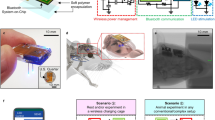
Abstract
Both in vivo neuropharmacology and optogenetic stimulation can be used to decode neural circuitry, and can provide therapeutic strategies for brain disorders. However, current neuronal interfaces hinder long-term studies in awake and freely behaving animals, as they are limited in their ability to provide simultaneous and prolonged delivery of multiple drugs, are often bulky and lack multifunctionality, and employ custom control systems with insufficiently versatile selectivity for output mode, animal selection and target brain circuits. Here, we describe smartphone-controlled, minimally invasive, soft optofluidic probes with replaceable plug-like drug cartridges for chronic in vivo pharmacology and optogenetics with selective manipulation of brain circuits. We demonstrate the use of the probes for the control of the locomotor activity of mice for over four weeks via programmable wireless drug delivery and photostimulation. Owing to their ability to deliver both drugs and photopharmacology into the brain repeatedly over long time periods, the probes may contribute to uncovering the basis of neuropsychiatric diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others

Data availability
The authors declare that all data supporting the results in this study are available within the paper and its Supplementary Information.
Code availability
All BLE firmware is available in the Supplementary Information.
References
Felix-Ortiz, A. C. et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013).
Jennings, J. H. et al. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228 (2013).
Kramer, R. H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nat. Neurosci. 16, 816–823 (2013).
McCall, J. G. & Jeong, J.-W. Minimally invasive probes for programmed microfluidic delivery of molecules in vivo. Curr. Opin. Pharmacol. 36, 78–85 (2017).
Sim, J. Y., Haney, M. P., Park, S. I., McCall, J. G. & Jeong, J.-W. Microfluidic neural probes: in vivo tools for advancing neuroscience. Lab. Chip 17, 1406–1435 (2017).
Park, S. et al. One-step optogenetics with multifunctional flexible polymer fibers. Nat. Neurosci. 20, 612–619 (2017).
Canales, A. et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat. Biotechnol. 33, 277–284 (2015).
Jeong, J.-W. et al. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell 162, 662–674 (2015).
McCall, J. G. et al. Preparation and implementation of optofluidic neural probes for in vivo wireless pharmacology and optogenetics. Nat. Protoc. 12, 219–237 (2017).
Hashimoto, M., Hata, A., Miyata, T. & Hirase, H. Programmable wireless light-emitting diode stimulator for chronic stimulation of optogenetic molecules in freely moving mice. Neurophotonics 1, 011002 (2014).
Iwai, Y., Honda, S., Ozeki, H., Hashimoto, M. & Hirase, H. A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci. Res. 70, 124–127 (2011).
Kim, T. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
McCall, J. G. et al. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat. Protoc. 8, 2413–2428 (2013).
Noh, K. N. et al. Miniaturized, battery-free optofluidic systems with potential for wireless pharmacology and optogenetics. Small 14, 1702479 (2018).
Park, S. I. et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 33, 1280–1286 (2015).
Shin, G. et al. Flexible near-field wireless optoelectronics as subdermal implants for broad applications in optogenetics. Neuron 93, 509–521 (2017).
Montgomery, K. L. et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat. Methods 12, 969–974 (2015).
Alam, M., Chen, X. & Fernandez, E. A low-cost multichannel wireless neural stimulation system for freely roaming animals. J. Neural Eng. 10, 066010 (2013).
Ativanichayaphong, T., He, J. W., Hagains, C. E., Peng, Y. B. & Chiao, J.-C. A combined wireless neural stimulating and recording system for study of pain processing. J. Neurosci. Methods 170, 25–34 (2008).
Chang, C.-W. & Chiou, J.-C. A wireless and batteryless microsystem with implantable grid electrode/3-dimensional probe array for ECoG and extracellular neural recording in rats. Sensors 13, 4624–4639 (2013).
Park, S. I. et al. Ultraminiaturized photovoltaic and radio frequency powered optoelectronic systems for wireless optogenetics. J. Neural Eng. 12, 056002 (2015).
Pinnell, R. C., Dempster, J. & Pratt, J. Miniature wireless recording and stimulation system for rodent behavioural testing. J. Neural Eng. 12, 066015 (2015).
Wentz, C. T. et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J. Neural Eng. 8, 046021 (2011).
Yeh, A. J. et al. Wirelessly powering miniature implants for optogenetic stimulation. Appl. Phys. Lett. 103, 163701 (2013).
Massey, L. K. Permeability Properties of Plastics and Elastomers:A Guide to Packaging and Barrier Materials 2nd edn (William Andrew, 2003).
Frank, R., Bronzi, W., Castignani, G. & Engel, T. Bluetooth low energy: an alternative technology for VANET applications. in 2014 11th Annual Conference on Wireless On-demand Network Systems and Services (WONS) 104–107 (IEEE, 2014).
Getting Started with SimbleeCOM v1.0 (RF Digital Corp, 2015).
Jenck, F., Bozarth, M. & Wise, R. A. Contraversive circling induced by ventral tegmental microinjections of moderate doses of morphine and [D-Pen2, D-Pen5]enkephalin. Brain Res. 450, 382–386 (1988).
Devine, D. P. & Wise, R. A. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J. Neurosci. 14, 1978–1984 (1994).
Namburi, P., Al-Hasani, R., Calhoon, G. G., Bruchas, M. R. & Tye, K. M. Architectural representation of valence in the limbic system. Neuropsychopharmacology 41, 1697–1715 (2016).
Jennings, J. H., Rizzi, G., Stamatakis, A. M., Ung, R. L. & Stuber, G. D. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 (2013).
Al-Hasani, R. et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077 (2015).
McCall, J. G. et al. CRH Engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87, 605–620 (2015).
Dagdeviren, C. et al. Miniaturized neural system for chronic, local intracerebral drug delivery. Sci. Transl. Med. 10, eaan2742 (2018).
Spieth, S., Schumacher, A., Kallenbach, C., Messner, S. & Zengerle, R. The neuromedicator—a micropump integrated with silicon microprobes for drug delivery in neural research. J. Micromech. Microeng. 22, 065020 (2012).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Qiu, Y. & Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 53, 321–339 (2001).
Jang, K.-I. et al. Soft network composite materials with deterministic and bio-inspired designs. Nat. Commun. 6, 6566 (2015).
Liu, Y. et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2, e1601185 (2016).
Banghart, M. R. & Sabatini, B. L. Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron 73, 249–259 (2012).
Tochitsky, I. et al. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 81, 800–813 (2014).
Banala, S. et al. Photoactivatable drugs for nicotinic optopharmacology. Nat. Methods 15, 347–350 (2018).
Mehrabian, A. & Abousleiman, Y. General solutions to poroviscoelastic model of hydrocephalic human brain tissue. J. Theor. Biol. 291, 105–118 (2011).
Liu, Q., Wang, Z., Lou, Y. & Suo, Z. Elastic leak of a seal. Extrem. Mech. Lett. 1, 54–61 (2014).
Bruchas, M. R., Land, B. B., Lemos, J. C. & Chavkin, C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS ONE 4, e8528 (2009).
Siuda, E. R. et al. Spatiotemporal control of opioid signaling and behavior. Neuron 86, 923–935 (2015).
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT (grant nos. NRF-2018R1C1B6001706 and NRF-2018025230, J.-W.J.). This work was also supported by Mallinckrodt Professorship (M.R.B.), NIH (grant no. R01DA037152), NIDA Diversity Supplement (grant no. R01DA033396-S1, M.R.B. and A.G.), and grant no. NIDA F32DA043999 (D.C.C). Support was also provided by the Hope Center Viral Vectors Core. We thank J.-W. Yu (KAIST) for providing equipment to conduct BLE wireless transmission tests.
Author information
Authors and Affiliations
Contributions
R.Q., A.G., M.R.B. and J.-W.J. conceptualized the project and designed the experiments with methodology. R.Q., J.A. and A.A. fabricated and prepared the devices for behavioural tests. R.Q., A.G. and D.C.C. performed the in vivo behavioural, tracing and imaging experiments. R.Q., A.G., Z.Z., J.X., J.Y.S., C.Y.K., S.-H.B., B.C.L., K.I.J., J.X., M.R.B. and J.-W.J. performed the investigation, analysed the data and wrote up the modelling results. R.Q., Y.X., A.A. and F.L., designed the firmware and software. R.Q., A.G., D.C.C, M.R.B. and J.-W.J. wrote the paper. M.R.B. and J.-W.J. acquired funding and supervised the project. M.R.B. and J.-W.J. are co-senior authors.
Corresponding authors
Ethics declarations
Competing interests
M.R.B. is a co-founder and scientific advisor for Neurolux, Inc., a neurotechnology company that offers neuroscience tools. The device described in this study is not currently sold or manufactured by this company.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, figures, tables and video captions.
Supplementary Video 1
Highly precise and wirelessly reprogrammable temporal control for the sequenced delivery of two distinct fluids, with a 1 s delay in between.
Supplementary Video 2
Wireless operation of the plug-n-play optofluidic device.
Supplementary Video 3
Spatiotemporal heat analysis during fluid actuation using the IR camera.
Supplementary Video 4
Smartphone App Graphical User Interface for the wireless, selective and programmable control of multimodal plug-n-play optofluidic devices.
Supplementary Video 5
Demonstration of a dependent closed-loop concept through the SimbleeCOM Protocol, using model mice.
Supplementary Video 6
Wireless chronic drug delivery in a freely moving mouse.
Supplementary Video 7
Wireless selective control triggering of intra-VTA DAMGO release in a group of four mice during a 60-min open-field test.
Rights and permissions
About this article
Cite this article
Qazi, R., Gomez, A.M., Castro, D.C. et al. Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation. Nat Biomed Eng 3, 655–669 (2019). https://doi.org/10.1038/s41551-019-0432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0432-1
This article is cited by
-
Multilayer stretchable electronics with designs enabling a compact lateral form
npj Flexible Electronics (2024)
-
Design and validation of a low-cost photomodulator for in vivo photoactivation of a mGluR5 inhibitor
Biomedical Engineering Letters (2024)
-
Wearable and Implantable Light-Emitting Diodes and Their Biomedical Applications
Korean Journal of Chemical Engineering (2024)
-
Recent developments in multifunctional neural probes for simultaneous neural recording and modulation
Microsystems & Nanoengineering (2023)
-
Customizable, wireless and implantable neural probe design and fabrication via 3D printing
Nature Protocols (2023)