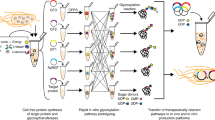
Abstract
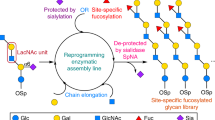
A major objective of synthetic glycobiology is to re-engineer existing cellular glycosylation pathways from the top down or construct non-natural ones from the bottom up for new and useful purposes. Here, we have developed a set of orthogonal pathways for eukaryotic O-linked protein glycosylation in Escherichia coli that installed the cancer-associated mucin-type glycans Tn, T, sialyl-Tn and sialyl-T onto serine residues in acceptor motifs derived from different human O-glycoproteins. These same glycoengineered bacteria were used to supply crude cell extracts enriched with glycosylation machinery that permitted cell-free construction of O-glycoproteins in a one-pot reaction. In addition, O-glycosylation-competent bacteria were able to generate an antigenically authentic Tn-MUC1 glycoform that exhibited reactivity with antibody 5E5, which specifically recognizes cancer-associated glycoforms of MUC1. We anticipate that the orthogonal glycoprotein biosynthesis pathways developed here will provide facile access to structurally diverse O-glycoforms for a range of important scientific and therapeutic applications.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this Article (and its Supplementary Information) or are available from the corresponding authors on reasonable request. All unique materials used in this work are available from the authors. Source Data are provided with this paper.
References
Khoury, G. A., Baliban, R. C. & Floudas, C. A. Proteome-wide post-translational modification statistics: frequency analysis and curation of the Swiss-Prot database. Sci. Rep. 1, 90 (2011).
Walsh, C. T., Garneau-Tsodikova, S. & Gatto, G. J. Jr Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. 44, 7342–7372 (2005).
Abu-Qarn, M., Eichler, J. & Sharon, N. Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Curr. Opin. Struct. Biol. 18, 544–550 (2008).
Varki, A. Biological roles of glycans. Glycobiology 27, 3–49 (2017).
Sethuraman, N. & Stadheim, T. A. Challenges in therapeutic glycoprotein production. Curr. Opin. Biotechnol. 17, 341–346 (2006).
Rappuoli, R. Glycoconjugate vaccines: principles and mechanisms. Sci. Transl. Med. 10, eaat4615 (2018).
Valderrama-Rincon, J. D. et al. An engineered eukaryotic protein glycosylation pathway in Escherichia coli. Nat. Chem. Biol. 8, 434–436 (2012).
Meuris, L. et al. GlycoDelete engineering of mammalian cells simplifies N-glycosylation of recombinant proteins. Nat. Biotechnol. 32, 485–489 (2014).
Hamilton, S. R. et al. Production of complex human glycoproteins in yeast. Science 301, 1244–1246 (2003).
Jaroentomeechai, T. et al. Single-pot glycoprotein biosynthesis using a cell-free transcription-translation system enriched with glycosylation machinery. Nat. Commun. 9, 2686 (2018).
Kightlinger, W. et al. A cell-free biosynthesis platform for modular construction of protein glycosylation pathways. Nat. Commun. 10, 5404 (2019).
Feldman, M. F. et al. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl Acad. Sci. USA 102, 3016–3021 (2005).
Tytgat, H. L. P. et al. Cytoplasmic glycoengineering enables biosynthesis of nanoscale glycoprotein assemblies. Nat. Commun. 10, 5403 (2019).
Aumiller, J. J., Hollister, J. R. & Jarvis, D. L. A transgenic insect cell line engineered to produce CMP-sialic acid and sialylated glycoproteins. Glycobiology 13, 497–507 (2003).
Chang, M. M. et al. Small-molecule control of antibody N-glycosylation in engineered mammalian cells. Nat. Chem. Biol. 15, 730–736 (2019).
Yang, Z. et al. Engineering mammalian mucin-type O-glycosylation in plants. J. Biol. Chem. 287, 11911–11923 (2012).
Elliott, S. et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat. Biotechnol. 21, 414–421 (2003).
Huang, W., Giddens, J., Fan, S. Q., Toonstra, C. & Wang, L. X. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J. Am. Chem. Soc. 134, 12308–12318 (2012).
Broecker, F. et al. Multivalent display of minimal Clostridium difficile glycan epitopes mimics antigenic properties of larger glycans. Nat. Commun. 7, 11224 (2016).
Umana, P., Jean-Mairet, J., Moudry, R., Amstutz, H. & Bailey, J. E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat. Biotechnol. 17, 176–180 (1999).
Ilyushin, D. G. et al. Chemical polysialylation of human recombinant butyrylcholinesterase delivers a long-acting bioscavenger for nerve agents in vivo. Proc. Natl Acad. Sci. USA 110, 1243–1248 (2013).
Schwarz, F. & Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21, 576–582 (2011).
Choi, B. K. et al. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc. Natl Acad. Sci. USA 100, 5022–5027 (2003).
Natarajan, A., Jaroentomeechai, T., Li, M., Glasscock, C. J. & DeLisa, M. P. Metabolic engineering of glycoprotein biosynthesis in bacteria. Emerg. Top. Life Sci. 2, 419–432 (2018).
Ollis, A. A., Zhang, S., Fisher, A. C. & DeLisa, M. P. Engineered oligosaccharyltransferases with greatly relaxed acceptor-site specificity. Nat. Chem. Biol. 10, 816–822 (2014).
Henderson, G. E., Isett, K. D. & Gerngross, T. U. Site-specific modification of recombinant proteins: a novel platform for modifying glycoproteins expressed in E. coli. Bioconjug. Chem. 22, 903–912 (2011).
Mueller, P. et al. High level in vivo mucin-type glycosylation in Escherichia coli. Microb. Cell Fact. 17, 168 (2018).
Du, T. et al. A bacterial expression platform for production of therapeutic proteins containing human-like O-linked glycans. Cell Chem. Biol. 26, 203–212 (2019).
Faridmoayer, A. et al. Extreme substrate promiscuity of the Neisseria oligosaccharyl transferase involved in protein O-glycosylation. J. Biol. Chem. 283, 34596–34604 (2008).
Pan, C. et al. Biosynthesis of conjugate vaccines using an O-linked glycosylation system. mBio 7, e00443–16 (2016).
Valentine, J. L. et al. Immunization with outer membrane vesicles displaying designer glycotopes yields class-switched, glycan-specific antibodies. Cell Chem. Biol. 23, 655–665 (2016).
Harding, C. M., Haurat, M. F., Vinogradov, E. & Feldman, M. F. Distinct amino acid residues confer one of three UDP-sugar substrate specificities in Acinetobacter baumannii PglC phosphoglycosyltransferases. Glycobiology 28, 522–533 (2018).
Fisher, A. C. et al. Production of secretory and extracellular N-linked glycoproteins in Escherichia coli. Appl. Environ. Microbiol. 77, 871–881 (2011).
Tarp, M. A. & Clausen, H. Mucin-type O-glycosylation and its potential use in drug and vaccine development. Biochim. Biophys. Acta 1780, 546–563 (2008).
Yang, G. et al. Fluorescence activated cell sorting as a general ultra-high-throughput screening method for directed evolution of glycosyltransferases. J. Am. Chem. Soc. 132, 10570–10577 (2010).
Glasscock, C. J. et al. A flow cytometric approach to engineering Escherichia coli for improved eukaryotic protein glycosylation. Metab. Eng. 47, 488–495 (2018).
Yates, L. E. et al. Glyco-recoded Escherichia coli: recombineering-based genome editing of native polysaccharide biosynthesis gene clusters. Metab. Eng. 53, 59–68 (2019).
Hershewe, J. M. et al. Improving cell-free glycoprotein synthesis by characterizing and enriching native membrane vesicles. Preprint at bioRxiv https://www.biorxiv.org/content/10.1101/2020.07.19.211201v2 (2020).
Lai, P. H., Everett, R., Wang, F. F., Arakawa, T. & Goldwasser, E. Structural characterization of human erythropoietin. J. Biol. Chem. 261, 3116–3121 (1986).
Maier, A. G. et al. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 9, 87–92 (2003).
Gendler, S., Taylor-Papadimitriou, J., Duhig, T., Rothbard, J. & Burchell, J. A highly immunogenic region of a human polymorphic epithelial mucin expressed by carcinomas is made up of tandem repeats. J. Biol. Chem. 263, 12820–12823 (1988).
Mazor, Y., Keydar, I. & Benhar, I. Humanization and epitope mapping of the H23 anti-MUC1 monoclonal antibody reveals a dual epitope specificity. Mol. Immunol. 42, 55–69 (2005).
Sorensen, A. L. et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance. Glycobiology 16, 96–107 (2006).
Ju, T. & Cummings, R. D. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl Acad. Sci. USA 99, 16613–16618 (2002).
Skretas, G. et al. Expression of active human sialyltransferase ST6GalNAcI in Escherichia coli. Micro. Cell Fact. 8, 50 (2009).
Schulz, B. L. et al. Identification of bacterial protein O-oligosaccharyltransferases and their glycoprotein substrates. PLoS ONE 8, e62768 (2013).
von Mensdorff-Pouilly, S. et al. Reactivity of natural and induced human antibodies to MUC1 mucin with MUC1 peptides and N-acetylgalactosamine (GalNAc) peptides. Int. J. Cancer 86, 702–712 (2000).
Apostolopoulos, V. et al. A glycopeptide in complex with MHC class I uses the GalNAc residue as an anchor. Proc. Natl Acad. Sci. USA 100, 15029–15034 (2003).
Ninkovic, T. & Hanisch, F. G. O-glycosylated human MUC1 repeats are processed in vitro by immunoproteasomes. J. Immunol. 179, 2380–2388 (2007).
Lakshminarayanan, V. et al. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl Acad. Sci. USA 109, 261–266 (2012).
Coyne, M. J. et al. Phylum-wide general protein O-glycosylation system of the Bacteroidetes. Mol. Microbiol. 88, 772–783 (2013).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Natarajan, A., Haitjema, C. H., Lee, R., Boock, J. T. & DeLisa, M. P. An engineered survival-selection assay for extracellular protein expression uncovers hypersecretory phenotypes in Escherichia coli. ACS Synth. Biol. 6, 875–883 (2017).
Shanks, R. M., Caiazza, N. C., Hinsa, S. M., Toutain, C. M. & O’Toole, G. A. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl. Environ. Microbiol. 72, 5027–5036 (2006).
Dykxhoorn, D. M., St Pierre, R. & Linn, T. A set of compatible tac promoter expression vectors. Gene 177, 133–136 (1996).
Apostolopoulos, V., Karanikas, V., Haurum, J. S. & McKenzie, I. F. Induction of HLA-A2-restricted CTLs to the mucin 1 human breast cancer antigen. J. Immunol. 159, 5211–5218 (1997).
Fierfort, N. & Samain, E. Genetic engineering of Escherichia coli for the economical production of sialylated oligosaccharides. J. Biotechnol. 134, 261–265 (2008).
Cox, E. C. et al. Antibody-mediated endocytosis of polysialic acid enables intracellular delivery and cytotoxicity of a glycan-directed antibody–drug conjugate. Cancer Res. 79, 1810–1821 (2019).
Dodev, T. S. et al. A tool kit for rapid cloning and expression of recombinant antibodies. Sci. Rep. 4, 5885 (2014).
Jewett, M. C. & Swartz, J. R. Mimicking the Escherichia coli cytoplasmic environment activates long-lived and efficient cell-free protein synthesis. Biotechnol. Bioeng. 86, 19–26 (2004).
Acknowledgements
We thank R. Lee and S. Murphy for their contributions working with GT enzymes, L. Yates for helpful discussions with glyco-recoding, D. Mills for helpful discussions regarding O-OSTs, M. Paszek, J. Hershewe, K. Warfel, J. Stark, and M. Jewett for helpful discussions and provision of reagents, M. Li for technical advice and J. Wilson, J. Brooks and J. Merritt for help with vector design and yeast-based recombineering. We are also grateful to R. Bhawal and S. Zhang of the Proteomics and Metabolomics Core Facility in the Cornell Institute of Biotechnology for assistance with LC-MS. This work was supported by the Defense Threat Reduction Agency (GRANT11631647 to M.P.D.), National Science Foundation (grant no. CBET-1605242 to M.P.D.) and National Institutes of Health (grant no. 1R01GM127578-01 to M.P.D.). Glycomics analysis was supported in part by the National Institutes of Health (grant no. 1S10OD018530 to P.A.). The work was also supported by seed project funding (to M.P.D.) through the National Institutes of Health-funded Cornell Center on the Physics of Cancer Metabolism (supporting grant no. 1U54CA210184-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. T.J. was supported by a Royal Thai Government Fellowship and also a Cornell Fleming Graduate Scholarship. E.C.C. was supported by a National Institutes of Health Chemical-Biology Interface (CBI) training fellowship (supporting grant no. T32GM008500).
Author information
Authors and Affiliations
Contributions
A.N. designed and performed all research, analyzed all data and wrote the paper. T.J. designed and performed research related to cell-free glycosylation and analyzed data. M.C.-S. and O.Y. performed research related to constructing and testing glycan biosynthetic pathways. J.C.M. performed research related to testing different proteins for glycosylation. E.C.C. performed research related to antibody-based detection of different MUC1 glycoforms. A.S., M.V., S.V., J.D.V. and P.A. performed mass spectrometry analysis and aided in data interpretation. M.P.D. directed research, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.P.D. has a financial interest in Glycobia, Inc. and Versatope, Inc. M.P.D.’s interests are reviewed and managed by Cornell University in accordance with their conflict of interest policies. All authors declare no other competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 MS/MS fragmentation analysis of Tn-modified glycoprotein.
EThcD fragmentation analysis of glycosylated peptide 397NVGGDLDWPAAAS(HexNAc)APQPGKPPR418 derived from MBPMOOR by trypsin digestion. The spectrum identifies the neutral loss pattern of the single HexNAc monosaccharide, corresponding oxonium ions, and fragments of the glycopeptide (c and z ions), validating the glycosylation and the site of glycosylation at S409 within the 8-residue WPAAASAP core sequence of MBPMOOR.
Extended Data Fig. 2 Flow cytometric screening of Gal transferases for biosynthesis of T antigen.
(a) Schematic of flow cytometric screen to evaluate candidate Gal transferases (GalTs) for their ability to generate lipid-linked T antigen. Once formed, the T antigen is subsequently flipped to periplasm by the native E. coli flippase, Wzx, transferred to lipid A core by the promiscuous O-antigen ligase WaaL native to E. coli, and ultimately displayed on the cell surface. Cells are labeled with FITC-conjugated PNA that specifically binds the T antigen. (b) Flow cytometric analysis of PNA-labeled E. coli MC4100 ΔwecA (MCΔw) (yellow) or MC4100 ΔwecA ΔwaaL (MCΔww) (gray) carrying no plasmid, plasmid pOG-Tn, or plasmid pOG-Tn modified with one of the candidate GalT enzymes as indicated. (c) Flow cytometric analysis of PNA-labeled MCΔw (yellow) or MCΔww (gray) carrying no plasmid, plasmid pOG-T (producing T antigen glycan with EcWbwC), or plasmid pOG-TΔgne (encoding T antigen pathway but lacking CjGne epimerase). In (b) and (c), unlabeled MCΔw cells (white) were included as negative controls. Inset histograms show representative flow cytometric data used to generate mean fluorescence intensity data. See Supplementary Fig. 1 for flow cytometry gating strategy.
Extended Data Fig. 3 MS/MS fragmentation analysis of T-modified glycoprotein.
EThcD fragmentation analysis of glycosylated peptide 397NVGGDLDWPAAAS(HexHexNAc)APQPGKPPR418 derived from MBPMOOR by trypsin digestion. The spectrum identifies the neutral loss pattern of the HexHexNAc disaccharide, corresponding oxonium ions, and fragments of the glycopeptide (c and z ions), validating the glycosylation and the site of glycosylation at S409 within the 8-residue WPAAASAP core sequence of MBPMOOR.
Extended Data Fig. 4 Orthogonal biosynthesis of sialylated O-glycoforms in E. coli.
(a) Nano-LC-MS/MS analysis of purified acceptor protein generated by nanA-deficient E. coli cells carrying plasmid pConNeuDBAC for CMP-NeuNAc biosynthesis along with pOG-T-NgPglO and pEXT-spDsbA-MBPMOOR-EcWbwA. Sequence coverage of 88% was obtained for the MBPMOOR protein in the analysis. Spectrum reveals a predominant species (80% abundance) corresponding to the indicated peptide fragment bearing a single HexHexNAc modification as well as three less abundant species bearing a single NeuNAcHexHexNAc, a single HexNAc, and no modification (16%, 2%, and 2%, respectively). (b) Same as in (a) but with purified acceptor protein generated by nanA-deficient glyco-recoded cells carrying pOG-Tn-NgPglO and pEXT-spDsbA-MBPMOOR-PspST6. Sequence coverage of 92% was obtained for MBPMOOR in the analysis. Spectrum reveals a predominant species (90% abundance) corresponding to the indicated peptide fragment bearing a single HexNAc modification as well as two less abundant species bearing a single NeuNAcHexNAc and no modification (2% and 9%, respectively). Arrow denotes modified serine (bold underlined font) as determined by EThcD fragmentation analysis.
Extended Data Fig. 5 MS/MS fragmentation analysis of ST- and STn-modified glycoproteins.
EThcD fragmentation analysis of glycosylated peptide 397NVGGDLDWPAAAS(NeuNAcHexHexNAc)APQPGKPPR418 derived from (a) ST-modified MBPMOOR and (b) STn-modified MBPMOOR that were subjected to trypsin digestion. The spectrum identifies the neutral loss pattern of the single NeuNAc and Hex monosaccharides, corresponding oxonium ions, and fragments of the glycopeptide (c and z ions), validating the glycosylation and site of glycosylation at S409 within the 8-residue WPAAASAP core sequence of MBPMOOR.
Extended Data Fig. 6 Yield determination for MBPMOOR modified with different O-glycans.
(a) Coomassie-stained SDS-PAGE gel showing MBPMOOR proteins purified from different strains. MBPMOOR bearing Tn or T antigens was produced in CLM25 cells co-transformed with pEXT-based plasmid for acceptor protein and appropriate sialyltransferase expression and either pOG-Tn-NgPglO or pOG-T-NgPglO plasmids, respectively. MBPMOOR bearing STn or ST antigens was produced in glyco-recoded cells carrying the CMP-NeuNAc biosynthesis pathway in the genome and co-transformed with pEXT-based plasmid for acceptor protein expression and either pOG-Tn-NgPglO or pOG-T-NgPglO plasmids, respectively. CLM25 cells co-transformed with only the pEXT-based plasmid for expressing MBPMOOR (agly) and appropriate sialyltransferase served as the control. Molecular weight (Mw) marker included on the left. SDS-PAGE gel is representative of three biological replicates. See Source Data for uncropped version of the image. (b) Yield of each glycoprotein calculated by multiplying the total yield times the percentage glycosylated (% gly), the latter of which was determined from nano-LC-MS/MS analysis of each glycoprotein product. Yield values are the average of three biological replicates and the error is the standard deviation of the mean.
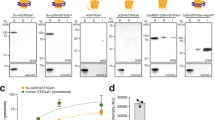
Extended Data Fig. 7 O-linked glycosylation of diverse protein targets.
(a) Immunoblot analysis of acceptor proteins purified from CLM25 cells co-transformed with pOG-T-NgPglO (+, top), pOG-T-NmPglL (+, bottom), or pOG-T without an O-OST (-) along with pEXT-based plasmid encoding each of the different protein targets as indicated. MBPMOOR and MBPMOORmut derived from the same cells served as positive and negative control, respectively. Blots were probed with anti-hexa-histidine antibody (6xHis) to detect acceptor proteins and PNA lectin to detect the T antigen. Molecular weight (Mw) markers are indicated on the left of each blot. All immunoblot results are representative of at least three biological replicates. (b, c) Same as in (a) with pOG-T-NmPglL (+) or pOG-T without NmPglL (-) along with pEXT-based plasmid encoding each of the different protein targets as indicated. See Source Data for uncropped versions of the images.
Extended Data Fig. 8 Secretion of O-glycoproteins in the culture supernatant.
Immunoblot analysis of culture supernatants derived from CLM24 ΔyaiW cells co-transformed with pOG-T-NgPglO or pOG-T-NmPglL along with pEXT-based plasmid encoding YebF-MBPMOOR or YebF-MBPMOORmut as indicated. Mutation of acceptor serine to glycine in YebF-MBPMOORmut served as negative control. Blots were probed with anti-hexa-histidine antibody (6xHis) to detect acceptor proteins and PNA lectin to detect the T antigen. Molecular weight (Mw) markers are indicated on the left of each blot. Immunoblot results are representative of at least three biological replicates. See Source Data for uncropped versions of the images.
Extended Data Fig. 9 Orthogonal biosynthesis of different MUC1 O-glycoforms in E. coli.
Nano-LC-MS/MS analysis of purified acceptor protein generated by CLM25 cells carrying plasmid pOG-T-NgPglO along with pEXT-based plasmid for expression of different MUC1 constructs including: (a) MUC1_8; (b) MUC1_20; (c) MUC1_24; and (d) MUC1_41. Sequence coverage of 77% was obtained for MUC1_8, 78% for MUC1_20, 88% for MUC1_24, and 75% for MUC1_41 in the analysis. All spectra reveal a predominant species corresponding to the indicated peptide fragments bearing a single HexHexNAc modification. Additional less abundant species bearing a single HexNAc and no modification were observed in all cases. For MUC1_41, several doubly glycosylated species were also identified as minor species. Arrow denotes modified serine (bold underlined font) as determined by EThcD fragmentation analysis.
Extended Data Fig. 10 MS/MS fragmentation analysis of MUC1 O-glycoforms bearing the T antigen.
EThcD fragmentation analysis of glycosylated peptides derived by trypsin digestion. The spectrum identifies the neutral loss pattern of HexHexNAc disaccharide, corresponding oxonium ions, and fragments of the glycopeptide (c and z ions), validating the glycosylation and the sites of glycosylation (S409 in MUC1_8; S415 in MUC1_20; S417 in MUC1_24 and S417 of MUC1_41) within relevant MUC1 peptides as indicated in the inset sequences.
Supplementary information
Supplementary Information
Supplementary Table 1 and Fig. 1.
Source data
Source Data Fig. 2
Unprocessed immunoblots.
Source Data Fig. 4
Unprocessed immunoblots.
Source Data Fig. 5
Unprocessed immunoblots.
Source Data Extended Data Fig. 6
Unprocessed immunoblots.
Source Data Extended Data Fig. 7
Unprocessed immunoblots.
Source Data Extended Data Fig. 8
Unprocessed immunoblots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Natarajan, A., Jaroentomeechai, T., Cabrera-Sánchez, M. et al. Engineering orthogonal human O-linked glycoprotein biosynthesis in bacteria. Nat Chem Biol 16, 1062–1070 (2020). https://doi.org/10.1038/s41589-020-0595-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0595-9
This article is cited by
-
Essential factors, advanced strategies, challenges, and approaches involved for efficient expression of recombinant proteins in Escherichia coli
Archives of Microbiology (2024)
-
A high-throughput screening platform for enzymes active on mucin-type O-glycoproteins
Nature Chemical Biology (2023)
-
Rapid biosynthesis of glycoprotein therapeutics and vaccines from freeze-dried bacterial cell lysates
Nature Protocols (2023)
-
Omics-guided bacterial engineering of Escherichia coli ER2566 for recombinant protein expression
Applied Microbiology and Biotechnology (2023)
-
Strategies for efficient production of recombinant proteins in Escherichia coli: alleviating the host burden and enhancing protein activity
Microbial Cell Factories (2022)