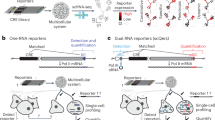
Abstract
The rise of the clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein (Cas) system has made it possible to induce double-strand breaks at almost any desired target site in the genome. In plant somatic cells, double-strand breaks are predominantly repaired by the error-prone nonhomologous end-joining pathway, which can lead to mutations at the break site upon repair. So far, it had only been possible to induce genomic changes of up to a few hundred kilobases in plants utilizing this mechanism. However, by combining the highly efficient Staphylococcus aureus Cas9 (SaCas9) with an egg-cell-specific promoter to facilitate heritable mutations, chromosomal rearrangements in the Mb range, such as inversion and translocations, were obtained in Arabidopsis thaliana recently. Here we describe the chromosome-engineering protocol used to generate these heritable chromosomal rearrangements in A. thaliana. The protocol is based on Agrobacterium-mediated transformation of A. thaliana with transfer DNA constructs containing SaCas9, which is driven by an egg-cell-specific promoter, and two guide RNAs that have been preselected based on their cutting efficiency. In the T1 generation, primary transformants are selected and, if required, analyzed by Droplet Digital PCR and propagated. In the following generations, junction-specific PCR screenings are carried out until plants that carry the rearrangement homozygously are identified. Using this protocol, overall rearrangement frequencies range between 0.03% and 0.5%, depending on the type of rearrangement. In total, it takes about 1 year to establish homozygous lines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schindele, A., Dorn, A. & Puchta, H. CRISPR/Cas brings plant biology and breeding into the fast lane. Curr. Opin. Biotechnol. 61, 7–14 (2020).
Rönspies, M., Dorn, A., Schindele, P. & Puchta, H. CRISPR–Cas-mediated chromosome engineering for crop improvement and synthetic biology. Nat. Plants 7, 566–573 (2021).
Huang, K. & Rieseberg, L. H. Frequency, origins, and evolutionary role of chromosomal inversions in plants. Front. Plant Sci. 11, 296 (2020).
Schmidt, C., Pacher, M. & Puchta, H. Efficient induction of heritable inversions in plant genomes using the CRISPR/Cas system. Plant J. 98, 577–589 (2019).
Schmidt, C. et al. Changing local recombination patterns in Arabidopsis by CRISPR/Cas mediated chromosome engineering. Nat. Commun. 11, 4418 (2020).
Beying, N., Schmidt, C., Pacher, M., Houben, A. & Puchta, H. CRISPR–Cas9-mediated induction of heritable chromosomal translocations in Arabidopsis. Nat. Plants 6, 638–645 (2020).
Durr, J., Papareddy, R., Nakajima, K. & Gutierrez-Marcos, J. Highly efficient heritable targeted deletions of gene clusters and non-coding regulatory regions in Arabidopsis using CRISPR/Cas9. Sci. Rep. 8, 4443 (2018).
Wu, R. et al. An efficient CRISPR vector toolbox for engineering large deletions in Arabidopsis thaliana. Plant Methods 14, 65 (2018).
Ordon, J. et al. Generation of chromosomal deletions in dicotyledonous plants employing a user-friendly genome editing toolkit. Plant J. 89, 155–168 (2017).
Schwartz, C. et al. CRISPR–Cas9-mediated 75.5-Mb inversion in maize. Nat. Plants 6, 1427–1431 (2020).
Schmidt, C., Schindele, P. & Puchta, H. From gene editing to genome engineering: restructuring plant chromosomes via CRISPR/Cas. aBIOTECH 1, 21–31 (2020).
Wolter, F., Klemm, J. & Puchta, H. Efficient in planta gene targeting in Arabidopsis using egg cell-specific expression of the Cas9 nuclease of Staphylococcus aureus. Plant J. 94, 735–746 (2018).
Miki, D., Zhang, W., Zeng, W., Feng, Z. & Zhu, J.-K. CRISPR/Cas9-mediated gene targeting in Arabidopsis using sequential transformation. Nat. Commun. 9, 1967 (2018).
Wolter, F. & Puchta, H. In planta gene targeting can be enhanced by the use of CRISPR/Cas12a. Plant J. 100, 1083–1094 (2019).
Merker, L., Schindele, P. & Puchta, H. Using CRISPR/ttLbCas12a for in planta gene targeting in A. thaliana. Curr. Protoc. Plant Biol. 5, e20117 (2020).
Veillet, F. et al. Transgene-free genome editing in tomato and potato plants using Agrobacterium-mediated delivery of a CRISPR/Cas9 cytidine base editor. Int. J. Mol. Sci. 20, https://doi.org/10.3390/ijms20020402 (2019).
Xu, R. et al. Gene targeting using the Agrobacterium tumefaciens–mediated CRISPR–Cas system in rice. Rice 7, 5 (2014).
Char, S. N. et al. An Agrobacterium-delivered CRISPR/Cas9 system for high-frequency targeted mutagenesis in maize. Plant Biotechnol. J. 15, 257–268 (2017).
Zhang, Z. et al. Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol. J. 17, 1623–1635 (2019).
Gasparis, S. et al. A simple and efficient CRISPR/Cas9 platform for induction of single and multiple, heritable mutations in barley (Hordeum vulgare L.). Plant Methods 14, 111 (2018).
Huang, T.-K. & Puchta, H. Novel CRISPR/Cas applications in plants: from prime editing to chromosome engineering. Transgenic Res. 30, 529–549 (2021).
Steinert, J., Schiml, S., Fauser, F. & Puchta, H. Highly efficient heritable plant genome engineering using Cas9 orthologues from Streptococcus thermophilus and Staphylococcus aureus. Plant J. 84, 1295–1305 (2015).
Wang, Z.-P. et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 144 (2015).
Altpeter, F. et al. Advancing crop transformation in the era of genome editing. Plant Cell 28, 1510–1520 (2016).
Lowe, K. et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28, 1998–2015 (2016).
Merker, L., Schindele, P., Huang, T.-K., Wolter, F. & Puchta, H. Enhancing in planta gene targeting efficiencies in Arabidopsis using temperature-tolerant CRISPR/LbCas12a. Plant Biotechnol. J. 18, 2382–2384 (2020).
Schindele, P., Wolter, F. & Puchta, H. CRISPR guide RNA design guidelines for efficient genome editing. Methods Mol. Biol. 2166, 331–342 (2020).
Steinert, J., Schmidt, C. & Puchta, H. Use of the Cas9 orthologs from Streptococcus thermophilus and Staphylococcus aureus for non-homologous end-joining mediated site-specific mutagenesis in Arabidopsis thaliana. Methods Mol. Biol. 1669, 365–376 (2017).
Alonso José, M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 (2003).
Graham, T. G. W., Carney, S. M., Walter, J. C. & Loparo, J. J. A single XLF dimer bridges DNA ends during nonhomologous end joining. Nat. Struct. Mol. Biol. 25, 877–884 (2018).
Kazda, A. et al. Chromosome end protection by blunt-ended telomeres. Genes Dev. 26, 1703–1713 (2012).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Brinkman, E. K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42, e168–e168 (2014).
Oberpichler, I. et al. Light affects motility and infectivity of Agrobacterium tumefaciens. Environ. Microbiol. 10, 2020–2029 (2008).
Salomon, S. & Puchta, H. Capture of genomic and T-DNA sequences during double-strand break repair in somatic plant cells. EMBO J. 17, 6086–6095 (1998).
Acknowledgements
This work was supported by the European Research Council (Advanced grant ERC-2016-AdG_741306 CRISBREED).
Author information
Authors and Affiliations
Contributions
All authors wrote the manuscript. M.R., P.S. and R.W. designed and created the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Protocols thanks Goetz Hensel, Seiichi Toki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Beying, N. et al. Nat. Plants 6, 638–645 (2020): https://doi.org/10.1038/s41477-020-0663-x
Schmidt, C. et al. Nat. Commun. 11, 4418 (2020): https://doi.org/10.1038/s41467-020-18277-z
Schmidt, C. et al. Plant J. 98, 577–589 (2019): https://doi.org/10.1111/tpj.14322
Supplementary information
Supplementary Data 1
Raw data from thermal gradient simplex ddPCR (Fig. 4)
Rights and permissions
About this article
Cite this article
Rönspies, M., Schindele, P., Wetzel, R. et al. CRISPR–Cas9-mediated chromosome engineering in Arabidopsis thaliana. Nat Protoc 17, 1332–1358 (2022). https://doi.org/10.1038/s41596-022-00686-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-022-00686-7
This article is cited by
-
Recent advances in therapeutic CRISPR-Cas9 genome editing: mechanisms and applications
Molecular Biomedicine (2023)
-
Massive crossover suppression by CRISPR–Cas-mediated plant chromosome engineering
Nature Plants (2022)
-
Redirecting meiotic recombination by CRISPR–Cas-mediated chromosome engineering
Nature Plants (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.