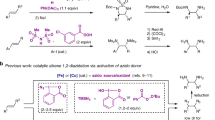
Abstract
This protocol describes an electrochemical synthesis of 1,2-diazides from alkenes. Organic azides are highly versatile intermediates for synthetic chemistry, materials, and biological applications. 1,2-Diazides are commonly reduced to form 1,2-diamines, which are prevalent structural motifs in bioactive natural products, therapeutic agents, and molecular catalysts. The electrochemical formation of 1,2-diazides involves the anodic generation of an azidyl radical from sodium azide, followed by two successive additions of this N-centered radical to the alkene, and is assisted by a Mn catalyst. The electrosynthesis of 1,2-diazides can be carried out using various experimental setups comprising custom-made or commercially available reaction vessels and a direct-current power supply. Readily accessible electrode materials can be used, including carbon (made from reticulated vitreous carbon and pencil lead), nickel foam, and platinum foil. This protocol is also demonstrated using ElectraSyn, a standardized electrochemistry kit. Compared with conventional synthetic approaches, electrochemistry allows for the precise control of the anodic potential input, eliminates the need for stoichiometric and often indiscriminate oxidants, and minimizes the generation of wasteful byproducts. As such, our electrocatalytic synthesis exhibits various advantages over existing methods for alkene diamination, including sustainability, operational simplicity, substrate generality, and exceptional functional-group compatibility. The resultant 1,2-diazides can be smoothly reduced to 1,2-diamines in a single step with high chemoselectivity. To exemplify this, we include a procedure for catalytic hydrogenation using palladium on carbon. This protocol, therefore, constitutes a general approach to accessing 1,2-diazides and 1,2-diamines from alkenes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bariwal, J. & Van der Eycken, E. C–N bond forming cross-coupling reactions: an overview. Chem. Soc. Rev. 42, 9283–9303 (2013).
McGrath, N. A., Brichacek, M. & Njardarson, J. T. A graphical journey of innovative organic architectures that have improved our lives. J. Chem. Ed. 87, 1348–1349 (2010).
Lucet, D., Le Gall, T. & Mioskowski, C. The chemistry of vicinal diamines. Angew. Chem. Int. Ed. Engl. 37, 2580–2627 (1998).
Cardona, F. & Goti, A. Metal-catalysed 1,2-diamination reactions. Nat. Chem. 1, 269–275 (2009).
Moeller, K. D. Synthetic applications of anodic electrochemistry. Tetrahedron 56, 9527–9554 (2000).
Yoshida, J., Kataoka, K., Horcajada, R. & Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Morofuji, T., Shimizu, A. & Yoshida, J. Heterocyclization approach for electrooxidative coupling of functional primary alkylamines with aromatics. J. Am. Chem. Soc. 137, 9816–9819 (2015).
Siu, T. & Yudin, A. K. Practical olefin aziridination with a broad substrate scope. J. Am. Chem. Soc. 124, 530–531 (2002).
Fu, N., Sauer, G. S., Saha, A., Loo, A. & Lin, S. Metal-catalyzed electrochemical diazidation of alkenes. Science 357, 575–579 (2017).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Zhang, W. & Jacobsen, E. N. Preparation of trans-1,2-diamino-1,2-dimethylcyclohexane via highly stereoselective olefin oxidation by dinitrogen tetroxide. Tetrahedron Lett. 32, 1711–1714 (1991).
Parry, J. B., Fu, N. & Lin, S. Electrocatalytic difunctionalization of olefins as a general approach to the synthesis of vicinal diamines. Synlett 29, 257–265 (2018).
Minisci, F. Free-radical additions to olefins in the presence of redox systems. Acc. Chem. Res. 8, 165–171 (1975).
Olson, D.-E., Su, J. Y., Roberts, D. A. & Du Bois, J. Vicinal diamination of alkenes under Rh-catalysis. J. Am. Chem. Soc. 136, 13506–13509 (2014).
Muñiz, K., Barreiro, L., Romero, R. M. & Martínez, C. Catalytic asymmetric diamination of styrenes. J. Am. Chem. Soc. 139, 4354–4357 (2017).
Zhang, B. & Studer, A. Copper-catalyzed intermolecular aminoazidation of alkenes. Org. Lett. 16, 1790–1793 (2014).
Zhao, B., Yuan, W., Du, H. & Shi, Y. Cu(I)-catalyzed intermolecular diamination of activated terminal olefins. Org. Lett. 9, 4943–4945 (2007).
Yuan, Y.-A., Lu, D.-F., Chen, Y.-R. & Xu, H. Iron-catalyzed direct diazidation for a broad range of olefins. Angew. Chem. Int. Ed. Engl. 55, 534–538 (2016).
Li, G., Wei, H. X., Kim, S. H. & Carducci, M. A novel electrophilic diamination reaction of alkenes. Angew. Chem. Int. Ed. Engl. 40, 4277–4280 (2001).
Snider, B. B. & Lin, H. An improved procedure for the conversion of alkenes and glycals to 1,2-diazides using Mn(OAc)3•2H2O in acetonitrile containing trifluoroacetic acid. Synth. Commun. 28, 1913–1922 (1998).
Fumagalli, G., Rabet, P. T. G., Boyd, S. & Greaney, M. F. Three-component azidation of styrene-type double bonds: light-switchable behavior of a copper photoredox catalyst. Angew. Chem. Int. Ed. Engl. 54, 11481–11484 (2015).
For an example, see: Pikul, S. & Corey, E. J. (1R,2R)-(+)- and (1S,2S)-(−)-1,2-diphenyl-1,2-ethylenediamine. Org. Synth. 71, 22 (1993).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemistry: calling all engineers. Angew. Chem. Int. Ed. Engl. 57, 4149–4155 (2018).
Zhu, H.-T., Arosio, L., Villa, R., Nebuloni, M. & Xu, H. Process safety assessment of the iron-catalyzed direct olefin diazidation for the expedient synthesis of vicinal primary diamines. Org. Process Res. Dev. 21, 2068–2072 (2017).
Fu, N., Sauer, G. S. & Lin, S. Electrocatalytic dichlorination of alkenes using nucleophilic chlorine sources. J. Am. Chem. Soc. 139, 15548–15553 (2017).
Ye, K.-Y. et al. Anodically coupled electrolysis for the heterodifunctionalization of alkenes. J. Am. Chem. Soc. 140, 2438–2441 (2018).
Xu, H.-C., Campbell, J. M. & Moeller, K. D. Cyclization reactions of anode-generated amidyl radicals. J. Org. Chem. 79, 379–391 (2014).
Finney, E. E., Ogawa, K. A. & Boydston, A. J. Organocatalyzed anodic oxidation of aldehydes. J. Am. Chem. Soc. 134, 12374–12377 (2012).
Zhu, L. et al. Electrocatalytic generation of amidyl radicals for olefin hydroamidation: use of solvent effects to enable anilide oxidation. Angew. Chem. Int. Ed. Engl. 55, 2226–2229 (2016).
Horn, E. J. et al. Scalable and sustainable electrochemical allylic C–H oxidation. Nature 533, 71–81 (2016).
Kawamata, Y. et al. Scalable, electrochemical oxidation of unactivated C–H bonds. J. Am. Chem. Soc. 139, 7448–7451 (2017).
Gütz, C., Klöckner, G. & Waldvogel, S. R. Electrochemical screening for electroorganic synthesis. Org. Process Res. Dev. 20, 26–32 (2016).
Wallace, K. J. et al. Preparation of 1,3,5-tris(aminomethyl)-2,4,6-triethylbenzene from two versatile 1,3,5-tri(halosubstituted) 2,4,6-triethylbenzene derivatives. Synthesis 12, 2080–2083 (2005).
Bayley, H., Standring, D. N. & Knowles, J. R. Propane-1,3-dithiol: a selective reagent for the efficient reduction of alkyl and aryl azides to amines. Tetrahedron Lett. 19, 3633–3634 (1978).
Chen, Y., Kamlet, A. S., Steinman, J. B. & Liu, D. R. A biomolecule-compatible visible-light-induced azide reduction from a DNA-encoded reaction-discovery system. Nat. Chem. 3, 146–153 (2011).
Rao, H. S. P. & Siva, P. Facile reduction of azides with sodium borohydride/copper (II) sulphate system. Synth. Commun. 24, 549–555 (1994).
Acknowledgements
Financial support was provided by Cornell University and the Atkinson Center for a Sustainable Future. S.L. is thankful to the National Science Foundation (NSF) for a CAREER Award (CHE-1751839). This study made use of the Cornell Center for Materials Research Shared Facilities supported by NSF MRSEC (DMR-1719875) and an NMR facility supported by the NSF (CHE-1531632). G.S.S. is grateful for an NSF Graduate Fellowship (DGE-1650441). We thank P. Baran and IKA for their generous gift of ElectraSyn.
Author information
Authors and Affiliations
Contributions
N.F. and S.L. designed the experiments. N.F. and G.S.S. carried out the experiments. N.F., G.S.S., and S.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related Links
Key references using this protocol
1. Fu, N., Sauer, G.S., Saha, A., Loo, A. & Lin, S. Science, 357, 575–579 (2017). https://doi.org/10.1126/science.aan6206
2. Fu, N., Sauer, G.S. & Lin, S. J. Am. Chem. Soc. 139, 15548–15553 (2017). https://doi.org/10.1021/jacs.7b09388
3. Ye, K.-Y., Pombar, G., Fu, N., Sauer, G.S., Keresztes, I. & Lin, S. J. Am. Chem. Soc. 140, 2438–2441 (2018). https://doi.org/10.1021/jacs.7b13387
Integrated supplementary information
Supplementary Figure 1.
Preparation of the nickel cathode and carbon anode for electrocatalytic diazidation of alkenes
Supplementary Figure 2. IKA EletraSyn setup: electrode assemblies.
Left: Ni foam and RVC electrodes and the electrode holders; Right: Ni foam and RVC electrode assemblies as described in Box 1
Supplementary Figure 3. IKA EletraSyn setup: parts for vial assembly.
Top: ElectraSyn vial cap; Bottom left: ElectraSyn vial (10 mL) with Teflon tape wrapped around the thread. Bottom middle: RVC electrode assembly. Bottom right: Ni foam electrode assembly
Supplementary Figure 4.
IKA EletraSyn setup: vial assembly
Supplementary Figure 5.
IKA EletraSyn setup: reactor assembly
Supplementary Figure 6.
Custom two-neck glass tube for small-scale electrolysis with standard no. 15 internal thread and 14/20 joint
Supplementary Figure 7. Custom Teflon cap components.
Left: Electrical feedthrough with 2 mm sockets created as described in Box 2. Right: Teflon cap as created in Box 2, including a #15 Teflon bushing, two electrical feedthroughs, and an O-ring; viewed from the side
Supplementary Figure 8. Custom Teflon cap components.
Left: Electrical feedthrough created as described in Box 2. Right: Teflon cap as created in Box 2; viewed from the bottom
Supplementary Figure 9. Custom Teflon cap components.
Left: Disassembled Teflon cap showing the hole drilled in the Teflon. Right: Electrical feedthrough created as described in Box 2
Supplementary Figure 10.
TGA data for (3,4-diazidobutyl)benzene (3)
Supplementary Figure 11.
TGA data for trans-2,3-diazido-1-tosylindoline (4)
Supplementary Figure 12.
TGA data for methyl 2,3-diazido-3-methyl-2-phenylbutanoate (7)
Supplementary Figure 13.
TGA data for 4-(1,2-diazidoethyl)benzaldehyde (9)
Supplementary Figure 14.
TGA data for 1,2-diazidoethyl ferrocene (13)
Supplementary Figure 15.
TGA data for sodium azide
Supplementary Figure 16.
1H NMR spectrum for a mixture of (2,3-diazidopropyl)benzene (16) and (1,2,3-triazidopropyl)benzene (17)
Supplementary Figure 17.
13C NMR spectrum for a mixture of (2,3-diazidopropyl)benzene (16) and (1,2,3-triazidopropyl)benzene (17)
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–17 and Supplementary Methods
Rights and permissions
About this article
Cite this article
Fu, N., Sauer, G.S. & Lin, S. A general, electrocatalytic approach to the synthesis of vicinal diamines. Nat Protoc 13, 1725–1743 (2018). https://doi.org/10.1038/s41596-018-0010-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-018-0010-0
This article is cited by
-
Energy transfer-enabled unsymmetrical diamination using bifunctional nitrogen-radical precursors
Nature Catalysis (2022)
-
Dual electrocatalysis enables enantioselective hydrocyanation of conjugated alkenes
Nature Chemistry (2020)
-
Practical and stereoselective electrocatalytic 1,2-diamination of alkenes
Nature Communications (2019)
-
Electricity-driven asymmetric Lewis acid catalysis
Nature Catalysis (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.