrial biochemistry. Genes Dev. 27, 2615–2627 (2013).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Weinberg, S. E., Sena, L. A. & Chandel, N. S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 42, 406–417 (2015).
Motori, E. et al. Neuronal metabolic rewiring promotes resilience to neurodegeneration caused by mitochondrial dysfunction. Sci. Adv. 6, eaba8271 (2020).
Fecher, C. et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat. Neurosci. 22, 1731–1742 (2019).
Bayraktar, E. C. et al. MITO-tag mice enable rapid isolation and multimodal profiling of mitochondria from specific cell types in vivo. Proc. Natl Acad. Sci. USA 116, 303–312 (2019).
Barros, L. F. et al. Current technical approaches to brain energy metabolism. Glia 66, 1138–1159 (2018).
Misgeld, T. & Schwarz, T. L. Mitostasis in neurons: maintaining mitochondria in an extended cellular architecture. Neuron 96, 651–666 (2017).
Plucinska, G. & Misgeld, T. Imaging of neuronal mitochondria in situ. Curr. Opin. Neurobiol. 39, 152–163 (2016).
Pekkurnaz, G. & Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 4, 802–812 (2022).
Bomba-Warczak, E., Edassery, S. L., Hark, T. J. & Savas, J. N. Long-lived mitochondrial cristae proteins in mouse heart and brain. J. Cell Biol. 220, e202005193 (2021).
Sanz, E. et al. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl Acad. Sci. USA 106, 13939–13944 (2009).
Franko, A. et al. Efficient isolation of pure and functional mitochondria from mouse tissues using automated tissue disruption and enrichment with anti-TOM22 magnetic beads. PLoS One. 8, e82392 (2013).
Horie, C., Suzuki, H., Sakaguchi, M. & Mihara, K. Characterization of signal that directs C-tail-anchored proteins to mammalian mitochondrial outer membrane. Mol. Biol. Cell 13, 1615–1625 (2002).
Chen, W. W., Freinkman, E., Wang, T., Birsoy, K. & Sabatini, D. M. Absolute quantification of matrix metabolites reveals the dynamics of mitochondrial metabolism. Cell 166, 1324–1337.e1311 (2016).
Zambrowicz, B. P. et al. Disruption of overlapping transcripts in the ROSA beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl Acad. Sci. USA 94, 3789–3794 (1997).
Meeusen, S., McCaffery, J. M. & Nunnari, J. Mitochondrial fusion intermediates revealed in vitro. Science 305, 1747–1752 (2004).
Breckwoldt, M. O. et al. Multiparametric optical analysis of mitochondrial redox signals during neuronal physiology and pathology in vivo. Nat. Med. 20, 555–560 (2014).
Fecher, C. Mitochondrial Diversity Probed in Mouse Cerebellum Elucidates Cell Type-Specific Fine-Tuning of Mitochondrial Biology. PhD thesis, LMU München (2020).
Wynne, M. E. et al. Heterogeneous expression of nuclear encoded mitochondrial genes distinguishes inhibitory and excitatory neurons. eNeuro. https://doi.org/10.1523/eneuro.0232-21.2021 (2021).
das Neves, R. P. et al. Connecting variability in global transcription rate to mitochondrial variability. PLoS Biol. 8, e1000560 (2010).
Medini, H., Cohen, T. & Mishmar, D. Mitochondrial gene expression in single cells shape pancreatic beta cells’ sub-populations and explain variation in insulin pathway. Sci. Rep. 11, 466 (2021).
Hubbard, W. B. et al. Fractionated mitochondrial magnetic separation for isolation of synaptic mitochondria from brain tissue. Sci. Rep. 9, 9656 (2019).
Suomalainen, A. & Battersby, B. J. Mitochondrial diseases: the contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 19, 77–92 (2018).
De Stefani, D., Rizzuto, R. & Pozzan, T. Enjoy the trip: calcium in mitochondria back and forth. Ann. Rev. Biochem. 85, 161–192 (2016).
Ahier, A., Onraet, T. & Zuryn, S. Cell-specific mitochondria affinity purification (CS-MAP) from Caenorhabditis elegans. STAR Protoc. 2, 100952 (2021).
Harbauer, A. B. et al. Neuronal mitochondria transport Pink1 mRNA via synaptojanin 2 to support local mitophagy. Neuron 110, 1516–1531.e1519 (2022).
Hansen, F. M. et al. Mitochondrial phosphoproteomes are functionally specialized across tissues. Preprint at bioRxiv https://doi.org/10.1101/2022.03.23.485457 (2022).
Cabrera-Orefice, A., Potter, A., Evers, F., Hevler, J. F. & Guerrero-Castillo, S. Complexome profiling-exploring mitochondrial protein complexes in health and disease. Front. Cell Dev. Biol. 9, 796128 (2021).
Roh, H. C. et al. Simultaneous transcriptional and epigenomic profiling from specific cell types within heterogeneous tissues in vivo. Cell Rep. 18, 1048–1061 (2017).
Bensley, R. R. & Hoerr, N. L. Studies on cell structure by the freezing-drying method V. The chemical basis of the organization of the cell. Anat. Rec. 60, 251–266 (1934).
Hogeboom, G. H., Schneider, W. C. & Pallade, G. E. Cytochemical studies of mammalian tissues: I. Isolation of intact mitochondria from rat liver; some biochemical properties of mitochondria and submicroscopic particulate material. J. Biol. Chem. 172, 619–635 (1948).
Frezza, C., Cipolat, S. & Scorrano, L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat. Protoc. 2, 287–295 (2007).
Sims, N. R. & Anderson, M. F. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat. Protoc. 3, 1228–1239 (2008).
Russo, G. L. et al. CRISPR-mediated induction of neuron-enriched mitochondrial proteins boosts direct glia-to-neuron conversion. Cell. Stem Cell. 28, 524–534.e527 (2021).
Rath, S. et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res. 49, D1541–D1547 (2021).
Alvarez-Castelao, B. et al. Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nat. Biotechnol. 35, 1196–1201 (2017).
Alvarez-Castelao, B., Schanzenbächer, C. T., Langer, J. D. & Schuman, E. M. Cell-type-specific metabolic labeling, detection and identification of nascent proteomes in vivo. Nat. Protoc. 14, 556–575 (2019).
Smith, A. C. & Robinson, A. J. MitoMiner v4.0: an updated database of mitochondrial localization evidence, phenotypes and diseases. Nucleic Acids Res. 47, D1225–D1228 (2019).
Benador, I. Y. et al. Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab. 27, 869–885.e866 (2018).
Misgeld, T., Kerschensteiner, M., Bareyre, F. M., Burgess, R. W. & Lichtman, J. W. Imaging axonal transport of mitochondria in vivo. Nat. Methods 4, 559–561 (2007).
Pham, A. H., McCaffery, J. M. & Chan, D. C. Mouse lines with photo-activatable mitochondria to study mitochondrial dynamics. Genesis 50, 833–843 (2012).
Agarwal, A. et al. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605.e587 (2017).
MacDonald, J. A. et al. A nanoscale, multi-parametric flow cytometry-based platform to study mitochondrial heterogeneity and mitochondrial DNA dynamics. Commun. Biol. 2, 258 (2019).
Cavelier, L., Johannisson, A. & Gyllensten, U. Analysis of mtDNA copy number and composition of single mitochondrial particles using flow cytometry and PCR. Exp. Cell Res. 259, 79–85 (2000).
Fieni, F. et al. Voltage-dependent inwardly rectifying potassium conductance in the outer membrane of neuronal mitochondria. J. Biol. Chem. 285, 27411–27417 (2010).
Zhang, S. et al. High-throughput multiparameter analysis of individual mitochondria. Anal. Chem. 84, 6421–6428 (2012).
Luo, L. et al. Optimizing nervous system-specific gene targeting with Cre driver lines: prevalence of germline recombination and influencing factors. Neuron 106, 37–65.e35 (2020).
Wanders, R. J. A., Waterham, H. R. & Ferdinandusse, S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front. Cell Dev. Biol. https://doi.org/10.3389/fcell.2015.00083 (2016).
Shai, N. et al. Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat. Commun. 9, 1761 (2018).
Herculano-Houzel, S. The glia/neuron ratio: how it varies uniformly across brain structures and species and what that means for brain physiology and evolution. Glia 62, 1377–1391 (2014).
Sharma, K. et al. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci. 18, 1819–1831 (2015).
Murgia, M. et al. Proteomics of cytochrome c oxidase-negative versus positive muscle fiber sections in mitochondrial myopathy. Cell Rep. 29, 3825–3834.e3824 (2019).
Schiaffino, S., Reggiani, C., Kostrominova, T. Y., Mann, M. & Murgia, M. Mitochondrial specialization revealed by single muscle fiber proteomics: focus on the Krebs cycle. Scand. J. Med. Sci. Sports 25, 41–48 (2015).
Gella, A. et al. Mitochondrial proteome of affected glutamatergic neurons in a mouse model of leigh syndrome. Front. Cell Dev. Biol. 8, 660 (2020).
Fünfschilling, U. & Reichardt, L. F. Cre-mediated recombination in rhombic lip derivatives. Genesis 33, 160–169 (2002).
Davidson, B. L. & Breakefield, X. O. Viral vectors for gene delivery to the nervous system. Nat. Rev. Neurosci. 4, 353–364 (2003).
Wang, M., Misgeld, T. & Brill, M. S. Neural labeling and manipulation by neonatal intraventricular viral injection in mice. STAR Protoc. 3, 101081 (2022).
Hughes, C. S. et al. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 14, 68–85 (2019).
Iuso, A., Repp, B., Biagosch, C., Terrile, C. & Prokisch, H. Assessing mitochondrial bioenergetics in isolated mitochondria from various mouse tissues using Seahorse XF96 analyzer. Methods Mol. Biol. 1567, 217–230 (2017).
Calvo, S. E., Clauser, K. R. & Mootha, V. K. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 44, D1251–D1257 (2016).
Wettmarshausen, J. & Perocchi, F. Assessing calcium-stimulated mitochondrial bioenergetics using the Seahorse XF96 analyzer. Methods Mol. Biol. 1925, 197–222 (2019).
Wettmarshausen, J. & Perocchi, F. Isolation of functional mitochondria from cultured cells and mouse tissues. Methods Mol. Biol. 1567, 15–32 (2017).
Zambrowicz, B. P. et al. Disruption of overlapping transcripts in the ROSA βgeo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl Acad. Sci. USA 94, 3789–3794 (1997).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e422 (2018).
Komatsu, M. et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 (2006).
Nectow, A. R. et al. Rapid molecular profiling of defined cell types using Viral TRAP. Cell Rep. 19, 655–667 (2017).
Acknowledgements
We thank all authors of the related article6 for their contribution to the establishment and validation of the MitoTag mouse model. Work in T.M.’s laboratory is supported by the DFG: CRC870 A11-ID 118803580, TRR 274/1 B03/ C02-ID 408885537, Mi 694/7-1, Mi 694/8-1, Mi 694/9-1 A03-ID 428663564, FOR ImmunoStroke and the ERC under the European Union’s Seventh Framework Program (FP/2007-2013; ERC grant agreement no. 616791). T.M. is a member of and supported by the German Center for Neurodegenerative Diseases (DZNE) and by the Munich Center for Systems Neurology (SyNergy EXC 2145; Project ID 390857198). N.P.d.M. and C.F. were enrolled in the Munich Graduate School of Systemic Neurosciences (GSC 82–ID 24184143). Work in F.P.’s laboratory is supported by the Munich Center for Systems Neurology (SyNergy EXC 2145; Project ID 390857198) and the ExNet-0041-Phase2-3 (‘SyNergy-HMGU’) through the Initiative and Network Fund of the Helmholtz Association. The authors thank H.C. Delgado de la Herran, Y. Hufnagel and M. Feng for help with material and data collection.
Author information
Authors and Affiliations
Contributions
N.P.d.M. and C.F. collected and analyzed the data. C.F. and N.P.d.M designed the figures with help from F.P. and T.M. A.M.P generated the video. All authors contributed to writing, editing and revision of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Elisenda Sanz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key reference using this protocol
Fecher, C. et al. Nat. Neurosci. 22, 1731–1742 (2019): https://doi.org/10.1038/s41593-019-0479-z
Extended data
Extended Data Fig. 1 MitoTag genetic strategy.
a, MitoTag locus recombination within the endogenous Rosa26 locus occurs during the crossing of MitoTag mice with Cre-driver lines generating recombined animals (F1) that express GFP-OMM in Cre+ cells (green element). e1/2, exon 1/2; P*, cell-type-specific promoter. b, MitoTag PCR result demonstrating PCR primer specificity for the MitoTag allele. PCR products: wild-type Rosa26 locus (WT), ~600 bp (see lane 3); MitoTag transgene, 324 bp (see lane 6). Consult Step 3A(iv–xiii) for genotyping protocol. The Rosa26-tm4(CAG-EGFP*) reporter mouse is another Rosa26 knock-in model that Cre-conditionally expresses GFP localized to the mitochondrial matrix (mito-EGFP). Note the absence of PCR product in this homozygous sample in lane 5. Primers are specific for both alleles, GFP-OMM and the endogenous Rosa26 locus. The sample in lane 5 is from a homozygous Rosa26-tm4(CAG-EGFP*) mouse. A PCR against GFP would not discriminate between the two Rosa26 reporter mouse models. All animal experiments were approved by the responsible regulatory agencies (Regierung von Oberbayern). a, adapted from ref. 6, Springer Nature Ltd.
Extended Data Fig. 2 MitoTag recombination in astrocytes through a number of Cre-driver lines and adeno-associated viral vector.
a-f, MitoTag mice were recombined with different Cre-driver lines for the expression in astrocytes: a, mGFAP:Cre (77.6); b, hGFAP:CreERT2; c, Aldh1¦1:Cre; d, Sept4:Cre; and e, Plp1:Cre/ERT; or neonatally injected with AAV9-CamkII.Cre virus (f, cerebellum and cortex). In cerebellum, two populations of astrocytes are present, namely Bergmann glia in the molecular layer (ML) with their cell body localized in the Purkinje cell layer (PCL) and protoplasmic astrocytes in the granule cell layer (GCL). Asterisks indicate leaky expression and off-target recombination. Scale bar, 1 mm for sagittal brain, 50 µm for detailed images. Further information on the Cre-driver lines is given in Table 1. All animal experiments were approved by the responsible regulatory agencies (Regierung von Oberbayern). a, adapted from ref. 6, Springer Nature Ltd.
Extended Data Fig. 3 Essential equipment for the tissue homogenization Step 16.
a, Dounce glass tissue homogenizers of different sizes (volume and clearance, see Table 4). #A can be used for soft tissue and homogenization by hand, while #20, 22-24 are used in combination with a motorized rotor (300 rpm for the present protocol) and for different tissue sources (brain, liver, muscle tissue). b, Sample pictures illustrating the final immunocapture in Step 29 and 31. Note, final samples can vary in size and color between IC Tom and IC GFP dependent on the abundance of GFP-OMM tagged mitochondria in tissue lysate and the amount of microbeads used. Microbeads are present as dark central spot within the sample pellet and we have not observed adverse effects due to their presence in functional downstream assays. All animal experiments were approved by the responsible regulatory agencies (Regierung von Oberbayern).
Extended Data Fig. 4 Troubleshooting of mitochondrial viability via oxygen consumption rate (OCR) measurement.
a, Protocol stage 2 illustrating the generation of a single-cell tissue homogenate in Step 16. For mitochondrial bioenergetics, two tissue lysate concentrations were generated in IB+: 5 mg/ml and 20 mg/ml, as recommended in our protocol. Immunocapture with IC Tom was performed from both samples as outlined in the PROCEDURE. b, Oxygen consumption ratio (OCR) via complex I (pyruvate/malate) from samples outlined in a (5 mg/ml, light orange; 20 mg/ml, orange) using 2 ug mitochondria/well supplemented with 10 mM pyruvate and 2 mM malate. The following compounds and concentrations were injected in the assay: ADP (4 mM), oligomycin A (Oligo, 1.5 µM), CCCP (10 µM), and rotenone (2 µM) together with antimycin A (4 µM; AA). The graph shows that mitochondrial functionality can be affected by the initial dilution during homogenisation, which is observed by the low OCR levels and unresponsiveness to the compounds of the 5 mg/ml sample (light orange) compared with the expected mitochondrial modulator responses of the standard sample (orange). Line graph: mean ± s.e.m. from ≥8 technical replicates. All animal experiments were approved by the responsible regulatory agencies (Regierung von Oberbayern).
Extended Data Fig. 5 Single-channel information from MitoTag/Gfap:Cre/Thy1:mitoRFP image represented in Fig. 4d.
Cortex from MitoTag/Gfap:Cre/Thy1:mitoRFP animals used as tissue source in the ‘spike-in’ experiment. a, Merged image shown in Fig. 4d and corresponding single channels. b, Detail from a. Scale bar, 20 µm for a, 10 µm for b.
Supplementary information
Supplementary Video 1
Description of how to perform Steps 17–20 corresponding to plasma membrane permeabilization using nitrogen cavitation.
Source data
Source Data
Unprocessed western blots and statistical source data for Figs. 4b,c,e,f and 5a–e and Extended Data Fig. 4b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Mello, N.P., Fecher, C., Pastor, A.M. et al. Ex vivo immunocapture and functional characterization of cell-type-specific mitochondria using MitoTag mice. Nat Protoc 18, 2181–2220 (2023). https://doi.org/10.1038/s41596-023-00831-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-023-00831-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.