Abstract
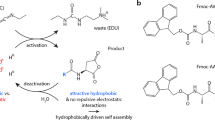
Many supramolecular materials in biological systems are driven to a nonequilibrium state by the irreversible consumption of high-energy molecules such as ATP or GTP. As a result, they exhibit unique dynamic properties such as a tunable lifetime, adaptivity or the ability to self-heal. In contrast, synthetic counterparts that exist in or close to equilibrium are controlled by thermodynamic parameters and therefore lack these dynamic properties. To mimic biological materials more closely, synthetic self-assembling systems have been developed that are driven out of equilibrium by chemical reactions. This protocol describes the synthesis and characterization of such an assembly, which is driven by carbodiimide fuels. Depending on the amount of chemical fuel added to the material, its lifetime can be tuned. In the first step, the protocol details the synthesis and purification of the peptide-based precursors for the fuel-driven assemblies by solid-phase peptide synthesis. Then, we explain how to analyze the kinetic response of the precursors to a carbodiimide-based chemical fuel by HPLC and kinetic models. Finally, we detail how to study the emerging assembly’s macro- and microscopic properties by time-lapse photography, UV-visible spectroscopy, shear rheology, confocal laser scanning microscopy and electron microscopy. The procedure is described using the example of a colloid-forming precursor Fmoc-E-OH and a fiber-forming precursor Fmoc-AAD-OH to emphasize the differences in characterization depending on the type of assembly. The characterization of a precursor’s transient assembly can be done within 5 d. The synthesis and purification of a peptide precursor requires 2 d of work.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
Data availability
The authors declare that all the data supporting the findings of this study are available within the article, the source data files and the Supplementary Information files. Source data are provided with this paper.
Software availability
The MATLAB code used in this protocol together with an exemplary dataset is provided at https://github.com/BoekhovenLab/Nature_protocols. Source data are provided with this paper.
References
Stupp, S. I. et al. Supramolecular materials: self-organized nanostructures. Science 276, 384–389 (1997).
Amabilino, D. B., Smith, D. K. & Steed, J. W. Supramolecular materials. Chem. Soc. Rev. 46, 2404–2420 (2017).
Zhou, J., Li, J., Du, X. & Xu, B. Supramolecular biofunctional materials. Biomaterials 129, 1–27 (2017).
Kato, T., Uchida, J., Ichikawa, T. & Soberats, B. Functional liquid-crystalline polymers and supramolecular liquid crystals. Polym. J. 50, 149–166 (2017).
Hegmann, T., Qi, H. & Marx, V. M. Nanoparticles in liquid crystals: synthesis, self-assembly, defect formation and potential applications. J. Inorg. Organomet. Polym. Mater. 17, 483–508 (2007).
Greaves, T. L. & Drummond, C. J. Ionic liquids as amphiphile self-assembly media. Chem. Soc. Rev. 37, 1709–1726 (2008).
de Greef, T. F. & Meijer, E. W. Materials science: supramolecular polymers. Nature 453, 171–173 (2008).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Weingarten, A. S. et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 6, 964–970 (2014).
Grimaldi, N. et al. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 45, 6520–6545 (2016).
Webber, M. J. & Langer, R. Drug delivery by supramolecular design. Chem. Soc. Rev. 46, 6600–6620 (2017).
Kadler, K. E., Holmes, D. F., Trotter, J. A. & Chapman, J. A. Collagen fibril formation. Biochem. J. 316, 1–11 (1996).
Zhu, J. & Kaufman, L. J. Collagen I self-assembly: revealing the developing structures that generate turbidity. Biophys. J. 106, 1822–1831 (2014).
Prockop, D. J. & Kivirikko, K. I. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 64, 403–434 (1995).
Zhao, B., Hu, H., Mandal, S. K. & Haddon, R. C. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chem. Mater. 17, 3235–3241 (2005).
Amendola, V. & Meneghetti, M. Self-healing at the nanoscale. Nanoscale 1, 74–88 (2009).
Tantakitti, F. et al. Energy landscapes and functions of supramolecular systems. Nat. Mater. 15, 469–476 (2016).
Lancia, F., Ryabchun, A. & Katsonis, N. Life-like motion driven by artificial molecular machines. Nat. Rev. Chem. 3, 536–551 (2019).
Needleman, D. & Dogic, Z. Active matter at the interface between materials science and cell biology. Nat. Rev. Mater. 2 (2017).
Korn, E., Carlier, M. & Pantaloni, D. Actin polymerization and ATP hydrolysis. Science 238, 638–644 (1987).
Desai, A. & Mitchison, T. J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117 (1997).
Kohler, S., Schaller, V. & Bausch, A. R. Structure formation in active networks. Nat. Mater. 10, 462–468 (2011).
Boekhoven, J. et al. Dissipative self-assembly of a molecular gelator by using a chemical fuel. Angew. Chem. Int. Ed. Engl. 49, 4825–4828 (2010).
Boekhoven, J., Hendriksen, W. E., Koper, G. J., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015).
van Esch, J. H., Klajn, R. & Otto, S. Chemical systems out of equilibrium. Chem. Soc. Rev. 46, 5474–5475 (2017).
Saha, B., Chatterjee, A., Reja, A. & Das, D. Condensates of short peptides and ATP for the temporal regulation of cytochrome c activity. Chem. Commun. 55, 14194–14197 (2019).
Post, E. A. J. & Fletcher, S. P. Dissipative self-assembly, competition and inhibition in a self-reproducing protocell model. Chem. Sci. 11, 9434–9442 (2020).
Deng, J. & Walther, A. Pathway complexity in fuel-driven DNA nanostructures with autonomous reconfiguration of multiple dynamic steady states. J. Am. Chem. Soc. 142, 685–689 (2020).
Cardona, M. A. & Prins, L. J. ATP-fuelled self-assembly to regulate chemical reactivity in the time domain. Chem. Sci. 11, 1518–1522 (2020).
Hossain, M. M., Atkinson, J. L. & Hartley, C. S. Dissipative assembly of macrocycles comprising multiple transient bonds. Angew. Chem. Int. Ed. Engl. 59, 13807–13813 (2020).
Bal, S., Ghosh, C., Ghosh, T., Vijayaraghavan, R. K. & Das, D. Non-equilibrium polymerization of cross-beta amyloid peptides for temporal control of electronic properties. Angew. Chem. Int. Ed. Engl. 59, 13506–13510 (2020).
Ragazzon, G. & Prins, L. J. Energy consumption in chemical fuel-driven self-assembly. Nat. Nanotechnol. 13, 882–889 (2018).
Astumian, R. D. Kinetic asymmetry allows macromolecular catalysts to drive an information ratchet. Nat. Commun. 10, 3837 (2019).
Maiti, S., Fortunati, I., Ferrante, C., Scrimin, P. & Prins, L. J. Dissipative self-assembly of vesicular nanoreactors. Nat. Chem. 8, 725–731 (2016).
van Ravensteijn, B. G. P., Hendriksen, W. E., Eelkema, R., van Esch, J. H. & Kegel, W. K. Fuel-mediated transient clustering of colloidal building blocks. J. Am. Chem. Soc. 139, 9763–9766 (2017).
Dambenieks, A. K., Vu, P. H. Q. & Fyles, T. M. Dissipative assembly of a membrane transport system. Chem. Sci. 5, 3396–3403 (2014).
Ogden, W.A. & Guan, Z. Redox chemical-fueled dissipative self-assembly of active materials. ChemSystemsChem https://doi.org/10.1002/syst.201900030 (2019).
Leira-Iglesias, J., Sorrenti, A., Sato, A., Dunne, P. A. & Hermans, T. M. Supramolecular pathway selection of perylenediimides mediated by chemical fuels. Chem. Commun. 52, 9009–9012 (2016).
Tena-Solsona, M. et al. Non-equilibrium dissipative supramolecular materials with a tunable lifetime. Nat. Commun. 8, 15895 (2017).
Kariyawasam, L. S. & Hartley, C. S. Dissipative assembly of aqueous carboxylic acid anhydrides fueled by carbodiimides. J. Am. Chem. Soc. 139, 11949–11955 (2017).
Dai, K. et al. Regulating chemically fueled peptide assemblies by molecular design. J. Am. Chem. Soc. 142, 14142–14149 (2020).
Riess, B. et al. Dissipative assemblies that inhibit their deactivation. Soft Matter 14, 4852–4859 (2018).
Grotsch, R. K. et al. Dissipative self-assembly of photoluminescent silicon nanocrystals. Angew. Chem. Int. Ed. Engl. 57, 14608–14612 (2018).
Grotsch, R. K. et al. Pathway dependence in the fuel-driven dissipative self-assembly of nanoparticles. J. Am. Chem. Soc. 141, 9872–9878 (2019).
Tena-Solsona, M., Wanzke, C., Riess, B., Bausch, A. R. & Boekhoven, J. Self-selection of dissipative assemblies driven by primitive chemical reaction networks. Nat. Commun. 9, 2044 (2018).
Wanzke, C., Tena-Solsona, M., Rieß, B., Tebcharani, L. & Boekhoven, J. Active droplets in a hydrogel release drugs with a constant and tunable rate. Mater. Horiz. 7, 1397–1403 (2020).
Donau, C. et al. Active coacervate droplets as a model for membraneless organelles and protocells. Nat. Commun. 11, 5167 (2020).
Kriebisch, B. A. K. et al. Reciprocal coupling in chemically fueled assembly: a reaction cycle regulates self-assembly and vice versa. J. Am. Chem. Soc. 142, 20837–20844 (2020).
Panja, S., Dietrich, B. & Adams, D. J. Chemically fuelled self-regulating gel-to-gel transition. ChemSystemsChem https://doi.org/10.1002/syst.201900038 (2019).
Bal, S., Das, K., Ahmed, S. & Das, D. Chemically fueled dissipative self-assembly that exploits cooperative catalysis. Angew. Chem. Int. Ed. Engl. 58, 244–247 (2019).
Coin, I., Beyermann, M. & Bienert, M. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences. Nat. Protoc. 2, 3247–3256 (2007).
Chung, B. K. W., White, C. J. & Yudin, A. K. Solid-phase synthesis, cyclization, and site-specific functionalization of aziridine-containing tetrapeptides. Nat. Protoc. 12, 1277–1287 (2017).
Murray, J. K. & Gellman, S. H. Parallel synthesis of peptide libraries using microwave irradiation. Nat. Protoc. 2, 624–631 (2007).
Sackett, D. L. & Wolff, J. Nile red as a polarity-sensitive fluorescent probe of hydrophobic protein surfaces. Anal. Biochem. 167, 228–234 (1987).
Stuart, M. C. A., van de Pas, J. C. & Engberts, J. B. F. N. The use of Nile Red to monitor the aggregation behavior in ternary surfactant-water-organic solvent systems. J. Phys. Org. Chem. 18, 929–934 (2005).
Schnitter, F. & Boekhoven, J. A method to quench carbodiimide-fueled self-assembly. ChemSystemsChem https://doi.org/10.1002/syst.202000037 (2020).
Draper, E. R. & Adams, D. J. Low-molecular-weight gels: the state of the art. Chem 3, 390–410 (2017).
Greenspan, P., Mayer, E. P. & Fowler, S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 100, 965–973 (1985).
Nakajima, N. & Ikada, Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjug. Chem. 6, 123–130 (1995).
Goodling, K., Johnson, K., Lefkowitz, L. & Williams, B. W. The modern student laboratory: luminescent characterization of sodium dodecyl sulfate micellar solution properties. J. Chem. Educ. 71, A8 (1994).
Brown, P. R. Effect of flow rates and the slope of the linear concentration gradient on peak areas in high pressure liquid chromatography. J. Chromatogr. A 57, 383–390 (1971).
Acknowledgements
This research was conducted within the Max Planck School Matter to Life supported by the German Federal Ministry of Education and Research (BMBF) in collaboration with the Max Planck Society. J.B., F.S., A.M.B., B.W. and O.L. acknowledge funding by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – SFB-863 – Project ID 111166240 (Project B11) and funding by the European Research Council (ERC starting grant 852187). J.R.F. acknowledges the Deutsche Forschungsgemeinschaft for project 411722921. Cryo-TEM measurements were performed using infrastructure contributed by the Dietz Lab and the TUM EM Core Facility. We acknowledge the technical support provided by F. Kohler.
Author information
Authors and Affiliations
Contributions
F.S. and A.M.B. contributed equally to this protocol. They designed and performed the experiments and wrote the manuscript. B.W. and J.R.F. carried out the experiments. J.B. designed experiments, outlined and wrote the manuscript, and supervised the project. O.L. designed the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Dibyendu Das and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Tena-Solsona, M. et al. Nat. Commun. 8, 15895 (2017): https://doi.org/10.1038/ncomms15895
Schnitter, F. & Boekhoven, J. ChemSystemsChem 3, e2000037 (2021): https://doi.org/10.1002/syst.202000037
Donau, C. et al. Nat. Commun. 11, 5157 (2020): https://doi.org/10.1038/s41467-020-18815-9
Dai, K. et al. J. Am. Chem. Soc. 142, 33 (2020): https://doi.org/10.1021/jacs.0c04203
Kriebisch, B. A. K. et al. J. Am. Chem. Soc. 142, 49 (2020): https://doi.org/10.1021/jacs.0c10486
Extended data
Extended Data Fig. 1 Cryo-TEM microscope micrograph.
The control micrograph of a 10 mM precursor 2 stock solution excludes preassembly of the inactivated precursor in the absence of EDC fuel.
Extended Data Fig. 2 EDC hydrolysis and Fmoc deprotection kinetics.
a,b, The addition of the quenching solution freezes the reaction cycle of Fmoc-E-OH (1) (a) and Fmoc-AAD-OH (2) (b). In the timescale of the HPLC analysis, the degradation of EDC and Fmoc deprotection does not falsify the concentration determination.
Extended Data Fig. 3 The absorbance of light as a measure for turbidity.
a, Usage of different sample containers affects the measurement quality as sedimentation in a 96-well plate wrongly extends the system’s lifetime. b, Regarding colloids formed by 1, a cuvette is a better choice as sample container.
Supplementary information
Supplementary Video 1
Macroscopic property of reaction cycle shown by evolution of turbidity
Supplementary Video 2
Macroscopic property of reaction cycle shown by evolution of a hydrogel
Source data
Source Data Fig. 2
HPLC signal areas.
Source Data Fig. 3
UV-VIS absorbance.
Source Data Fig. 4
Rheology storage and loss modulus.
Source Data Fig. 10
Nile Red fluorescence intensity.
Source Data Fig. 11
HPLC calibration.
Source Data Extended Data Fig. 2
EDC hydrolysis and Fmoc deprotection kinetics.
Source Data Extended Data Fig. 3
Effect of sample container on UV-VIS measurements.
Rights and permissions
About this article
Cite this article
Schnitter, F., Bergmann, A.M., Winkeljann, B. et al. Synthesis and characterization of chemically fueled supramolecular materials driven by carbodiimide-based fuels. Nat Protoc 16, 3901–3932 (2021). https://doi.org/10.1038/s41596-021-00563-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-021-00563-9
This article is cited by
-
Memory, switches, and an OR-port through bistability in chemically fueled crystals
Nature Communications (2022)
-
Viscoelastic behavior of chemically fueled supramolecular hydrogels under load and influence of reaction side products
Communications Materials (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.