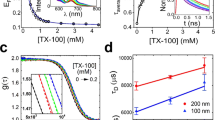
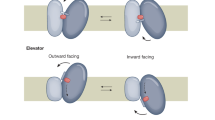
Abstract
The passive permeability of cell membranes is of key importance in biology, biomedical research and biotechnology as it determines the extent to which various molecules such as drugs, products of metabolism, and toxins can enter or leave the cell unaided by dedicated transport proteins. The quantification of passive solute permeation is possible with radio-isotope distribution experiments, spectroscopic measurements and molecular dynamics simulations. This protocol describes stopped-flow fluorimetry measurements performed on lipid vesicles and living yeast cells to estimate the osmotic permeability of water and solutes across (bio)membranes. Encapsulation of the fluorescent dye calcein into lipid vesicles allows monitoring of volume changes upon osmotic shifts of the medium via (de)quenching of the fluorophore, which we interpret using a well-defined physical model that takes the dynamics of the vesicles into account to calculate the permeability coefficients of solutes. We also present analogous procedures to probe weak acid and base permeability in vesicles and cells by using the read-out of encapsulated or expressed pH-sensitive probes. We describe the preparation of synthetic vesicles of varying lipid composition and determination of vesicle size distribution by dynamic light scattering. Data on membrane permeation are obtained using either conventional or stopped-flow kinetic fluorescence measurements on instruments available in most research institutes and are analyzed with a suite of user-friendly MATLAB scripts (https://doi.org/10.5281/zenodo.6511116). Collectively, these procedures provide a comprehensive toolbox for determining membrane permeability coefficients in a variety of experimental systems, and typically take 2–3 d.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
Raw data for Figs. 9 and 10 can be obtained from the corresponding author upon request and at https://github.com/jacopofrallicciardi/Data-Determining-small-molecule-permeations.
Code availability
All the MATLAB scripts used in this study are available at https://doi.org/10.5281/zenodo.6511116 (ref. 36).
References
Krämer, S. D. et al. When barriers ignore the “rule-of-five”. Adv. Drug Deliv. Rev. 101, 62–74 (2016).
Drew, D., North, R. A., Nagarathinam, K. & Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 121, 5289–5335 (2021).
Henderson, R. K., Fendler, K. & Poolman, B. Coupling efficiency of secondary active transporters. Curr. Opin. Biotechnol. 58, 62–71 (2019).
Fricke, W. Water transport and energy. Plant. Cell Environ. 40, 977–994 (2017).
Hannesschlaeger, C., Horner, A. & Pohl, P. Intrinsic membrane permeability to small molecules. Chem. Rev. 119, 5922–5953 (2019).
Kerns, E. H. & Li, D. Drug-like Properties: Concepts, Structure Optimization, Design and Methods from ADME to Toxicity (Academic, 2008).
Bangham, A. D., De Gier, J. & Greville, G. D. Osmotic properties and water permeability of phospholipid liquid crystals. Chem. Phys. Lipids 1, 225–246 (1967).
Gabba, M. et al. Weak acid permeation in synthetic lipid vesicles and across the yeast plasma membrane. Biophys. J. 118, 422–434 (2020).
Neijssel, O. M., Buurman, E. T. & de Mattos, M. J. T. The role of futile cycles in the energetics of bacterial growth. Biochim. Biophys. Acta 1018, 252–255 (1990).
Poolman, B. & Konings, W. N. Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J. Bacteriol. 170, 700–707 (1988).
Borgnia, M. J., Kozono, D., Calamita, G., Maloney, P. C. & Agre, P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol. 291, 1169–1179 (1999).
Hill, W. G. & Zeidel, M. L. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J. Biol. Chem. 275, 30176–30185 (2000).
Krylov, A. V., Pohl, P., Zeidel, M. L. & Hill, W. G. Water permeability of asymmetric planar lipid bilayers. J. Gen. Physiol. 118, 333–340 (2001).
Frallicciardi, J., Melcr, J., Siginou, E., Marrink, S. J. & Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commum. 13, 1605 (2022).
Chen, P. Y., Pearce, D. & Verkman, A. S. Membrane water and solute permeability determined quantitatively by self-quenching of an entrapped fluorophore. Biochemistry 27, 5713–5718 (1988).
Sha’afi, R. I., Rich, G. T., Sidel, V. W., Bossert, W. & Solomon, A. K. The effect of the unstirred layer on human red cell water permeability. J. Gen. Physiol. 50, 1377–1399 (1967).
Zeidel, M. L., Ambudkar, S. V., Smith, B. L. & Agre, P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry 31, 7436–7440 (1992).
Tsunoda, S. P., Wiesner, B., Lorenz, D., Rosenthal, W. & Pohl, P. Aquaporin-1, nothing but a water channel. J. Biol. Chem. 279, 11364–11367 (2004).
Preston, G. M., Carroll, T. P., Guggino, W. B. & Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256, 385–387 (1992).
Mlekoday, H. J., Moore, R. & Levitt, D. G. Osmotic water permeability of the human red cell. Dependence on direction of water flow and cell volume. J. Gen. Physiol. 81, 213–220 (1983).
Mathai, J. C., Sprott, G. D. & Zeidel, M. L. Molecular mechanisms of water and solute transport across archaebacterial lipid membranes. J. Biol. Chem. 276, 27266–27271 (2001).
Verkman, A. S. Water permeability measurement in living cells and complex tissues. J. Membr. Biol. 173, 73–87 (2000).
Saparov, S. M., Antonenko, Y. N., Koeppe, R. E. & Pohl, P. Desformylgramicidin: a model channel with an extremely high water permeability. Biophys. J. 79, 2526–2534 (2000).
Shatil-cohen, A. et al. Measuring the osmotic water permeability coefficient (Pf) of spherical cells: isolated plant protoplasts as an example. J. Vis. Exp. 92, e51652 (2014).
Vitali, V., Sutka, M., Amodeo, G., Chara, O. & Ozu, M. The water to solute permeability ratio governs the osmotic volume dynamics in beetroot vacuoles. Front. Plant Sci. 7, 1388 (2016).
Massari, S., Frigeri, L. & Azzone, G. F. Permeability to water, dimension of surface, and structural changes during swelling in rat liver mitochondria. J. Membr. Biol. 9, 57–70 (1972).
Brahm, J. Diffusional water permeability of human erythrocytes and their ghosts. J. Gen. Physiol. 79, 791–819 (1982).
Mathai, J. C. et al. Functional analysis of aquaporin-1 deficient red cells. J. Biol. Chem. 271, 1309–1313 (1996).
Pfeuffer, J. et al. Expression of aquaporins in Xenopus laevis oocytes and glial cells as detected by diffusion-weighted 1H NMR spectroscopy and photometric swelling assay. Biochim. Biophys. Acta 1448, 27–36 (1998).
Ye, R. & Verkman, A. S. Simultaneous optical measurement of osmotic and diffusional water permeability in cells and liposomes. Biochemistry 28, 824–829 (1989).
Karan, D. M. & Macey, R. I. The permeability of the human red cell to deuterium oxide (heavy water). J. Cell. Physiol. 104, 209–214 (1980).
Zhang, R. B. & Verkman, A. S. Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am. J. Physiol. Physiol. 260, C26–C34 (1991).
Lande, M. B., Priver, N. A. & Zeidel, M. L. Determinants of apical membrane permeabilities of barrier epithelia. Am. J. Physiol. Physiol. 267, C367–C374 (1994).
Levitt, D. G. & Mlekoday, H. J. Reflection coefficient and permeability of urea and ethylene glycol in the human red cell membrane. J. Gen. Physiol. 81, 239–253 (1983).
Gabba, M. & Poolman, B. Physiochemical modeling of vesicle dynamics upon osmotic upshift. Biophys. J. 118, 435–447 (2020).
Frallicciardi, J., Gabba, M. & Poolman, B. Determining small molecule permeation through lipid membranes. GitHub https://doi.org/10.5281/zenodo.6511116 (2022).
Rawicz, W., Smith, B. A., McIntosh, T. J., Simon, S. A. & Evans, E. Elasticity, strength, and water permeability of bilayers that contain raft microdomain-forming lipids. Biophys. J. 94, 4725–4736 (2008).
Lindahl, L., Genheden, S., Eriksson, L. A., Olsson, L. & Bettiga, M. Sphingolipids contribute to acetic acid resistance in Zygosaccharomyces bailii. Biotechnol. Bioeng. 113, 744–753 (2016).
Di, L. et al. The critical role of passive permeability in designing successful drugs. ChemMedChem 15, 1862–1874 (2020).
Dahan, A. & Miller, J. M. The solubility–permeability interplay and its implications in formulation design and development for poorly soluble drugs. AAPS J. 14, 244–251 (2012).
Pols, T. et al. A synthetic metabolic network for physicochemical homeostasis. Nat. Commun. 10, 4239 (2019).
Costa, A. P., Xu, X. & Burgess, D. J. Freeze–anneal–thaw cycling of unilamellar liposomes: effect on encapsulation efficiency. Pharm. Res. 31, 97–103 (2014).
Llu, L. & Yonetani, T. Preparation and characterization of liposome-encapsulated haemoglobin by a freeze–thaw method. J. Microencapsul. 11, 409–421 (1994).
Geertsma, E. R., Nik Mahmood, N. A. B., Schuurman-Wolters, G. K. & Poolman, B. Membrane reconstitution of ABC transporters and assays of translocator function. Nat. Protoc. 3, 256–266 (2008).
Lee, C. et al. A two-domain elevator mechanism for sodium/proton antiport. Nature 501, 573–577 (2013).
Herget, M. et al. Purification and reconstitution of the antigen transport complex TAP. J. Biol. Chem. 284, 33740–33749 (2009).
Sacerdote, M. G. & Szostak, J. W. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc. Natl Acad. Sci. USA 102, 6004–6008 (2005).
Hannesschlaeger, C., Barta, T., Pechova, H. & Pohl, P. The effect of buffers on weak acid uptake by vesicles. Biomolecules 9, 63 (2019).
Shi, S. et al. Hidden complexity in membrane permeabilization behavior of antimicrobial polycations. Phys. Chem. Chem. Phys. 23, 1475–1488 (2021).
Dutta, S., Watson, B., Mattoo, S. & Rochet, J.-C. Calcein release assay to measure membrane permeabilization by recombinant alpha-synuclein. Bio Protoc. 10, e3690 (2020).
Allen, T. M. & Cleland, L. G. Serum-induced leakage of liposome contents. Biochim. Biophys. Acta 597, 418–426 (1980).
De Gier, J., Mandersloot, J. G. & Van Deenen, L. L. M. Lipid composition and permeability of liposomes. Biochim. Biophys. Acta 150, 666–675 (1968).
De Gier, J., Mandersloot, J. G., Hupkes, J. V., McElhaney, R. N. & Van Beek, W. P. On the mechanism of non-electrolyte permeation through lipid bilayers and through biomembranes. Biochim. Biophys. Acta 233, 610–618 (1971).
Allen, T. M., Hong, K. & Papahadjopoulos, D. Membrane contact, fusion and hexagonal (HII) transitions in phosphatidylethanolamine liposomes. Biochemistry 29, 2976–2985 (1990).
D'Souza, G. G. M. Liposomes 1522 (Springer, 2017).
Hamann, S. et al. Measurement of cell volume changes by fluorescence self-quenching. J. Fluoresc. 12, 139–145 (2002).
Foctave & Scilab. MINUIT—a Binding to Minuit for Matlab (1996).
Blicher, A., Wodzinska, K., Fidorra, M., Winterhalter, M. & Heimburg, T. The temperature dependence of lipid membrane permeability, its quantized nature, and the influence of anesthetics. Biophys. J. 96, 4581–4591 (2009).
James, F. Minuit: Function Minimization and Error Analysis Version 94.1 (CERN, 1994).
El Tayar, N., Tsai, R.-S., Carrupt, P.-A. & Testa, B. Octan-1-ol–water partition coefficients of zwitterionic α-amino acids. Determination by centrifugal partition chromatography and factorization into steric/hydrophobic and polar components. J. Chem. Soc. Perkin Trans. 2, 79–84 (1992).
Perkins, R. & Vaida, V. Phenylalanine increases membrane permeability. J. Am. Chem. Soc. 139, 14388–14391 (2017).
Acknowledgements
The research was funded by an ERC Advanced grant (ABCVolume; no. 670578) and the EU CoFund program ALERT. We thank C. Presutti for testing the protocol.
Author information
Authors and Affiliations
Contributions
B.P. and M.G. conceived the idea and developed the design guidelines; M.G. performed the initial fluorescence measurements and developed the mathematical model for the data processing; J.F. performed the majority of the experiments, developed the protocol further and improved the computational data processing; J.F. and B.P. wrote the manuscript. B.P. supervised, resourced and led the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Ulo Langel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Gabba, M. et al. Biophys. J. 118, 422–434 (2020): https://doi.org/10.1016/j.bpj.2019.11.3384
Gabba, M. & Poolman, B. Biophys. J. 118, 435–447 (2020): https://doi.org/10.1016/j.bpj.2019.11.3383
Frallicciardi, J. et al. Nat. Commun. 13, 1605 (2022): https://doi.org/10.1038/s41467-022-29272-x
Key data used in this protocol
Frallicciardi, J. et al. Nat. Commun. 13, 1605 (2022): https://doi.org/10.1038/s41467-022-29272-x
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Figs. 1–3.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frallicciardi, J., Gabba, M. & Poolman, B. Determining small-molecule permeation through lipid membranes. Nat Protoc 17, 2620–2646 (2022). https://doi.org/10.1038/s41596-022-00734-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00734-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.