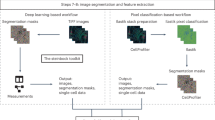
Abstract
Cell morphology encodes essential information on many underlying biological processes. It is commonly used by clinicians and researchers in the study, diagnosis, prognosis, and treatment of human diseases. Quantification of cell morphology has seen tremendous advances in recent years. However, effectively defining morphological shapes and evaluating the extent of morphological heterogeneity within cell populations remain challenging. Here we present a protocol and software for the analysis of cell and nuclear morphology from fluorescence or bright-field images using the VAMPIRE algorithm (https://github.com/kukionfr/VAMPIRE_open). This algorithm enables the profiling and classification of cells into shape modes based on equidistant points along cell and nuclear contours. Examining the distributions of cell morphologies across automatically identified shape modes provides an effective visualization scheme that relates cell shapes to cellular subtypes based on endogenous and exogenous cellular conditions. In addition, these shape mode distributions offer a direct and quantitative way to measure the extent of morphological heterogeneity within cell populations. This protocol is highly automated and fast, with the ability to quantify the morphologies from 2D projections of cells seeded both on 2D substrates or embedded within 3D microenvironments, such as hydrogels and tissues. The complete analysis pipeline can be completed within 60 minutes for a dataset of ~20,000 cells/2,400 images.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from GitHub: Micropattern Data (https://github.com/kukionfr/Micropattern_MEF_LMNA_Image) and Aging Data (https://github.com/kukionfr/Aging_human_dermal_fibroblast_nucleus). A smaller example dataset is provided as Supplementary Data 1 and is also deposited on GitHub: https://github.com/kukionfr/VAMPIRE_open/releases/download/v1.0/Supplementary.Data.zip.
Code availability
The VAMPIRE source code is available on GitHub: https://github.com/kukionfr/VAMPIRE_open. The code can be accessed and used by readers without restriction.
References
Wu, P. H. et al. Evolution of cellular morpho-phenotypes in cancer metastasis. Sci. Rep. 5, 1–10 (2015).
Chen, W.-C. et al. Functional interplay between the cell cycle and cell phenotypes. Integr. Biol. 5, 523–34 (2013).
Chambliss, A. B., Wu, P. H., Chen, W. C., Sun, S. X. & Wirtz, D. Simultaneously defining cell phenotypes, cell cycle, and chromatin modifications at single-cell resolution. FASEB J 27, 2667–2676 (2013).
Bakal, C., Aach, J., Church, G. & Perrimon, N. Quantitative morphological signatures define local signaling networks regulating cell morphology. Science 316, 1753–1756 (2007).
Rohban, M. H. et al. Systematic morphological profiling of human gene and allele function via cell painting. eLife 6, e24060 (2017).
Wu, P.-H. et al. Single-cell morphology encodes metastatic potential. Sci. Adv. https://doi.org/10.1126/sciadv.aaw6938 (2020).
Driscoll, M. K. et al. Robust and automated detection of subcellular morphological motifs in 3D microscopy images. Nat. Methods https://doi.org/10.1038/s41592-019-0539-z (2019).
Yeung, T. et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton https://doi.org/10.1002/cm.20041 (2005).
Guo, Q. et al. Modulation of keratocyte phenotype by collagen fibril nanoarchitecture in membranes for corneal repair. Biomaterials 34, 9365–9372 (2013).
Sero, J. E. et al. Cell shape and the microenvironment regulate nuclear translocation of NF-κB in breast epithelial and tumor cells. Mol. Syst. Biol. 11, 790 (2015).
Simm, J. et al. Repurposing high-throughput image assays enables biological activity prediction for drug discovery. Cell Chem. Biol. https://doi.org/10.1016/j.chembiol.2018.01.015 (2018).
Bray, M.-A. et al. A dataset of images and morphological profiles of 30 000 small-molecule treatments using the Cell Painting assay. Gigascience 6, 1–5 (2017).
Wawer, M. J. et al. Toward performance-diverse small-molecule libraries for cell-based phenotypic screening using multiplexed high-dimensional profiling. Proc. Natl Acad. Sci. USA 111, 10911–10916 (2014).
Bray, M. A. et al. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 11, 1757–1774 (2016).
Beghin, A. et al. Localization-based super-resolution imaging meets high-content screening. Nat. Methods 14, 1184–1190 (2017).
Meijering, E., Carpenter, A. E., Peng, H., Hamprecht, F. A. & Olivo-Marin, J. C. Imagining the future of bioimage analysis. Nat. Biotechnol. 34, 1250–1255 (2016).
Ruan, X. & Murphy, R. F. Evaluation of methods for generative modeling of cell and nuclear shape. Bioinformatics https://doi.org/10.1093/bioinformatics/bty983 (2019).
Piccinini, F. et al. Advanced cell classifier: user-friendly machine-learning-based software for discovering phenotypes in high-content imaging data. Cell Syst 4, 651–655.e5 (2017).
Danuser, G. Computer vision in cell biology. Cell 147, 973–978 (2011).
Falk, T. et al. U-Net: deep learning for cell counting, detection, and morphometry. Nat. Methods 16, 67–70 (2019).
Chicco, D. Ten quick tips for machine learning in computational biology. BioData Mining https://doi.org/10.1186/s13040-017-0155-3 (2017).
Gabril, M. Y. & Yousef, G. M. Informatics for practicing anatomical pathologists: Marking a new era in pathology practice. Modern Pathol. 23, 349–358 (2010).
Fuchs, T. J. & Buhmann, J. M. Computational pathology: challenges and promises for tissue analysis. Computer. Med. Imag. Graphics 35, 515–530 (2011).
Sarnecki, J. S. et al. A robust nonlinear tissue-component discrimination method for computational pathology. Lab. Investig 96, 450–458 (2016).
Beck, A. H. et al. Systematic analysis of breast cancer morphology uncovers stromal features associated with survival. Sci. Transl. Med. 3, 108ra113–108ra113 (2011).
Phillip, J. M. et al. Biophysical and biomolecular determination of cellular age in humans. Nat. Biomed. Eng. 1, 0093 (2017).
Pegoraro, G. & Misteli, T. High-throughput imaging for the discovery of cellular mechanisms of disease. Trends Genet. 33, 604–615 (2017).
Lang, P., Yeow, K., Nichols, A. & Scheer, A. Cellular imaging in drug discovery. Nat. Rev. Drug Discov. 5, 343–356 (2006).
Loo, L. H., Wu, L. F. & Altschuler, S. J. Image-based multivariate profiling of drug responses from single cells. Nat. Methods 4, 445–453 (2007).
Sailem, H. Z., Sero, J. E. & Bakal, C. Visualizing cellular imaging data using PhenoPlot. Nat. Commun. 6, 1–6 (2015).
McQuin, C. et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. https://doi.org/10.1371/journal.pbio.2005970 (2018).
Carpenter, A. E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. https://doi.org/10.1186/gb-2006-7-10-r100 (2016).
Schindelin, J. et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods https://doi.org/10.1038/nmeth.2019 (2012).
Jayatilaka, H. et al. EB1 and cytoplasmic dynein mediate protrusion dynamics for efficient 3-dimensional cell migration. FASEB J. https://doi.org/10.1096/fj.201700444RR (2018).
Jayatilaka, H. et al. Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat. Commun. 8, 15584 (2017).
Jayatilaka, H. et al. Tumor cell density regulates matrix metalloproteinases for enhanced migration. Oncotarget 9, 32556–32569 (2018).
Phillip, J. M., Aifuwa, I., Walston, J. & Wirtz, D. The mechanobiology of aging. Annu. Rev. Biomed. Eng. 17, 113–141 (2015).
Kim, D.-H. et al. Volume regulation and shape bifurcation in the cell nucleus. J. Cell Sci. 129, 457–457 (2016).
Yu, Y. et al. Inhibition of spleen tyrosine kinase potentiates paclitaxel-induced cytotoxicity in ovarian cancer cells by stabilizing microtubules. Cancer Cell 28, 82–96 (2015).
Driscoll, M. K. et al. Automated image analysis of nuclear shape: What can we learn from a prematurely aged cell? Aging 4, 119–132 (2012).
Bookstein, F. L. Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Med. Image Anal. https://doi.org/10.1016/S1361-8415(97)85012-8 (1997).
Dryden, I. L. & Mardia, K. V. Statistical Shape Analysis, with Applications in R 2nd edn. https://doi.org/10.1002/9781119072492 (2016).
Keren, K. et al. Mechanism of shape determination in motile cells. Nature 453, 475–480 (2008).
Pincus, Z. & Theriot, J. A. Comparison of quantitative methods for cell-shape analysis. J. Microsc. 227, 140–156 (2007).
MacLeod, N. Generalizing and extending the eigenshape method of shape space visualization and analysis. Paleobiology 25, 107–138 (1999).
Tsai, A. et al. A shape-based approach to the segmentation of medical imagery using level sets. IEEE Trans. Med. Imaging https://doi.org/10.1109/TMI.2002.808355 (2003).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. (2011).
Ester, M., Kriegel, H.-P., Sander, J. & Xu, X. A Density-based algorithm for discovering clusters in large spatial databases with noise. in Proceedings of the 2nd International Conference on Knowledge Discovery and Data Mining (1996).
Ankerst, M., Breunig, M. M., Kriegel, H. P. & Sander, J. OPTICS: ordering points to identify the clustering structure. SIGMOD Rec. 28, 49–60 (1999).
Kim, D. H. & Wirtz, D. Focal adhesion size uniquely predicts cell migration. FASEB J. 27, 1351–1361 (2013).
Kim, J.-K. et al. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 8, 2123 (2017).
Zheng, W., Thorne, N. & McKew, J. C. Phenotypic screens as a renewed approach for drug discovery. Drug Discov. Today https://doi.org/10.1016/j.drudis.2013.07.001 (2003).
Kashyap, A., Jain, M., Shukla, S. & Andley, M. Role of nuclear morphometry in breast cancer and its correlation with cytomorphological grading of breast cancer: a study of 64 cases. J. Cytol. https://doi.org/10.4103/JOC.JOC_237_16 (2003).
Seethala, R. R. et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod. Pathol. https://doi.org/10.1038/modpathol.2017.130 (2018).
Legland, D., Arganda-Carreras, I. & Andrey, P. MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics https://doi.org/10.1093/bioinformatics/btw413 (2016).
Abdi, H. & Williams, L. J. Principal component analysis. Wiley Interdisciplinary Reviews: Computational Statistics 2, 433–459 (2010).
Shlens, J. A tutorial on principal component analysis. Preprint at https://arxiv.org/abs/1404.1100 (2014).
Lee, H. C., Liao, T., Zhang, Y. J. & Yang, G. Shape component analysis: Structure-preserving dimension reduction on biological shape spaces. Bioinformatics https://doi.org/10.1093/bioinformatics/btv648 (2016).
Hinton, G. E. & Salakhutdinov, R. R. Reducing the dimensionality of data with neural networks. Science https://doi.org/10.1126/science.1127647 (2006).
Goodfellow, I. J. et al. Generative adversarial nets. GitHub http://www.github.com/goodfeli/adversarial.
Osokin, A., Chessel, A., Salas, R. E. C. & Vaggi, F. GANs for biological image synthesis. Proc. IEEE Int. Conf. Comput. Vis. 2017, 2252–2261 (2017).
Johnson, G. R., Donovan-Maiye, R. M. & Maleckar, M. M. Generative modeling with conditional autoencoders: building an integrated cell. Preprint at arXiv https://arxiv.org/abs/1705.00092 (2017).
Liberti, L. Distance geometry and data science. TOP 28, 271–339 (2020).
Donaldson, J. G. Immunofluorescence staining. Curr. Protoc. Cell Biol. 60, 4.3.1–4.3.6 (1998).
Giri, A. et al. The Arp2/3 complex mediates multigeneration dendritic protrusions for efficient 3-dimensional cancer cell migration. FASEB J 27, 4089–4099 (2013).
Fraley, S. I. et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. https://doi.org/10.1038/ncb2062 (2010).
Artym, V. V. & Matsumoto, K. Imaging cells in three-dimensional collagen matrix. Curr. Protoc. Cell Biol. https://doi.org/10.1002/0471143030.cb1018s48 (2010).
Fischer, A. H., Jacobson, K. A., Rose, J. & Zeller, R. Hematoxylin and eosin staining of tissueand cell sections. Cold Spring Harb. Protoc. 3, pdb.prot4986 (2008).
Kim, S. W., Roh, J. & Park, C. S. Immunohistochemistry for pathologists: Protocols, pitfalls, and tips. J. Pathol. Transl. Med. 50, 411–418 (2016).
Hale, C. M. et al. SMRT analysis of MTOC and nuclear positioning reveals the role of EB1 and LIC1 in single-cell polarization. J. Cell Sci. 124, 4267–4285 (2011).
Kim, D. H. & Wirtz, D. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials 48, 161–172 (2015).
Hale, C. M. et al. SMRT analysis of MTOC and nuclear positioning reveals the role of EB1 and LIC1 in single-cell polarization. J. Cell Sci. 124, 4267–4285 (2011).
Acknowledgements
This work was supported in part by National Institutes of Health grants U54CA143868 (D.W.), R01CA174388 (D.W.), P30AG021334 (P.H.W. and J.M.P.) and U01AG060903 (D.W., J.M.P. and P.H.W.).
Author information
Authors and Affiliations
Contributions
J.M.P. and P.H.W. designed and conducted experiments; P.H.W., J.M.P., D.W. and W.C. conceived analysis and workflow of VAMPIRE; P.H.W. developed the original VAMPIRE software; K.S.H. converted the VAMPIRE software from MATLAB to Python; K.S.H. developed the graphical user interface of VAMPIRE; K.S.H. and J.M.P. analyzed and plotted data; P.H.W. and D.W. supervised the study; J.M.P., D.W., K.S.H. and P.H.W. wrote and edited the protocol; D.W., J.M.P., and P.H.W. secured funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Wu, P. -H. et al. Sci. Rep. 5, 1–10 (2015): https://www.nature.com/articles/srep18437
Wu, P.-H. et al. Sci. Adv. 6, eaaw6938 (2020): https://advances.sciencemag.org/content/6/4/eaaw6938
Phillip, J. M. et al. Nat. Biomed. Eng. 1, 0093 (2017): https://www.nature.com/articles/s41551-017-0093
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Data 1
Example input files for the protocol procedures, including fluorescence images of phalloidin-stained mouse embryonic fibroblast, their segmented images, CellProfiler segmentation workflow pipeline file, lists of segmented images for building and applying the model in CSV format, and example output files generated during the analysis.
Rights and permissions
About this article
Cite this article
Phillip, J.M., Han, KS., Chen, WC. et al. A robust unsupervised machine-learning method to quantify the morphological heterogeneity of cells and nuclei. Nat Protoc 16, 754–774 (2021). https://doi.org/10.1038/s41596-020-00432-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00432-x
This article is cited by
-
Morphological entropy encodes cellular migration strategies on multiple length scales
npj Systems Biology and Applications (2024)
-
Orientation-invariant autoencoders learn robust representations for shape profiling of cells and organelles
Nature Communications (2024)
-
A method for real-time mechanical characterisation of microcapsules
Biomechanics and Modeling in Mechanobiology (2023)
-
Machine learning-based morphological quantification of replicative senescence in human fibroblasts
GeroScience (2023)
-
Predicting multipotency of human adult stem cells derived from various donors through deep learning
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.