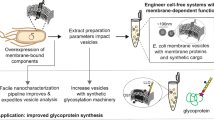
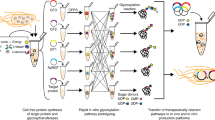
Abstract
The advent of distributed biomanufacturing platforms promises to increase agility in biologic production and expand access by reducing reliance on refrigerated supply chains. However, such platforms are not capable of robustly producing glycoproteins, which represent the majority of biologics approved or in development. To address this limitation, we developed cell-free technologies that enable rapid, modular production of glycoprotein therapeutics and vaccines from freeze-dried Escherichia coli cell lysates. Here, we describe a protocol for generation of cell-free lysates and freeze-dried reactions for on-demand synthesis of desired glycoproteins. The protocol includes construction and culture of the bacterial chassis strain, cell-free lysate production, assembly of freeze-dried reactions, cell-free glycoprotein synthesis, and glycoprotein characterization, all of which can be completed in one week or less. We anticipate that cell-free technologies, along with this comprehensive user manual, will help accelerate development and distribution of glycoprotein therapeutics and vaccines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data discussed in this manuscript were generated as part of our previously published work22,23,24. Source data are provided with this paper.
References
US Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases 13th edn (eds Hamborsky, J., Korger, A. & Wolfe, C.) (Public Health Foundation, 2015).
FDA okays marketing of human insulin. Chem. Eng. News Arch. 60, 5 (1982).
Mullin, R. Cost to develop new pharmaceutical drug now exceeds $2.5 B. Scientific American https://www.scientificamerican.com/article/cost-to-develop-new-pharmaceutical-drug-now-exceeds-2-5b/ (24 November 2014).
Thiel, K. A. Biomanufacturing, from bust to boom…to bubble? Nat. Biotechnol. 22, 1365 (2004).
Jayapal, K. P., Wlaschin, K. F., Hu, W. & Yap, M. G. S. Recombinant protein therapeutics from CHO cells-20 years and counting. Chem. Eng. Prog. 103, 40 (2007).
Dolsten, M. & Sogaard, M. Precision medicine: an approach to R&D for delivering superior medicines to patients. Clin. Transl. Med. 1, 7 (2012).
Ashok, A., Brison, M. & LeTallec, Y. Improving cold chain systems: challenges and solutions. Vaccine 35, 2217–2223 (2017).
Kumru, O. S. et al. Vaccine instability in the cold chain: mechanisms, analysis and formulation strategies. Biologicals 42, 237–259 (2014).
Choi, E. J. & Ling, G. S. Battlefield medicine: paradigm shift for pharmaceuticals manufacturing. PDA J. Pharm. Sci. Technol. 68, 312 (2014).
Perez-Pinera, P. et al. Synthetic biology and microbioreactor platforms for programmable production of biologics at the point-of-care. Nat. Commun. 7, 12211 (2016).
Crowell, L. E. et al. On-demand manufacturing of clinical-quality biopharmaceuticals. Nat. Biotechnol. 36, 988–995 (2018).
Pardee, K. et al. Portable, on-demand biomolecular manufacturing. Cell 167, 248–259.e12 (2016).
Salehi, A. S. et al. Cell-free protein synthesis of a cytotoxic cancer therapeutic: Onconase production and a just-add-water cell-free system. Biotechnol. J. 11, 274–281 (2016).
Adiga, R. et al. Point-of-care production of therapeutic proteins of good-manufacturing-practice quality. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-018-0259-1 (2018).
Adiga, R. et al. Manufacturing biological medicines on demand: Safety and efficacy of granulocyte colony-stimulating factor in a mouse model of total body irradiation. Biotechnol. Prog. 36, e2970 (2020).
Kightlinger, W., Warfel, K. F., DeLisa, M. P. & Jewett, M. C. Synthetic glycobiology: parts, systems, and applications. ACS Synth. Biol. 9, 1534–1562 (2020).
Sethuraman, N. & Stadheim, T. A. Challenges in therapeutic glycoprotein production. Curr. Opin. Biotechnol. 17, 341–346 (2006).
Sola, R. J. & Griebenow, K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 98, 1223–1245 (2009).
Lin, C. W. et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc. Natl Acad. Sci. USA 112, 10611–10616 (2015).
Elliott, S. et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat. Biotechnol. 21, 414–421 (2003).
Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 (2009).
Stark, J. C. et al. On-demand biomanufacturing of protective conjugate vaccines. Sci. Adv. 7, eabe9444 (2021).
Jaroentomeechai, T. et al. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat. Commun. 9, 2686 (2018).
Hershewe, J. M. et al. Improving cell-free glycoprotein synthesis by characterizing and enriching native membrane vesicles. Nat. Commun. 12, 2363 (2021).
Schoborg, J. A. et al. A cell-free platform for rapid synthesis and testing of active oligosaccharyltransferases. Biotechnol. Bioeng. 115, 739–750 (2017).
Jaroentomeechai, T. et al. A pipeline for studying and engineering single-subunit oligosaccharyltransferases. Methods Enzymol. 597, 55–81 (2017).
Kightlinger, W. et al. A cell-free biosynthesis platform for modular construction of protein glycosylation pathways. Nat. Commun. 10, 5404 (2019).
Lin, L. et al. Sequential glycosylation of proteins with substrate-specific N-glycosyltransferases. ACS Cent. Sci. 6, 144–154 (2020).
Natarajan, A. et al. Engineering orthogonal human O-linked glycoprotein biosynthesis in bacteria. Nat. Chem. Biol. 16, 1062–1070 (2020).
Warfel, K. F. et al. A low-cost, thermostable, cell-free protein synthesis platform for on demand production of conjugate vaccines. ACS Synth. Biol. 12, 95–107 (2023).
Silverman, A. D., Karim, A. S. & Jewett, M. C. Cell-free gene expression: an expanded repertoire of applications. Nat. Rev. Genet. 21, 151–170 (2020).
Perez, J. G., Stark, J. C. & Jewett, M. C. Cell-free synthetic biology: engineering beyond the cell. Cold Spring Harb. Perspect. Biol. 8, a023853 (2016).
Hershewe, J., Kightlinger, W. & Jewett, M. C. Cell-free systems for accelerating glycoprotein expression and biomanufacturing. J. Ind. Microbiol. Biotechnol. 47, 977–991 (2020).
Williams, A. J. et al. A low-cost recombinant glycoconjugate vaccine confers immunogenicity and protection against enterotoxigenic Escherichia coli infections in mice. Preprint at bioRxiv https://doi.org/10.1101/2022.10.31.514630 (2022).
Tarui, H., Imanishi, S. & Hara, T. A novel cell-free translation/glycosylation system prepared from insect cells. J. Biosci. Bioeng. 90, 508–514 (2000).
Moreno, S. N., Ip, H. S. & Cross, G. A. An mRNA-dependent in vitro translation system from Trypanosoma brucei. Mol. Biochem. Parasitol. 46, 265–274 (1991).
Shibutani, M., Kim, E., Lazarovici, P., Oshima, M. & Guroff, G. Preparation of a cell-free translation system from PC12 cell. Neurochem. Res. 21, 801–807 (1996).
Mikami, S., Kobayashi, T., Yokoyama, S. & Imataka, H. A hybridoma-based in vitro translation system that efficiently synthesizes glycoproteins. J. Biotechnol. 127, 65–78 (2006).
Brodel, A. K. et al. IRES-mediated translation of membrane proteins and glycoproteins in eukaryotic cell-free systems. PLoS ONE 8, e82234 (2013).
Gurramkonda, C. et al. Improving the recombinant human erythropoietin glycosylation using microsome supplementation in CHO cell-free system. Biotechnol. Bioeng. 115, 1253–1264 (2018).
Lingappa, V. R., Lingappa, J. R., Prasad, R., Ebner, K. E. & Blobel, G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc. Natl Acad. Sci. USA 75, 2338–2342 (1978).
Rothblatt, J. A. & Meyer, D. I. Secretion in yeast: reconstitution of the translocation and glycosylation of alpha-factor and invertase in a homologous cell-free system. Cell 44, 619–628 (1986).
Valderrama-Rincon, J. D. et al. An engineered eukaryotic protein glycosylation pathway in Escherichia coli. Nat. Chem. Biol. 8, 434–436 (2012).
Feldman, M. F. et al. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl Acad. Sci. USA 102, 3016–3021 (2005).
Kightlinger, W. et al. Design of glycosylation sites by rapid synthesis and analysis of glycosyltransferases. Nat. Chem. Biol. 14, 627–635 (2018).
Ollis, A. A. et al. Substitute sweeteners: diverse bacterial oligosaccharyltransferases with unique N-glycosylation site preferences. Sci. Rep. 5, 15237 (2015).
Pan, C. et al. Biosynthesis of conjugate vaccines using an O-linked glycosylation system. MBio 7, e00443–16 (2016).
Harding, C. M. et al. A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host. Nat. Commun. 10, 891 (2019).
Kowarik, M. et al. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J. 25, 1957–1966 (2006).
Li, M. et al. Shotgun scanning glycomutagenesis: a simple and efficient strategy for constructing and characterizing neoglycoproteins. Proc. Natl Acad. Sci. USA 118, e2107440118 (2021).
Kwon, Y. C. & Jewett, M. C. High-throughput preparation methods of crude extract for robust cell-free protein synthesis. Sci. Rep. 5, 8663 (2015).
Kim, D. M. & Swartz, J. R. Efficient production of a bioactive, multiple disulfide-bonded protein using modified extracts of Escherichia coli. Biotechnol. Bioeng. 85, 122–129 (2004).
Cai, Q. et al. A simplified and robust protocol for immunoglobulin expression in Escherichia coli cell-free protein synthesis systems. Biotechnol. Prog. 31, 823–831 (2015).
Calhoun, K. A. & Swartz, J. R. An economical method for cell-free protein synthesis using glucose and nucleoside monophosphates. Biotechnol. Prog. 21, 1146–1153 (2005).
Jewett, M. C. & Swartz, J. R. Mimicking the Escherichia coli cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. Biotechnol. Bioeng. 86, 19–26 (2004).
Cuccui, J. et al. Exploitation of bacterial N-linked glycosylation to develop a novel recombinant glycoconjugate vaccine against Francisella tularensis. Open Biol. 3, 130002 (2013).
Celik, E. et al. Glycoarrays with engineered phages displaying structurally diverse oligosaccharides enable high-throughput detection of glycan–protein interactions. Biotechnol. J. 10, 199–209 (2015).
Valvano, M. A. & Crosa, J. H. Molecular cloning and expression in Escherichia coli K-12 of chromosomal genes determining the O7 lipopolysaccharide antigen of a human invasive strain of E. coli O7:K1. Infect. Immun. 57, 937–943 (1989).
Ollis, A. A., Zhang, S., Fisher, A. C. & DeLisa, M. P. Engineered oligosaccharyltransferases with greatly relaxed acceptor-site specificity. Nat. Chem. Biol. 10, 816–822 (2014).
Acknowledgements
J.C.S. acknowledges support from NIH/NCI F32 Postdoctoral Fellowship 1F32CA250324-01 and American Cancer Society Postdoctoral Fellowship PF-20-143-01-LIB. T.J. acknowledges support from the European Molecular Biology Organization Postdoctoral Fellowship 336-2021. K.F.W. acknowledges support from the National Defense Science and Engineering (NDSEG) Fellowship Program (ND-CEN-013-096). M.C.J. acknowledges support from the David and Lucile Packard Foundation, the Camille Dreyfus Teacher-Scholar Program, the Defense Threat Reduction Agency Grants HDTRA1-15-10052, HDTRA-12-11-0038 and HDTRA-12-01-0004, the Army Research Office Grants W911NF-20-1-0195, W911NF-18-1-0200 and W911NF-16-1-0372, the Army Contracting Command Contract W52P1J-21-9-3023 and DARPA Grant W911NF-23-2-0039.
Author information
Authors and Affiliations
Contributions
J.C.S., T.J., K.F.W. and J.M.H. wrote and edited the manuscript. J.C.S., M.P.D. and M.C.J. conceptualized the manuscript. M.P.D. and M.C.J. directed the research and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
M.C.J. is a cofounder of SwiftScale Biologics, Stemloop, Inc., Design Pharmaceuticals, and Pearl Bio. M.P.D. has interests in Glycobia Inc. and Versatope Inc. M.P.D. and M.C.J. have an interest in SwiftScale Biologics. M.C.J.’s and M.P.D.’s interests are reviewed and managed by Northwestern University and Cornell University, respectively, in accordance with their conflict of interest policies.
Peer review
Peer review information
Nature Protocols thanks Nigel Forest Reuel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Stark, J. C. et al. Sci. Adv. 7, eabe9444 (2021): https://doi.org/10.1126/sciadv.abe9444
Jaroentomeechai, T. et al. Nat. Commun. 9, 2686 (2018): https://doi.org/10.1038/s41467-018-05110-x
Natarajan, A. et al. Nat. Chem. Biol. 16, 1062–1070 (2020): https://doi.org/10.1038/s41589-020-0595-9
Hershewe, J. M. et al. Nat. Commun. 12, 2363 (2021): https://doi.org/10.1038/s41467-021-22329-3
Warfel, K. F. et al. ACS Synth. Biol. 12, 95–107 (2023): https://doi.org/10.1021/acssynbio.2c00392
Source data
Source Data Fig. 3
Statistical source data for Fig. 3b,c and uncropped western blot for Fig. 3c.
Source Data Fig. 4
Statistical source data for Fig. 4c and uncropped western blots for Fig. 4b,d.
Source Data Fig. 5
Uncropped western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stark, J.C., Jaroentomeechai, T., Warfel, K.F. et al. Rapid biosynthesis of glycoprotein therapeutics and vaccines from freeze-dried bacterial cell lysates. Nat Protoc 18, 2374–2398 (2023). https://doi.org/10.1038/s41596-022-00799-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00799-z
This article is cited by
-
Cell-free systems for a multi-pronged approach to next-generation therapeutics and diagnostics
Biotechnology and Bioprocess Engineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.